Possible Roles of Permafrost Melting, Atmospheric Transport, and Solar Irradiance in the Development of Major Coronavirus and Influenza Pandemics
Abstract
:1. Introduction and Background
- (1)
- Invention of airplanes has not hastened onsets: early cases of the 1889 influenza pandemic appeared in St Petersburg 10 days before eruption in London and other European cities, and only 5 weeks prior to onset in New York [2]. This timing outpaces initial development of COVID-19, where the first appearance in China preceded surges in Italy and Iran by ~5 weeks and the US by ~9 weeks. Instead, changes in spreading rates within any given country of MRVPs over the past ~230 years, as presented by Sanders-Hasting and Krewski [1], strongly correlate with advances in ground transportation. Thus, more than one mechanism of spreading exists.
- (2)
- Although jet travel dispersed the first SARS coronavirus in 2003, it only provided 1–3 cases in many countries, whereas >7500 out of ~8100 total cases occurred in China or nearby [3]. Since below 0.0002% of the global population was infected, SARS-2003 is best described as an epidemic with outliers, and indicates that jet travel is insufficient to produce large numbers of infections worldwide.
- (3)
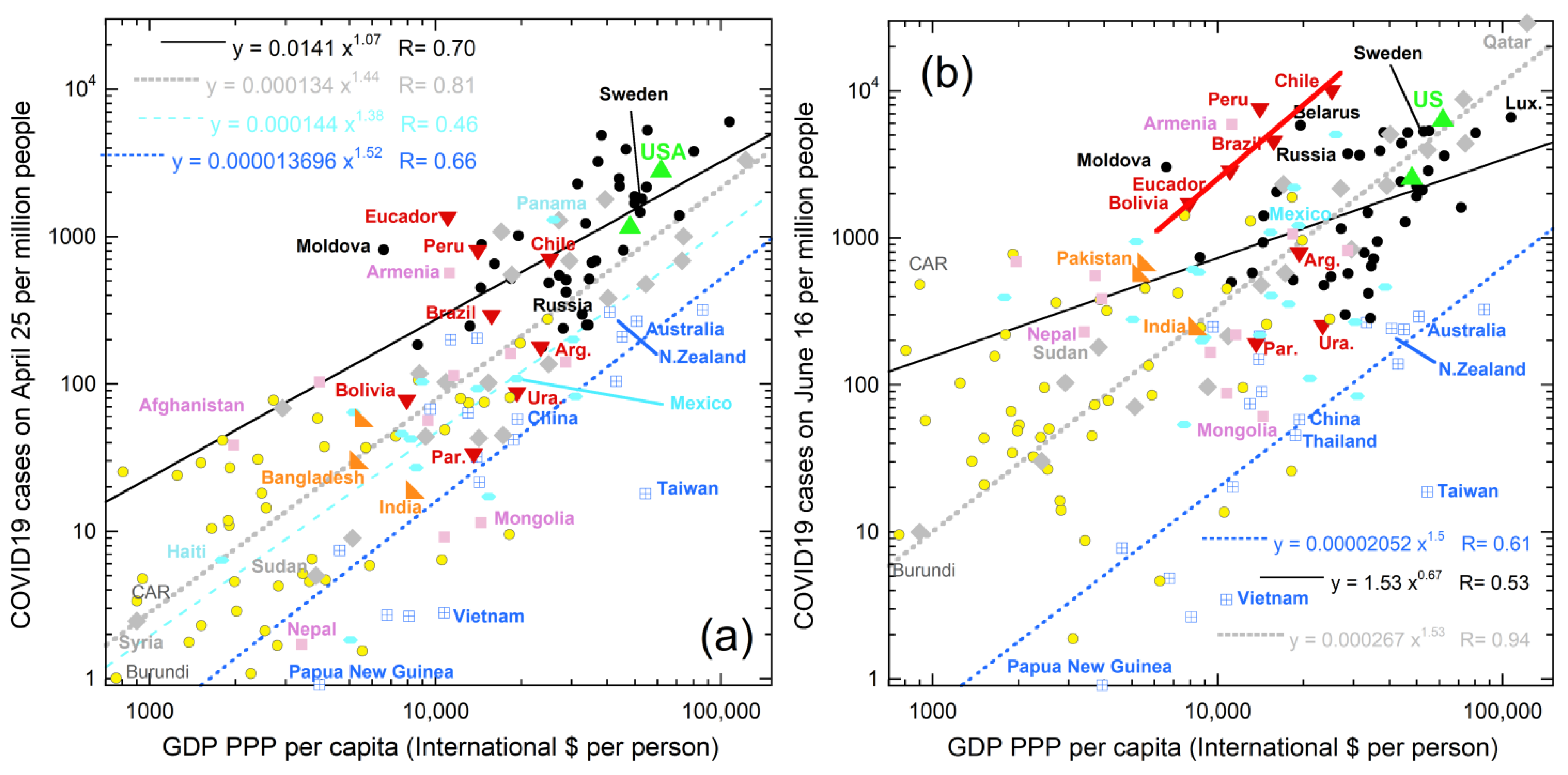
- Location of COVID-19 case surges in June 2020 across the southernmost US, but not in the northern and eastern cities which drew large crowds of protestors in May 2020, strongly suggests that factors besides human proximity cause spreading.
- (4)
- Viruses with very high genetic identity are found in very distant and different environments, which can be explained by atmospheric transport of organic aerosols [4]. Rapid distribution of high volumes of viruses over large distances characterizes pandemics. This can be realized through airborne transport, and is considered as the mechanism for the annual minor influenza pandemic, after Hammond et al. [5]. Viruses in general are transported and transmitted via aerosols [6]. Section 1.2 summarizes data on atmospheric transport of viruses.
