A 20-Year Follow-Up Study of Objectively Measured Physical Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Procedure and Equipment
2.3. Anthropometry
2.4. Data Preparation and Statistical Analysis
2.5. Ethics
3. Results
3.1. Sample Characteristics
3.2. Differences in Steps per Days between the Six Measurement Points
3.3. Tracking of Steps per Day across the Six Measurement Points
3.4. Steps per Day during Weekdays and Weekend Days from Adolescence to Adulthood
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U.; Lancet Physical Activity Series Working, G. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- Nelson, M.C.; Story, M.; Larson, N.I.; Neumark-Sztainer, D.; Lytle, L.A. Emerging adulthood and college-aged youth: An overlooked age for weight-related behavior change. Obesity 2008, 16, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Konstabel, K.; Pasquali, E.; Ruiz, J.R.; Hurtig-Wennlof, A.; Maestu, J.; Lof, M.; Harro, J.; Bellocco, R.; Labayen, I.; et al. Objectively measured physical activity and sedentary time during childhood, adolescence and young adulthood: A cohort study. PLoS ONE 2013, 8, e60871. [Google Scholar] [CrossRef] [PubMed]
- Heath, G.W.; Parra, D.C.; Sarmiento, O.L.; Andersen, L.B.; Owen, N.; Goenka, S.; Montes, F.; Brownson, R.C. Evidence-based intervention in physical activity: Lessons from around the world. Lancet 2012, 380, 272–281. [Google Scholar] [CrossRef]
- Kristensen, P.L.; Korsholm, L.; Møller, N.C.; Wedderkopp, N.; Andersen, L.B.; Froberg, K. Sources of variation in habitual physical activity of children and adolescents: The european youth heart study. Scand. J. Med. Sci. Sports 2008, 18, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Treuth, M.S.; Catellier, D.J.; Schmitz, K.H.; Pate, R.R.; Elder, J.P.; McMurray, R.G.; Blew, R.M.; Yang, S.; Webber, L. Weekend and weekday patterns of physical activity in overweight and normal-weight adolescent girls. Obesity 2007, 15, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Nordman, M.; Matthiessen, J.; Biltoft-Jensen, A.; Ritz, C.; Hjorth, M.F. Weekly variation in diet and physical activity among 4-75-year-old danes. Public Health Nutr. 2020, 23, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Malina, R.M. Physical activity and fitness: Pathways from childhood to adulthood. Am. J. Hum. Biol. 2001, 13, 162–172. [Google Scholar] [CrossRef]
- Shephard, R.J.; Tudor-Locke, C. The Objective Monitoring of Physical Activity: Contributions of Accelerometry to Epidemiology, Exercise Science and Rehabilitation; Cham Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Craigie, A.M.; Lake, A.A.; Kelly, S.A.; Adamson, A.J.; Mathers, J.C. Tracking of obesity-related behaviours from childhood to adulthood: A systematic review. Maturitas 2011, 70, 266–284. [Google Scholar] [CrossRef]
