SARS-CoV-2 Infection and Inflammatory Response in a Twin Pregnancy
Abstract
:1. Introduction
2. Material and Methods
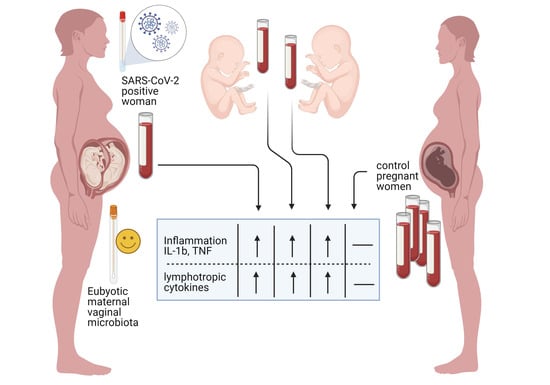
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Savasi, V.M.; Parisi, F.; Patanè, L.; Ferrazzi, E.; Frigerio, L.; Pellegrino, A.; Spinillo, A.; Tateo, S.; Ottoboni, M.; Veronese, P.; et al. Clinical Findings and Disease Severity in Hospitalized Pregnant Women with Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2020, 136, 252–258. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Wei, M.; Cheng, B.H.; Zhou, X.C.; Li, J.; Tian, J.H.; Dong, L.; Hu, R.H. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi 2020, 55, E009. [Google Scholar]
- Regan, A.K.; Moore, H.C.; Sullivan, S.G.; De Klerk, N.; Effler, P.V. Epidemiology of seasonal influenza infection in pregnant women and its impact on birth outcomes. Epidemiol. Infect. 2017, 145, 2930–2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Toro, F.; Gjoka, M.; Di Lorenzo, G.; De Santo, D.; De Seta, F.; Maso, G.; Risso, F.M.; Romano, F.; Wiesenfeld, U.; Levi-D’Ancona, R.; et al. Impact of COVID-19 on maternal and neonatal outcomes: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.-D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 2020, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.L.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Segal, J.P.; Mak, J.W.Y.; Mullish, B.H.; Alexander, J.L.; Ng, S.C.; Marchesi, J.R. The gut microbiome: An under-recognised contributor to the COVID-19 pandemic? Ther. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.E.; Lash, G.E.; Pretlove, S.J.; Chan, B.C.; Holder, R.; Kilby, M.D. Maternal plasma and amniotic fluid cytokines in monochorionic, diamniotic twin pregnancies complicated by twin-to-twin transfusion syndrome. Fetal Diagn. Ther. 2014, 35, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Campisciano, G.; Zanotta, N.; Licastro, D.; De Seta, F.; Comar, M. In vivo microbiome and associated immune markers: New insights into the pathogenesis of vaginal dysbiosis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L.; et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020, 146, 119–127. [Google Scholar] [CrossRef]
- Galván-Peña, S.; Leon, J.; Chowdhary, K.; Michelson, D.A.; Vijaykumar, B.; Yang, L.; Magnuson, A.; Manickas-Hill, Z.; Piechocka-Trocha, A.; Worrall, D.P.; et al. Profound Treg perturbations correlate with COVID-19 severity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Stein, R.R.; Tanoue, T.; Szabady, R.L.; Bhattarai, S.K.; Olle, B.; Norman, J.M.; Suda, W.; Oshima, K.; Hattori, M.; Gerber, G.K.; et al. Computer-guided design of optimal microbial consortia for immune system modulation. eLife 2018, 7, e30916. [Google Scholar] [CrossRef]
- Smigiel, K.S.; Srivastava, S.; Stolley, J.M.; Campbell, D.J. Regulatory T-cell homeostasis: Steady-state maintenance and modulation during inflammation. Immunol. Rev. 2014, 259, 40–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosa, Y.; Kwon, D.; De Oliveira, T.; Wong, E.B. Determinants of Vaginal Microbiota Composition. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Flannery, D.D.; Gouma, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Triebwasser, J.E.; Gerber, J.S.; Morris, J.S.; Weirick, M.E.; et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021. [Google Scholar] [CrossRef]