1.1. Permafrost as a Possible Source of Large Amounts of Novel Viruses
1.2. Long-Distance Atmospheric Transport of Microbes, Including Coronaviruses
1.3. Roles of Particulates and Pollution in Virus Transport and COVID-19 Cases
1.4. Effect of Solar Ultraviolet Light on Temperature, Viruses, and Immune Response
1.5. Hypothesis and Organization of the Paper
2. Available Data and Methods
3. Permafrost Melting as the Source of Pandemic-Generating Viruses
3.1. Links of Early COVID-19 Cases with Permafrost Distribution

3.2. Correlations in the Timing of MRVPs, Solar Cycles, and Permafrost Melting
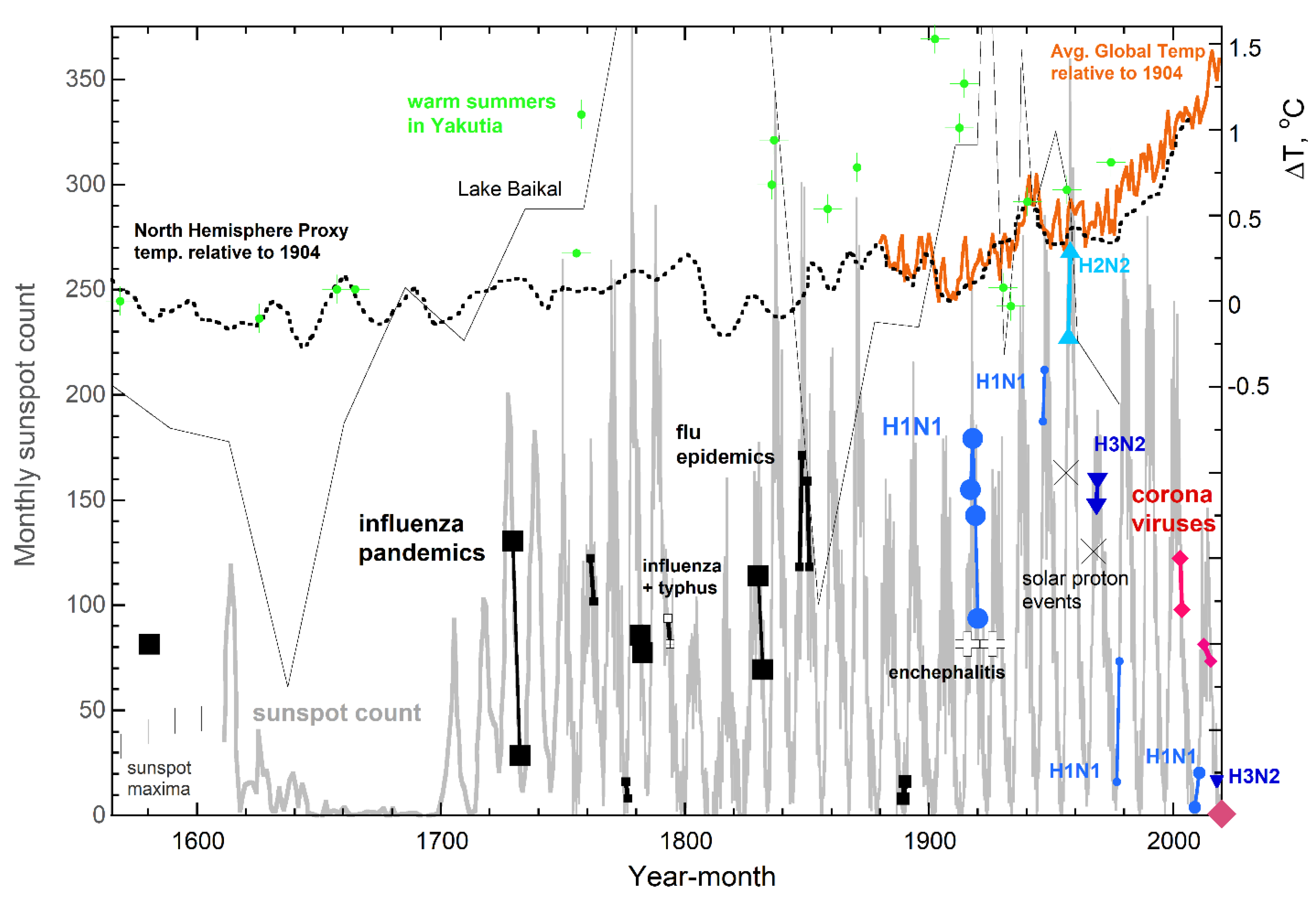