- Telama, R. Tracking of physical activity from childhood to adulthood: A review. Obes. Facts 2009, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.; Dowd, K.P.; MacDonncha, C.; Donnelly, A.E. Tracking of physical activity and sedentary behavior from adolescence to young adulthood: A systematic literature review. J. Adolesc. Health 2019, 65, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, H.J.; Brage, S.; Warren, J.; Besson, H.; Ekelund, U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.S.; Miller, K. Objective monitoring of physical activity using motion sensors and heart rate. Res. Q. Exerc. Sport 2000, 71, S21–S29. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.I.; Van Hirtum, H.W.; Bakker, I.; Hopman-Rock, M.; Hirasing, R.A.; Van Mechelen, W. Validity and reproducibility of motion sensors in youth: A systematic update. Med. Sci. Sports Exerc. 2009, 41, 818–827. [Google Scholar] [CrossRef]
- De Vries, S.I.; Bakker, I.; Hopman-Rock, M.; Hirasing, R.A.; van Mechelen, W. Clinimetric review of motion sensors in children and adolescents. J. Clin. Epidemiol. 2006, 59, 670–680. [Google Scholar] [CrossRef]
- Raustorp, A.; Pangrazi, R.P.; Stahle, A. Physical activity level and body mass index among schoolchildren in south-eastern sweden. Acta Paediatr. 2004, 93, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Raustorp, A.; Stahle, A.; Gudasic, H.; Kinnunen, A.; Mattsson, E. Physical activity and self-perception in school children assessed with the children and youth--physical self-perception profile. Scand. J. Med. Sci. Sports 2005, 15, 126–134. [Google Scholar] [CrossRef]
- Raustorp, A.; Mattsson, E.; Svensson, K.; Stahle, A. Physical activity, body composition and physical self-esteem: A 3-year follow-up study among adolescents in sweden. Scand. J. Med. Sci. Sports 2006, 16, 258–266. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Sisson, S.B.; Lee, S.M.; Craig, C.L.; Plotnikoff, R.C.; Bauman, A. Evaluation of quality of commercial pedometers. Can. J. Public Health 2006, 97 (Suppl. 1), S10–S16. [Google Scholar] [CrossRef]
- Rowe, D.A.; Mahar, M.T.; Raedeke, T.D.; Lore, J. Measuring physical activity in children with pedometers: Reliability, reactivity, and replacement of missing data. Pediatr. Exerc. Sci. 2004, 16, 343–354. [Google Scholar] [CrossRef]
- Pigott, T.D. A review of methods for missing data. Educ. Res. Eval. 2001, 7, 353–383. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the united states measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Telama, R.; Yang, X. Decline of physical activity from youth to young adulthood in finland. Med. Sci. Sports Exerc. 2000, 32, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Van Mechelen, W.; Twisk, J.W.; Post, G.B.; Snel, J.; Kemper, H.C. Physical activity of young people: The Amsterdam longitudinal growth and health study. Med. Sci. Sports Exerc. 2000, 32, 1610–1616. [Google Scholar] [CrossRef]