- Kerget, B.; Kerget, F.; Aksakal, A.; Aşkın, S.; Sağlam, L.; Akgün, M. Evaluation of alpha defensin, IL-1 receptor antagonist, and IL-18 levels in COVID-19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J. Med Virol. 2021, 93, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Quirch, M.; Lee, J.; Rehman, S. Hazards of the cytokine storm and cytokine-targeted therapy in COVID-19 patients: A Review (Preprint). J. Med. Internet Res. 2020, 22, e20193. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, Y.; Mao, J.; Du, R. T cell immunobiology and cytokine storm of COVID-19. Scand. J. Immunol. 2021, 93, e12989. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, A.; Grassi, G.; Bordoni, V.; Lorenzini, P.; Cimini, E.; Casetti, R.; Tartaglia, E.; Marchioni, L.; Petrosillo, N.; Palmieri, F.; et al. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Dis. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [Green Version]
- Shibabaw, T. Inflammatory Cytokine: IL-17A Signaling Pathway in Patients Present with COVID-19 and Current Treatment Strategy. J. Inflamm. Res. 2020, 13, 673–680. [Google Scholar] [CrossRef]
- Jenkins, C.; Roberts, J.; Wilson, R.; MacLean, M.A.; Shilito, J.; Walker, J.J. Evidence of a TH 1 type response associated with recurrent miscarriage. Fertil. Steril. 2000, 73, 1206–1208. [Google Scholar] [CrossRef]
- Kaislasuo, J.; Simpson, S.; Petersen, J.F.; Peng, G.; Aldo, P.; Lokkegaard, E.; Paidas, M.; Pal, L.; Guller, S.; Mor, G. IL-10 to TNFα ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am. J. Reprod. Immunol. 2020, 83, e13195. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lu, Z.; Zhou, X.; Ding, Y.; Guan, L. Effects of intrahepatic cholestasis of pregnancy on hepatic function, changes of inflammatory cytokines and fetal outcomes. Exp. Ther. Med. 2019, 17, 2979–2984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Hu, L.; Cui, Y.; Qi, Z.; Huang, X.; Cai, L.; Zhang, T.; Yin, Y.; Lu, Z.; Xiang, J. Roles of PPARγ/NF-κB Signaling Pathway in the Pathogenesis of Intrahepatic Cholestasis of Pregnancy. PLoS ONE 2014, 9, e87343. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.C.M.; Ramírez, O.V.; Jiménez, M.M.; Garcia, A.A.; Alfonso, J.M.; Baéz, G.G.; Arrieta, R.R.; Simón, D.R.; Gainza, D.A.; Vázquez, B.S.; et al. Interferon gamma, TGF-β1 and RANTES expression in upper airway samples from SARS-CoV-2 infected patients. Clin. Immunol. 2020, 220, 108576. [Google Scholar] [CrossRef] [PubMed]
- Cerbulo-Vazquez, A.; Zavala-Barrios, B.; Briones-Garduno, J.C.; Guerrero-Avendano, G.M.L.; Arriaga-Pizano, L.; Ferat-Osorio, E.; Cabrera-Rivera, G.L.; Miranda-Cruz, P.; de la Rosa, M.T.G.; Prieto-Chavez, J.L.; et al. Title Serological Cytokine and chemokine profile in pregnant women with COVID19 in Mexico City Running title Cytokine and chemokines in COVID19 pregnant patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Vanders, R.L.; Gibson, P.G.; Murphy, V.E.; Wark, P.A.B. Plasmacytoid Dendritic Cells and CD8 T Cells from Pregnant Women Show Altered Phenotype and Function Following H1N1/09 Infection. J. Infect. Dis. 2013, 208, 1062–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
| Before Delivery | ||
|---|---|---|
| Samples | Real-Time PCR SARS-CoV-2 (copies/reactions) | Microbiome analysis |
| * TNF swab (6/10, 34 gestational week) | POS (5.1 × 103) | − |
| TNF swab (12/10, 34 + 6 gestational week) | POS (1.35 × 105) | − |
| TNF swab (19/10, 35 + 6 gestational week) | POS (4.39 × 100) | − |
| TNF swab (22/10, 36 + 2 gestational week) | NEG | − |