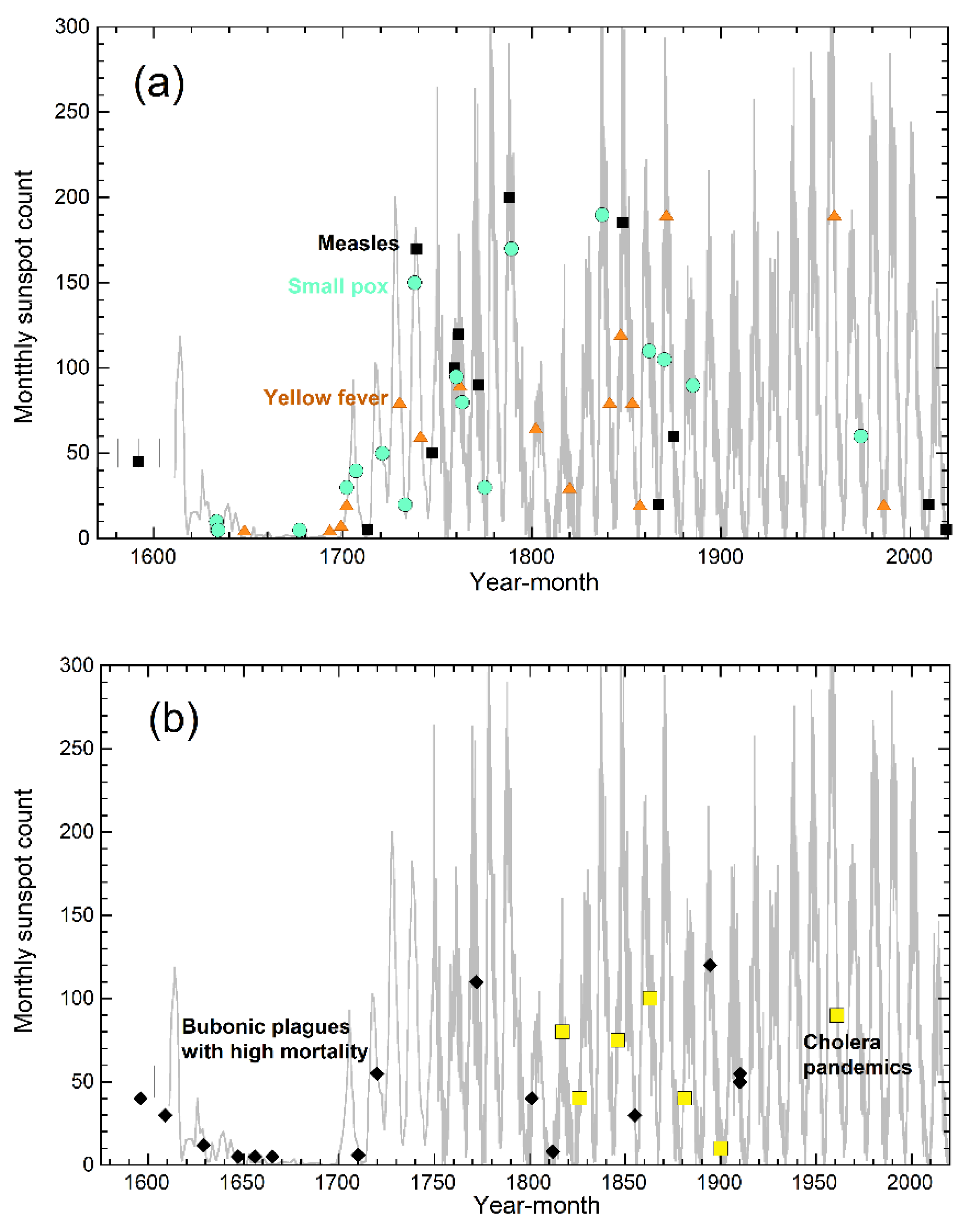
3.3. Why Pandemics Rarely Onset in Arctic Regions
4. Transport of Newly Released Viruses by the Northern Polar Jet Stream
4.1. Emergence of COVID-19
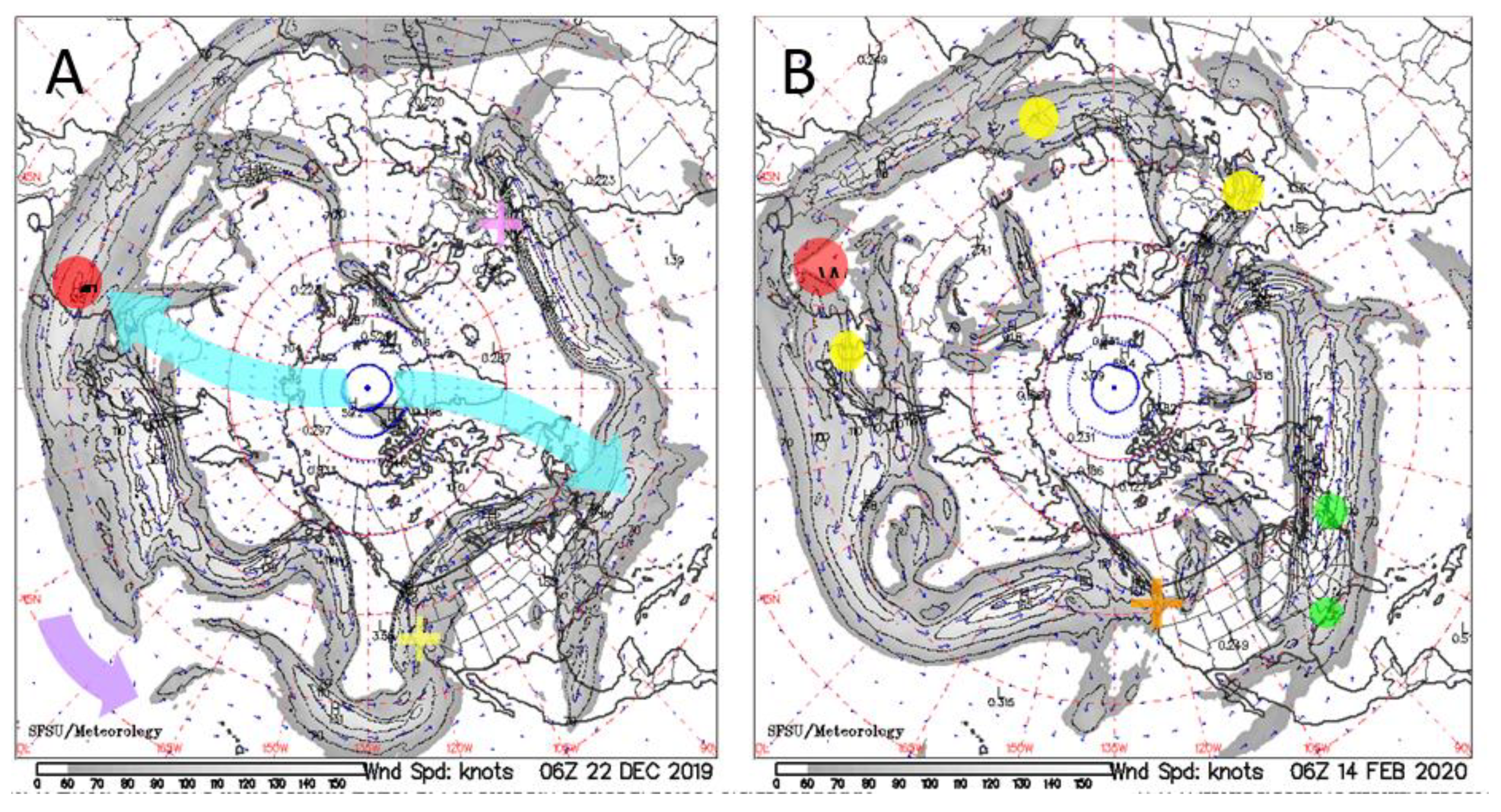
4.2. Genomic and Other Evidence for Multiple Points of Origin
5. Environmental and Human Factors Propelling COVID-19 Growth
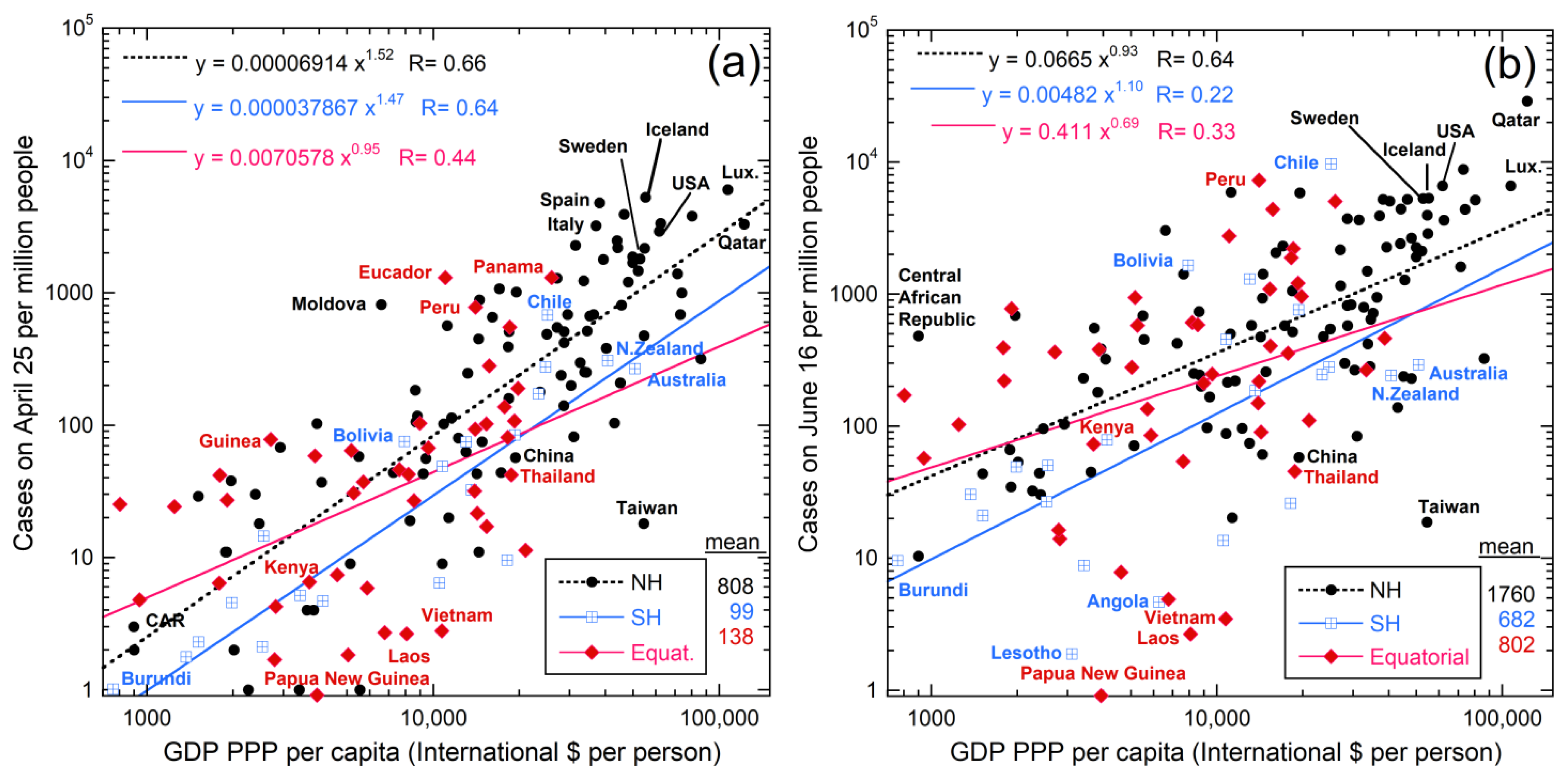
5.1. Links with Latitude and Climate
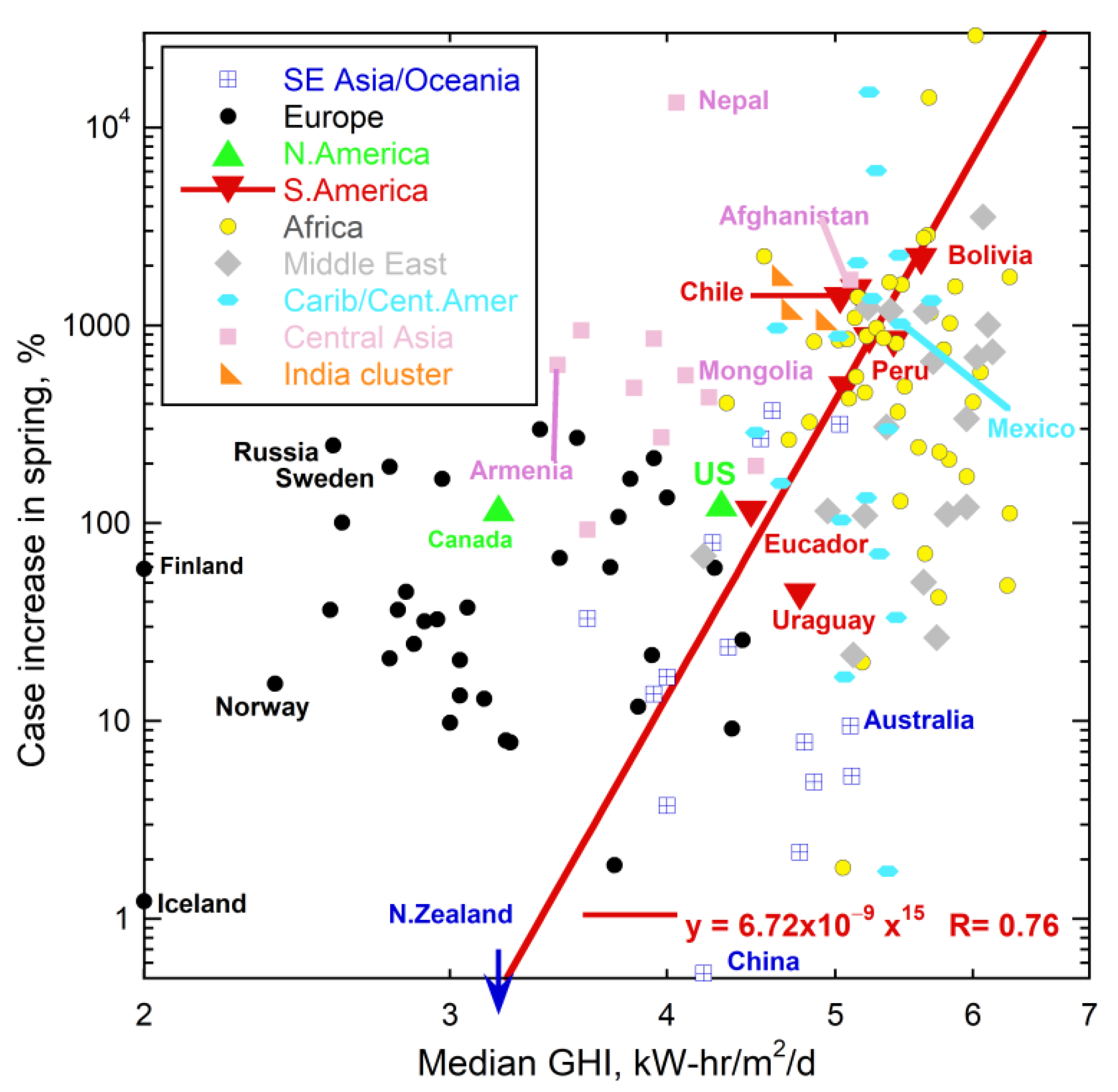
5.2. Links with Solar Irradiance
5.3. Human Immune Response to UV
5.4. Links with Poor 2020 Air Quality in Los Angeles
6. A Model for Natural Causes of Major Respiratory Virus Pandemics
7. Model Predictions and Tests
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunders-Hasting, P.; Krewski, D. Reviewing the History of Pandemic Influenza: Understanding Patterns of Emergence and Transmission. Pathogens 2016, 5, 66. [Google Scholar] [CrossRef] [Green Version]
- Vallerona, A.-J.; Coria, A.; Valtata, S.; Meurissec, S.; Carrata, F.; Boëllea, P.-Y. Transmissibility and geographic spread of the 1889 influenza pandemic. Proc. Natl. Acad. Sci. USA 2010, 107, 8778–8781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003. Available online: https://www.who.int/csr/sars/country/table2004_04_21/en/ (accessed on 16 June 2020).