- Beets, M.W.; Bornstein, D.; Beighle, A.; Cardinal, B.J.; Morgan, C.F. Pedometer-measured physical activity patterns of youth: A 13-country review. Am. J. Prev. Med. 2010, 38, 208–216. [Google Scholar] [CrossRef]
- Tudor-Locke, C.E.; Myers, A.M. Methodological considerations for researchers and practitioners using pedometers to measure physical (ambulatory) activity. Res. Q. Exerc. Sport 2001, 72, 1–12. [Google Scholar] [CrossRef]
- Matthiessen, J.; Andersen, E.W.; Raustorp, A.; Knudsen, V.K.; Sørensen, M.R. Reduction in pedometer-determined physical activity in the adult danish population from 2007 to 2012. Scand. J. Soc. Med. 2015, 43, 525–533. [Google Scholar] [CrossRef]
- Harris, T.; Kerry, S.M.; Limb, E.S.; Furness, C.; Wahlich, C.; Victor, C.R.; Iliffe, S.; Whincup, P.H.; Ussher, M.; Ekelund, U.; et al. Physical activity levels in adults and older adults 3-4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in uk primary care. PLoS Med. 2018, 15, e1002526. [Google Scholar] [CrossRef]
- Raustorp, A.; Ekroth, Y. Tracking of pedometer-determined physical activity: A 10-year follow-up study from adolescence to adulthood in sweden. J. Phys. Act. Health 2013, 10, 1186–1192. [Google Scholar] [CrossRef]
- Raustorp, A.; Archer, T.; Svensson, K.; Perlinger, T.; Alricsson, M. Physical self-esteem, a five year follow-up study on swedish adolescents. Int. J. Adolesc. Med. Health 2009, 21, 497–507. [Google Scholar] [CrossRef]
- Malina, R. Tracking of physical activity across the lifespan. Pres. Counc. Phys. Fit. Sports Res. Dig. 2001, 3, 1–8. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Bassett, D.R., Jr.; Rutherford, W.J.; Ainsworth, B.E.; Chan, C.B.; Croteau, K.; Giles-Corti, B.; Le Masurier, G.; Moreau, K.; Mrozek, J.; et al. Bmi-referenced cut points for pedometer-determined steps per day in adults. J. Phys. Act. Health 2008, 5 (Suppl. 1), S126–S139. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; McClain, J.J.; Brychta, R.J.; Chen, K.Y. Evolution of accelerometer methods for physical activity research. Br. J. Sports Med. 2014, 48, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Beets, M.W.; Welk, G.J. Everything you wanted to know about selecting the “right” actigraph accelerometer cut-points for youth, but…: A systematic review. J. Sci. Med. Sport 2012, 15, 311–321. [Google Scholar] [CrossRef]
- Tison, G.H.; Avram, R.; Kuhar, P.; Abreau, S.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Worldwide effect of COVID-19 on physical activity: A descriptive study. Ann. Intern. Med. 2020, 173, 767–770. [Google Scholar] [CrossRef] [PubMed]
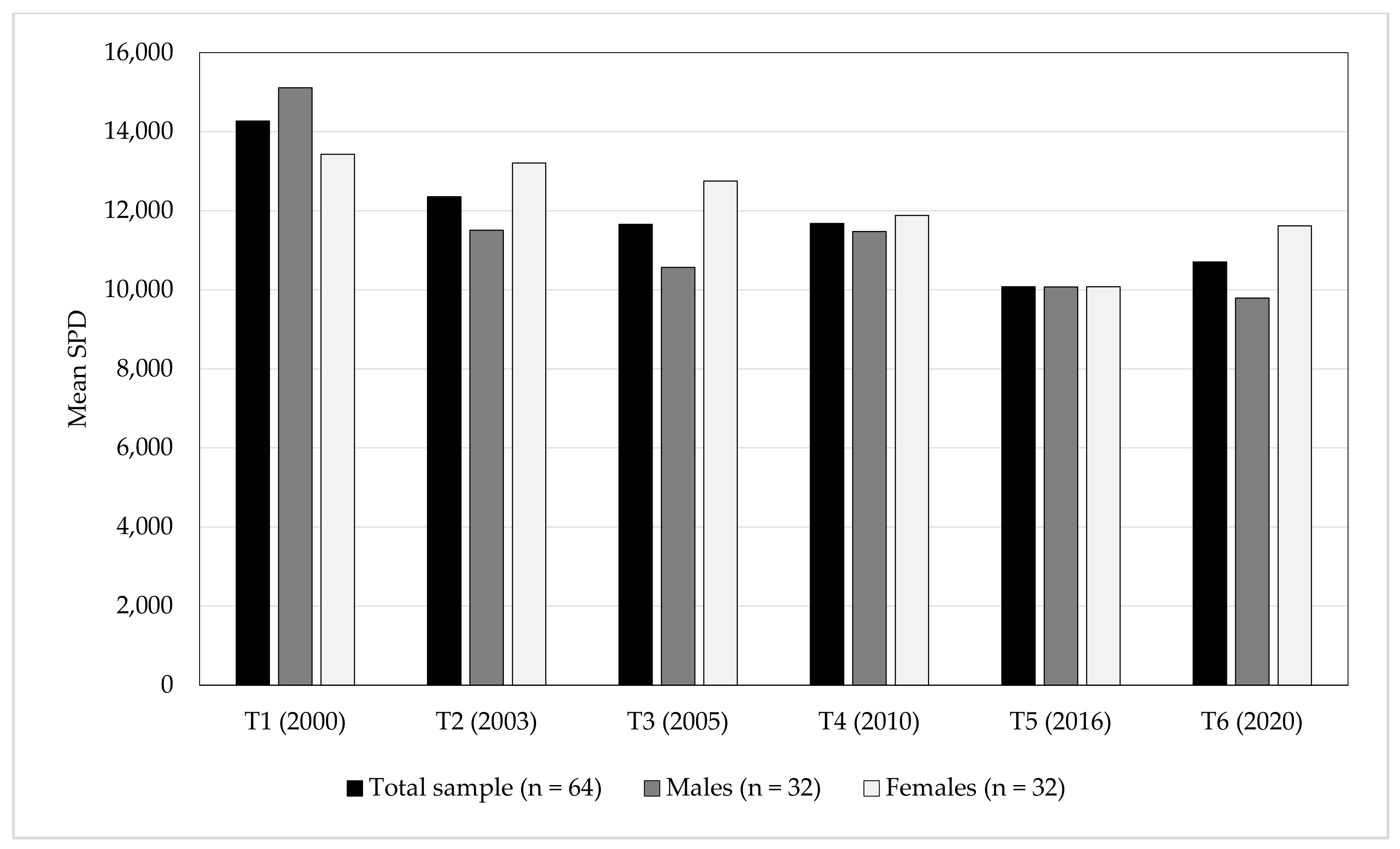
| Time | Total Sample (n = 64) * | Malese (n = 32) ** | Femalese (n = 32) *** | |||||
|---|---|---|---|---|---|---|---|---|