| Vaginal Swab | NEG | Lactobacillus spp. 97% Mycoplasma hominis 1% Streptococcus spp. 1% Ureaplasma parvum serovar 6 1% |
| After Delivery | ||
| Samples | Real-Time PCR SARS-CoV-2 (copies/reactions) | ELISA IgG SARS-CoV-2 (seropositivity index) |
| Placental biopsy I twin | NEG | − |
| Placental biopsy II twin | NEG | − |
| TNF swab I twin | NEG | − |
| TNF swab II twin | NEG | − |
| Maternal Serum | − | POS (2.032) |
| Umbilical blood of the first twin | − | POS (1.816) |
| Umbilical blood of the second twin | − | POS (1.816) |
| Immune Proteins (pg/mL) | Pregnant Women SARS-CoV-2 Negative Serum (n = 20) | Maternal Serum | Umbilical Blood of the First Twin | Umbilical Blood of the Second Twin |
|---|---|---|---|---|
| IL-1b | n.d. (<0.47) | 2.1 | 1.59 | 2.27 |
| IL-1ra | 152.2 (117.7 ± 172.4) | 384.61 | 232.05 | 355.43 |
| IL-2 | 13.1 (9.24 ± 16.34) | 11.18 | 7.75 | 9.49 |
| IL-4 | 7.10 (6.08 ± 8.4) | 3.25 | 2.15 | 2.53 |
| IL-5 | n.d. (<0.58) | 7.49 | 4.81 | 20.26 |
| IL-6 | 11.42 (8.14 ± 13.61) | 6.85 | 0.82 | 2.51 |
| IL-7 | 0.6 (0.26 ± 1.06) | 32.11 | 24.01 | 29.67 |
| IL-8 | 31.91 (10.26 ± 196.6) | 20.52 | 7.99 | 14.26 |
| IL-9 | 2.01 (1.26 ± 3.34) | 104.13 | 84.59 | 97.97 |
| IL-10 | 13.7 (8.39 ± 20.81) | 2.65 | 1.42 | 5.68 |
| IL-12(p70) | 2.37(1.08 ± 5.09) | 5.09 | 1.68 | 8.28 |
| IL-13 | 0.75 (0.49 ± 0.97) | 5.12 | 2.79 | 5.12 |
| IL-15 | 1.34 (0.42 ± 6.62) | nd | nd | nd |
| IL-17 | 104.18 (83.31 ± 128.3) | 29.48 | 21.27 | 25.39 |
| Eotaxin | 3.37 (1.78 ± 8.21) | 32.83 | 30.77 | 18.64 |
| FGF basic | 6.84 (5.76 ± 23.45) | 50.68 | 42.59 | 48.1 |
| G-CSF | 58.36 (12.1 ± 166.7) | 204.87 | 148.69 | 138.74 |
| GM-CSF | 44.8 (41.33 ± 80.91) | 4.09 | 2.36 | 4.09 |
| IFN-g | 135.9 (109.6 ± 158.3) | 7.15 | 1.4 | 6.65 |
| IP-10 | 48.32 (17.98 ± 293.3) | 1746.14 | 178.15 | 117.95 |
| MCP-1 | 2.53 (2.02 ± 4.75) | 30.13 | 13.02 | 16.82 |
| MIP-1a° | 6.80 (6.09 ± 7.51) | 3.61 | 2.88 | 3.05 |
| PDGF-bb | 6455 (5979 ± 7582) | 1752.45 | 1526.48 | 595.1 |
| MIP-1b | 1.47 (0.60 ± 3.24) | 62.22 | 66.09 | 65.65 |
| RANTES | 7825 (4564 ± 5684) | 8153.9 | 6683.47 | 6467.94 |
| TNFα | 1.84 (1.63 ± 4.69) | 58.97 | 45.54 | 57.48 |
| VEGF | 7.41 (1.45 ± 32.68) | 13.58 | 26.86 | 96.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombetta, A.; Comar, M.; Tommasini, A.; Canton, M.; Campisciano, G.; Zanotta, N.; Cason, C.; Maso, G.; Risso, F.M. SARS-CoV-2 Infection and Inflammatory Response in a Twin Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 3075. https://doi.org/10.3390/ijerph18063075
Trombetta A, Comar M, Tommasini A, Canton M, Campisciano G, Zanotta N, Cason C, Maso G, Risso FM. SARS-CoV-2 Infection and Inflammatory Response in a Twin Pregnancy. International Journal of Environmental Research and Public Health. 2021; 18(6):3075. https://doi.org/10.3390/ijerph18063075
Chicago/Turabian StyleTrombetta, Andrea, Manola Comar, Alberto Tommasini, Melania Canton, Giuseppina Campisciano, Nunzia Zanotta, Carolina Cason, Gianpaolo Maso, and Francesco Maria Risso. 2021. "SARS-CoV-2 Infection and Inflammatory Response in a Twin Pregnancy" International Journal of Environmental Research and Public Health 18, no. 6: 3075. https://doi.org/10.3390/ijerph18063075
APA StyleTrombetta, A., Comar, M., Tommasini, A., Canton, M., Campisciano, G., Zanotta, N., Cason, C., Maso, G., & Risso, F. M. (2021). SARS-CoV-2 Infection and Inflammatory Response in a Twin Pregnancy. International Journal of Environmental Research and Public Health, 18(6), 3075. https://doi.org/10.3390/ijerph18063075