- Reche, I.; D’Orta, G.; Mladenov, N.; Winget, D.M.; Suttle, C.A. Deposition rates of viruses and bacteria above the atmospheric boundary layer. ISME J. 2018, 12, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, G.W.; Raddatz, R.L.; Gelskey, D.E. Impact of atmospheric dispersion and transport of viral aerosols on the epidemiology of influenza. Rev. Infect. Dis. 1989, 11, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Bakarat, T.; Muylkens, B.; Su, B.-L. Is Particulate Matter of Air Pollution a Vector of Covid-19 Pandemic? Matter 2020, 3, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Niewijk, G. Controversy Aside, Why the source of COVID-19 matters. Gen. Engr. Biotech. News 2020. Available online: https://www.genengnews.com/insights/controversy-aside-why-the-source-of-covid-19-matters/ (accessed on 31 January 2021).
- Gorman, J. Some Scientists Question W.H.O. Inquiry into the Coronavirus Pandemic’s Origins. New York Times. 4 March 2021. Available online: https://www.nytimes.com/2021/03/04/health/covid-virus-origins.html?smid=em-share (accessed on 5 March 2021).
- Gale, J. Where Are We in Finding the Coronavirus’s Origin? Bloomberg News 2021. Available online: https://www.bloomberg.com/news/articles/2021-03-04/where-are-we-in-hunting-for-the-coronavirus-s-origin-quicktake (accessed on 6 March 2021).
- Ruan, Y.H.; Wen, H.; He, X.; Wu, C.-I. A theoretical exploration of the origin and early evolution of a pandemic. Sci. Bull. 2020. [Google Scholar] [CrossRef]
- Mathai, V.; Das, A.; Bailey, J.A. Airflows inside passenger cars and implications for airborne disease transmission. Sci. Adv. 2020. [Google Scholar] [CrossRef]
- Lofgren, E.; Fefferman, N.H.; Naumov, Y.N.; Gorski, J.; Naumova, E.N. Influenza Seasonality: Underlying Causes and Modeling Theorie. J. Virol. 2007, 81, 5429–5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilbourne, E.D. Influenza Pandemics of the 20th Century. Emerg. Infect. Dis. 2006, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Bartoli, J.; Shmakova, L.; Jeudya, S.; Labadie, K.; Adrait, A.; Lescot, M.; Poirot, O.; Bertaux, L.; Bruley, C.; et al. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proc. Natl. Acad. Sci. USA 2017, 111, 4274–4279. Available online: https://www.pnas.org/content/111/11/4274 (accessed on 31 January 2021). [CrossRef] [PubMed] [Green Version]
- Jansson, J.K.; Tas, N. The microbial ecology of permafrost. Nat. Rev. 2014, 414, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Revich, B.; Tokarevich, N.; Parkinson, A.J. Climate change and zoonotic infections in the Russian Arctic. Intl. J. Circumpolar Health 2012, 71, 18792. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.B.; Roux, S.; Brum, J.R.; Bolduc, B.; Woodcroft, B.J.; Jang, H.B.; Singleton, C.M.; Solden, L.M.; Naas, A.E.; Boyd, J.A.; et al. Host-linked soil viral ecology along a permafrost thaw gradient. Nat. Microbiol. 2018, 3, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Langlois, V.; Vigneron, A.; Vincent, W.F.; Culley, A.I. Seasonal regime shift in the viral communities of a permafrost thaw lake. Viruses 2020, 12, 1204. [Google Scholar] [CrossRef] [PubMed]
- Wacharapluesadee, S.; Tan, C.W.; Maneeorn, P.; Duengkae, P.; Zhu, F.; Joyjinda, Y.; Kaewpom, T.; Ni Chia, W.; Ampoot, W.; Lim, B.L.; et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Coronaviruses closely related to the pandemic virus discovered in Japan and Cambodia. Nature 2020, 558, 15–16. [Google Scholar]
- Griffin, D.W.; Gonzalez-Martin, C.; Hoose, C.; Smith, D.J. Global-Scale Atmospheric Dispersion of Microorganisms. In Microbiology of Aerosols; Delort, A.M., Amato, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 155–194. [Google Scholar]
- Cáliza, J.; Triadó-Margarita, X.; Camareroa, L.; Casamayora, E.O. A long-term survey unveils strong seasonal patterns in the airborne microbiome coupled to general and regional atmospheric circulations. Proc. Natl. Acad. Sci. USA 2018, 115, 12229–12234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tignat-Perrier, R.; Dommergue, A.; Thollot, A.; Keuschnig, C.; Magand, O.; Vogel, T.M.; LaRose, C. Global airborne microbial communities controlled by surrounding landscapes and wind conditions. Sci. Rep. 2019, 9, 14441. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Passarini, F.; Gennaro, G.D.; Barbieri, P.; Perrone, M.G.; Borelli, M.; Palmisani, J.; Gilio, A.D.; Torboli, V.; Pallavicini, A.; et al. SARS-CoV-2 RNA found on particulate matter of Bergamo in Northern Italy: First preliminary evidence. Environ. Res. 2020, 188, 109754. [Google Scholar] [CrossRef] [PubMed]
- Breibart, M.; Rohwer, F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005, 13, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Ravichandar, J.D.; Jain, S.; Jain, D.W.; Yu, H.; Tan, Q.; Thissen, J.; Lusby, T.; Nicoll, P.; Shedler, S. Airborne bacteria in Earth’s lower stratosphere resemble taxa detected in the troposphere: Results from a new NASA Aircraft Bioaerosol Collector (ABC). Front. Microbiol. 2018, 9, 1752–1772. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Retuerta, E.; Passow, U.; Duarte, C.M.; Reche, I. Effects of ultraviolet B radiation on (not so) transparent exopolymer particles. Biogeosciences 2009, 6, 3071–3080. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Kong, C.; Liu, G.; Wu, C.; Deng, H.; Zhang, Y.; Zhuang., Q. Changes of urban wetlands in Wuhan, China, from 1987 to 2005. Prog. Phys. Geog. 2010, 34, 207–220. Available online: http://ppg.sagepub.com/cgi/content/abstract/34/2/207 (accessed on 31 January 2021).