| Age (y) | SPD (SD±) | Age (y) | SPD (SD ±) | Age (y) | SPD (SD±) | |||
| T1 | 12.9 | 14,271 (± 2876) | 12.9 | 15,114 (± 2933) | 12.9 | 13,427 (± 2595) | ||
| T2 | 15.9 | 12,356 (± 2790) | 15.9 | 11,507 (± 2926) | 15.9 | 13,206 (± 2403) | ||
| T3 | 17.9 | 11,658 (± 3142) | 17.9 | 10,567 (± 2300) | 17.9 | 12,749 (± 3509) | ||
| T4 | 22.9 | 11,680 (± 3391) | 22.9 | 11,474 (± 3670) | 22.9 | 11,885 (± 3132) | ||
| T5 | 28.9 | 10,076 (± 2483) | 28.9 | 10,075 (± 2712) | 28.9 | 10,076 (± 2295) | ||
| T6 | 32.9 | 10,705 (±2787) | 32.9 | 9792 (± 2542) | 32.9 | 11,618 (± 2759) | ||
| Total Sample (n = 64) | ||||||
| T1 | T2 | T3 | T4 a | T5 a | T6 a | |
| T1 | ||||||
| T2 | 0.18 | |||||
| T3 | −0.01 | 0.31 * | ||||
| T4 a | −0.03 | −0.00 | 0.40 ** | |||
| T5a | 0.03 | −0.00 | 0.01 | 0.41 ** | ||
| T6a | −0.10 | −0.02 | −0.02 | 0.21 | 0.51 ** | |
| Males (n = 32) | ||||||
| T1 | T2 | T3 | T4 a | T5 a | T6 a | |
| T1 | ||||||
| T2 | 0.41 * | |||||
| T3 | 0.38 * | 0.32 | ||||
| T4 a | 0.13 | −0.05 | 0.43 * | |||
| T5 a | 0.15 | −0.04 | 0.32 | 0.50 ** | ||
| T6 a | −0.09 | −0.15 | 0.19 | 0.33 | 0.34 | |
| Females (n = 32) | ||||||
| T1 | T2 | T3 | T4 a | T5 a | T6 a | |
| T1 | ||||||
| T2 | 0.15 | |||||
| T3 | −0.09 | 0.18 | ||||
| T4 a | −0.16 | −0.13 | 0.40 * | |||
| T5 a | 0.00 | −0.06 | −0.26 | 0.31 | ||
| T6 a | 0.01 | 0.04 | −0.35 | −0.06 | 0.63 ** | |
| Total Samplee (n = 64) | Malese (n = 32) | Femalese (n = 32) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SPD | p | SPD | p | SPD | p | ||||
| Weekdays | Weekend Days | Weekdays | Weekend Days | Weekdays | Weekend Days | ||||
| T3 | 11,658 | 10,320 | 0.017 | 10,567 | 9484 | 0.096 | 12,749 | 11,156 | 0.084 |
| T4 | 11,680 | 11,295 | 0.402 | 11,475 | 10,418 | 0.126 | 11,885 | 12,173 | 0.636 |
| T5 | 10,076 | 8612 | <0.001 | 10,076 | 7993 | <0.001 | 10,077 | 9230 | 0.013 |
| T6 | 10,705 | 10,064 | 0.105 | 9792 | 9249 | 0.325 | 11,619 | 10,879 | 0.202 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raustorp, A.; Fröberg, A. A 20-Year Follow-Up Study of Objectively Measured Physical Activity. Int. J. Environ. Res. Public Health 2021, 18, 3076. https://doi.org/10.3390/ijerph18063076
Raustorp A, Fröberg A. A 20-Year Follow-Up Study of Objectively Measured Physical Activity. International Journal of Environmental Research and Public Health. 2021; 18(6):3076. https://doi.org/10.3390/ijerph18063076
Chicago/Turabian StyleRaustorp, Anders, and Andreas Fröberg. 2021. "A 20-Year Follow-Up Study of Objectively Measured Physical Activity" International Journal of Environmental Research and Public Health 18, no. 6: 3076. https://doi.org/10.3390/ijerph18063076
APA StyleRaustorp, A., & Fröberg, A. (2021). A 20-Year Follow-Up Study of Objectively Measured Physical Activity. International Journal of Environmental Research and Public Health, 18(6), 3076. https://doi.org/10.3390/ijerph18063076