- Hubei Government. Wetlands of International Importance in Hubei. Available online: http://en.hubei.gov.cn/news/newslist/201901/t20190110_1377819.shtml (accessed on 4 January 2020).
- Kim, J.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Internatl. 2015, 74, 136–143. Available online: https://www.sciencedirect.com/science/article/pii/S0160412014002992?via%3Dihub (accessed on 31 January 2021). [CrossRef] [PubMed]
- Hutter, H.-P.; Poteser, M.; Moshammer, H.; Lemmerer, K.; Mayer, M.; Weitensfelder, L.; Wallner, P.; Kundi, M. Air Pollution is associated with COVID-19 incidence and mortality in Vienna, Austria. Int. J. Environ. Res. Public Health 2020, 17, 9275. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Han, J. Electrostatic fine particles emitted from laser printers as potential vectors for airborne transmission of COVID-19. Environ. Chem. Lett. 2021, 19, 5–16. [Google Scholar] [CrossRef]
- Wheeling, K. The Rise of Zombie Fires, EOS 2020, 101. Available online: https://doi.org/10.1029/2020EO146119 (accessed on 30 June 2020).
- Unprecedented Wildfires in the Arctic. Available online: https://public.wmo.int/en/media/news/unprecedented-wildfires-arctic (accessed on 4 January 2020).
- Pappas, C. Zombie Wildfires are Blazing through the Arctic, Causing Record Burning. Live Science News. 2020. Available online: https://www.livescience.com/zombie-wildfires-burn-arctic.html (accessed on 4 January 2020).
- Siberian Town Hits Highest Temperature in Recorded History for the Arctic Circle. Available online: https://www.wusa9.com/article/weather/accuweather/russian-city-verkhoyansk-highest-record-temperature-arctice-circle/507-e3d7ada6-8272-470d-bd2c-9c0f338b7ea9 (accessed on 30 June 2020).
- Anthony, L. Arctic Permafrost is Thawing. Canadian Geographic. 2020. Available online: https://www.canadiangeographic.ca/article/arctic-permafrost-thawing-heres-what-means-canadas-north-and-world (accessed on 25 July 2020).
- Norval, M. The effect of ultraviolet radiation on human viral infections. Photochem. Photobiol. 2008, 82, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.M.; Ulrich, S.E. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes: An essential role for keratinocyte-derived IL-10. J. Immunol. 1992, 149, 3865–3871. [Google Scholar] [PubMed]
- Rivas, J.M.; Ullrich, S.E. The role of IL-4, IL-10, and TNF-α in the immune suppression induced by ultraviolet radiation. J. Leukoc. Biol. 1994, 56, 769–775. [Google Scholar] [CrossRef]
- González Maglio, D.H.; Paz, M.L.; Leoni, J. Sunlight Effects on Immune System: Is There Something Else in addition to UV-Induced Immunosuppression? BioMed Res. Int. 2016, 1934518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loser, K.; Apelt, J.; Voskort, M.; Mohaupt, M.; Balkow, S.; Schwarz, T.; Grabbe, S.; Beissert, S. IL-10 controls ultraviolet-induced carcinogenesis in mice. J. Immunol. 2007, 179, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roser, M.; Ritchie, H.; Ortiz-Ospina, E.; Hasell, J. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 22 February 2021).
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet 2020, 20, 533–534. [Google Scholar] [CrossRef]
- An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Available online: https://github.com/CSSEGISandData/COVID-19 (accessed on 22 February 2021).
- Zhang, T.; Heginbottom, J.A.; Barry, R.G.; Brown, J. Further statistics on the distribution of permafrost and ground ice in the Northern Hemisphere. Polar Geog. 2000, 24, 126–131. [Google Scholar] [CrossRef]
- Dobinski, W. Permafrost. Earth-Sci. Rev. 2011, 108, 158–169. [Google Scholar] [CrossRef]
- All about Frozen Ground. Available online: https://nsidc.org/cryosphere/frozenground/index.html (accessed on 24 February 2021).
- Coronavirus (COVID-19) Confirmed Cases, Active Cases, Recoveries, and Deaths in Russia as of 24 July 2020, by Region. Available online: https://www.statista.com/statistics/1102935/coronavirus-cases-by-region-in-russia/ (accessed on 24 July 2020).
- Population of Russian Provinces. Available online: https://en.wikipedia.org/wiki/List_of_federal_subjects_of_Russia_by_population (accessed on 25 July 2020).
- Land Resources of Russia—Maps of Permafrost and Ground Ice. Available online: https://nsidc.org/fgdc/maps/perm_ext_browse.html (accessed on 25 July 2020).
- COVID-19 Pandemic by Country and Territory. Available online: https://en.wikipedia.org/wiki/COVID-19_pandemic_by_country_and_territory#Asia (accessed on 25 July 2020).
- Permafrost and Treeline. Available online: https://www.eea.europa.eu/data-and-maps/figures/permafrost-and-tree-line (accessed on 4 July 2020).
- Template: COVID-19 Pandemic Data/Canada Medical Cases by Province. Available online: https://en.wikipedia.org/wiki/Template:COVID-19_pandemic_data/Canada_medical_cases_by_province (accessed on 25 July 2020).
- List of Canadian Provinces and Territories by Area. Available online: https://en.wikipedia.org/wiki/List_of_Canadian_provinces_and_territories_by_area (accessed on 25 July 2020).
- SARS-CoV-2 Mink-Associated Variant Strain—Denmark. Available online: https://www.who.int/csr/don/06-november-2020-mink-associated-sars-cov2-denmark/en/ (accessed on 23 December 2020).
- Lovelace, B.; Feuer, W. Five Things to Know about the New Covid Strain Spreading in the UK. Available online: https://www.cnbc.com/2020/12/22/new-covid-strain-5-things-to-know-about-virus-spreading-in-the-uk.html (accessed on 23 December 2020).
- Gebbie, G.; Huyburs, P. The Little Ice Age and 20th-century deep Pacific cooling. Science 2019, 363, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Hoyt, D.V.; Schatten, K.H. Group sunspot numbers: A new solar activity reconstruction. Part 2. Sol. Phys. 1998, 181, 491–512. [Google Scholar] [CrossRef]
- Hofmeister, A.M. Measurements, Mechanisms, and Models of Heat Transport; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Fröhlich, C. Total solar irradiance: What have we learned from the last three cycles and the recent minimum? Space Sci. Rev. 2013, 176, 237–252. [Google Scholar] [CrossRef]
- Wu, C.J. Solar Variability over the Last 9000 Years. Ph.D. Thesis, Georg-August-Universität, Göttingen, Germany, 2018. [Google Scholar]
- Enushchenko, I.V.; Melgunov, M.S.; Fedotov, A.P. Reconstruction of summer temperatures in East Siberia (Russia) for the last 850 years, inferred from records in lake sediments of non-biting midges (Diptera: Chironomidae). Intl. J. Environ. Stud. 2014, 71, 647–655. [Google Scholar] [CrossRef]
- Hughes, M.K.; Vaganov, E.A.; Shiyatov, S.; Touchan, R.; Funkhouser, G. Twentieth-century summer warmth in northern Yakutia in a 600-year context. Holocene 1999, 9, 629–634. [Google Scholar] [CrossRef]
- Mann, M.E.; Zhang, Z.; Rutherford, S.; Bradley, R.S.; Hughes, M.K.; Shindell, D.; Ammann, C.; Faluvegi, G.; Ni, F. Global Signatures and Dynamical Origins of the Little Ice Age and Medieval Climate Anomaly. Science 2009, 326, 1256–1260. [Google Scholar] [CrossRef] [Green Version]
- SILSO, World Data Center—Sunspot Number and Long-Term Solar Observations from the Royal Observatory of Belgium, on-Line Sunspot Number Catalogue. Covering 1749 to 2020. Available online: http://www.sidc.be/silso/datafiles (accessed on 16 June 2016).
- Shea, M.A.; Smart, D.F. A summary of major proton events. Sol. Phys. 1990, 127, 297–320. [Google Scholar] [CrossRef]
- Knipp, D.J.; Ramsay, A.C.; Beard, E.D.; Boright, A.L.; Cade, W.B.; Hewins, I.M.; McFadden, R.H.; Denig, W.F.; Kilcommons, L.M.; Shea, M.A.; et al. The May 1967 great storm and radio disruption event: Extreme space weather and extraordinary responses. Space Weather 2016, 14, 614–633. [Google Scholar] [CrossRef] [Green Version]
- List of Epidemics. Available online: https://en.wikipedia.org/wiki/List_of_epidemics (accessed on 17 June 2020).
- Climate at a Glance: Global Time Series, NOAA National Centers for Environmental Information. Available online: https://www.ncdc.noaa.gov/cag/ (accessed on 15 June 2020).
- Cutchins, E.C.; Dayhuff, T.R. Photoinactivation of measles virus. Virology 1962, 17, 420–425. [Google Scholar] [CrossRef]
- Bagnold, R.A. The Physics of Blown Sand and Desert Dunes; William Morrow and Co.: New York, NY, USA, 1941. [Google Scholar]
- Coronavirus Disease 2019 (COVID-19) Situation Report–37. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200226-sitrep-37-covid-19.pdf (accessed on 15 January 2021).
- Monthly Weather-Wuhan, Hubei, People’s Republic of China. Available online: https://weather.com/weather/monthly/l/2637660151899903e8cbdd23636051470b6731863286ec74b3033421cb87e1e8 (accessed on 16 June 2020).
- Average weather in December in Wuhan. Available online: https://weatherspark.com/m/128408/12/Average-Weather-in-December-in-Wuhan-China (accessed on 24 February 2021).
- La Rosa, G.; Mancini, P.; Ferraro, G.B.; Veneri, C.; Iaconelli, M.; Bonadonna, L.; Lucentini, L.; Suffredini, E. COVID-19 has been circulating in northern Italy since December 2019: Evidence from environmental monitoring. Sci. Total Environ. 2019, 750, 141711. [Google Scholar] [CrossRef] [PubMed]
- Jet Stream Archive. Available online: https://squall.sfsu.edu/crws/archive/jetstream_archive.html (accessed on 25 February 2021).
- Deslandes, A.; Berti, V.; Tandjaoui-Lambotte, Y.; Alloui, C.; Carbonnelle, E.; Zahar, J.R.; Brichler, S.; Cohen, Y. COVID-19 was already spreading in France in late December 2019. Int. J. Antimicrob. Agents 2020, 55, 106006. [Google Scholar] [CrossRef] [PubMed]
- Jorden, M.A.; Rudman, S.L.; Villarino, E.; Hoferka, S.; Patel, M.T.; Bemis, K.; Simmons, C.R.; Jespersen, M.; Johnson, J.I.; Mytty, E.; et al. Evidence for limited early spread of COVID-19 within the United States, January–February 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 680–684. [Google Scholar] [CrossRef]
- Tang, X.; Wu, C.; Li, X.; Song, Y.; Yao, X.; Wu, X.; Duan, Y.; Zhang, H.; Wang, Y.; Qian, Z.; et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020, 7, 1012–1023. [Google Scholar] [CrossRef] [Green Version]
- van Dorp, L.; Acmana, M.; Richard, D.; Shaw, L.P.; Ford, C.E.; Ormond, L.; Owen, C.J.; Pang, J.; Tan, C.C.S.; Boshier, F.A.T.; et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020, 83, 104351. [Google Scholar] [CrossRef]
- Shen, Z.; Xiao, Y.; Kang, L.; Ma, W.; Shi, L.; Zhang, L.; Zhou, Z.; Yang, J.; Zhong, J.; Yang, D.; et al. Genomic Diversity of Severe Acute Respiratory Syndrome–Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Davis, B.D.; Shen, S.S.; Martinez, J.M.S.; Plummer, J.T.; Vail, E. Emergence of a novel SARS-CoV-2 variant in Southern California. JAMA 2021. [Google Scholar] [CrossRef] [PubMed]
- List of Countries by GDP (PPP). Available online: https://en.wikipedia.org/wiki/List_of_countries_by_GDP_(PPP) (accessed on 26 April 2020).
- List of Countries by Population (United Nations). Available online: https://en.wikipedia.org/wiki/List_of_countries_by_population_(United_Nations) (accessed on 17 June 2020).
- List of Countries and Dependencies by Area. Available online: https://en.wikipedia.org/wiki/List_of_countries_and_dependencies_by_area (accessed on 17 June 2020).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Solar Atlas 2.0. This is a Free, Web-Based Application is Developed and Operated by the Company Solargis s.r.o. on Behalf of the World Bank Group, Utilizing Solargis Data, with Funding Provided by the Energy Sector Management Assistance Program (ESMAP). Available online: https://globalsolaratlas.info (accessed on 1 June 2020).
- Schwarz, T. Mechanisms of UV-induced immunosuppression. Keio J. Med. 2010, 54, 165–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, A.; Schwarz, T. UVR-induced regulatory T cells switch antigen-presenting cells from a stimulatory to a regulatory phenotype. J. Investig. Derm. 2010, 130, 1914–1921. [Google Scholar] [CrossRef] [Green Version]
- Ghoreishi, M.; Dutz, J.P. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4 + CD25 + T regulatory cells and is dependent on host-derived IL-10. J. Immunol. 2006, 176, 2635–2644. [Google Scholar] [CrossRef] [Green Version]
- Moan, J.; Dahlback, A.; Ma, L.; Juzeniene, A. Influenza, solar radiation and vitamin D. Dermato-Endocrinology 2009, 1, 307–309. [Google Scholar] [CrossRef] [Green Version]
- Arnson, Y.; Amital, H.; Shoenfeld, Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007, 66, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Carleton, T.; Cornetet, J.; Huybers, P.; Meng, K.C.; Proctor, J. Global evidence for ultraviolet radiation decreasing COVID-19 growth rates. Proc. Natl. Acad. Sci. USA 2021, 118, e2012370118. [Google Scholar] [CrossRef] [PubMed]
- Moozhipurath, R.K.; Kraft, L.; Skiera, B. Evidence of protective role of Ultraviolet-B (UVB) radiation in reducing COVID-19 deaths. Nat. Sci. Rep. 2020, 10, 17705. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 10, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Haagen-Smit, A.J. The air pollution problem in Los Angeles. Eng. Sci. 1950, 14, 7–13. [Google Scholar]
- Lozona, A.V. Los Angeles becomes First County to Hit 1 Million Covid-19 Cases. NBC News. 16 January 2021. Available online: https://www.nbcnews.com/news/us-news/los-angeles-becomes-first-county-hit-1-million-covid-19-n1254498 (accessed on 31 January 2021).
- Barboza, T.L.A. Began 2020 with a Clean-Air Streak but Ended with Its Worst Smog in Decades. Los Angeles Times. 6 December 2021. Available online: https://www.latimes.com/california/story/2020-12-06/2020-la-air-quality-southern-california-pollution-analysis (accessed on 19 January 2021).
- Devlin, R.B.; Raub, J.A.; Folinsbee, L.J. Health effects of ozone. Sci. Med. 1997, 4, 8–17. [Google Scholar]
- Moretti, E.; Neidell, M. Pollution, Health, and Avoidance Behavior Evidence from the Ports of Los Angeles. J. Hum. Resour. 2011, 46, 154–175. [Google Scholar] [CrossRef] [Green Version]
- Weil, T.; De Filippo, C.; de Albanese, D.; Donati, C.; Pindo, M.; Pavarini, L.; Carotenuto, F.; Pasqui, M.; Poto, L.; Gabrieli, J.; et al. Legal Immigrants: Invasion of Alien Microbial Communities during Winter Occurring Desert Dust Storms. Microbiome 2017, 5, 32. Available online: https://link.springer.com/article/10.1186/s40168-017-0249-7 (accessed on 31 January 2021). [CrossRef] [PubMed] [Green Version]
- Gorillas at San Diego Zoo Safari Park Recover from COVID-19. Available online: https://www.latimes.com/california/story/2021-02-13/gorillas-san-diego-zoo-covid (accessed on 22 February 2021).
| Stage | Natural Process | Key Evidence or Reason |
|---|---|---|
| Novel virus source | Permafrost melting | Massive reservoir of ancient infectious agents |
| Sporadic timing | Climate and solar cycles | Semi-regular; seasonality of flu; rekindling |
| Virus transport | North Polar Jet stream | Rapid onset at similar locations historically |
| Virus deposition | Rainout; pollution | Viruses and disease linked to particulates |
| Case growth | UV immunosuppression | Amplification in sunny regions after flu season |
| Multi-year duration | Atmospheric circulation | Infected hosts increase virus content in air; more permafrost melting augments supply |
| Historic cessation | Arctic freeze; immunity | Minimal supply with reduced amplification |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmeister, A.M.; Seckler, J.M.; Criss, G.M. Possible Roles of Permafrost Melting, Atmospheric Transport, and Solar Irradiance in the Development of Major Coronavirus and Influenza Pandemics. Int. J. Environ. Res. Public Health 2021, 18, 3055. https://doi.org/10.3390/ijerph18063055
Hofmeister AM, Seckler JM, Criss GM. Possible Roles of Permafrost Melting, Atmospheric Transport, and Solar Irradiance in the Development of Major Coronavirus and Influenza Pandemics. International Journal of Environmental Research and Public Health. 2021; 18(6):3055. https://doi.org/10.3390/ijerph18063055
Chicago/Turabian StyleHofmeister, Anne M., James M. Seckler, and Genevieve M. Criss. 2021. "Possible Roles of Permafrost Melting, Atmospheric Transport, and Solar Irradiance in the Development of Major Coronavirus and Influenza Pandemics" International Journal of Environmental Research and Public Health 18, no. 6: 3055. https://doi.org/10.3390/ijerph18063055
APA StyleHofmeister, A. M., Seckler, J. M., & Criss, G. M. (2021). Possible Roles of Permafrost Melting, Atmospheric Transport, and Solar Irradiance in the Development of Major Coronavirus and Influenza Pandemics. International Journal of Environmental Research and Public Health, 18(6), 3055. https://doi.org/10.3390/ijerph18063055






