Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
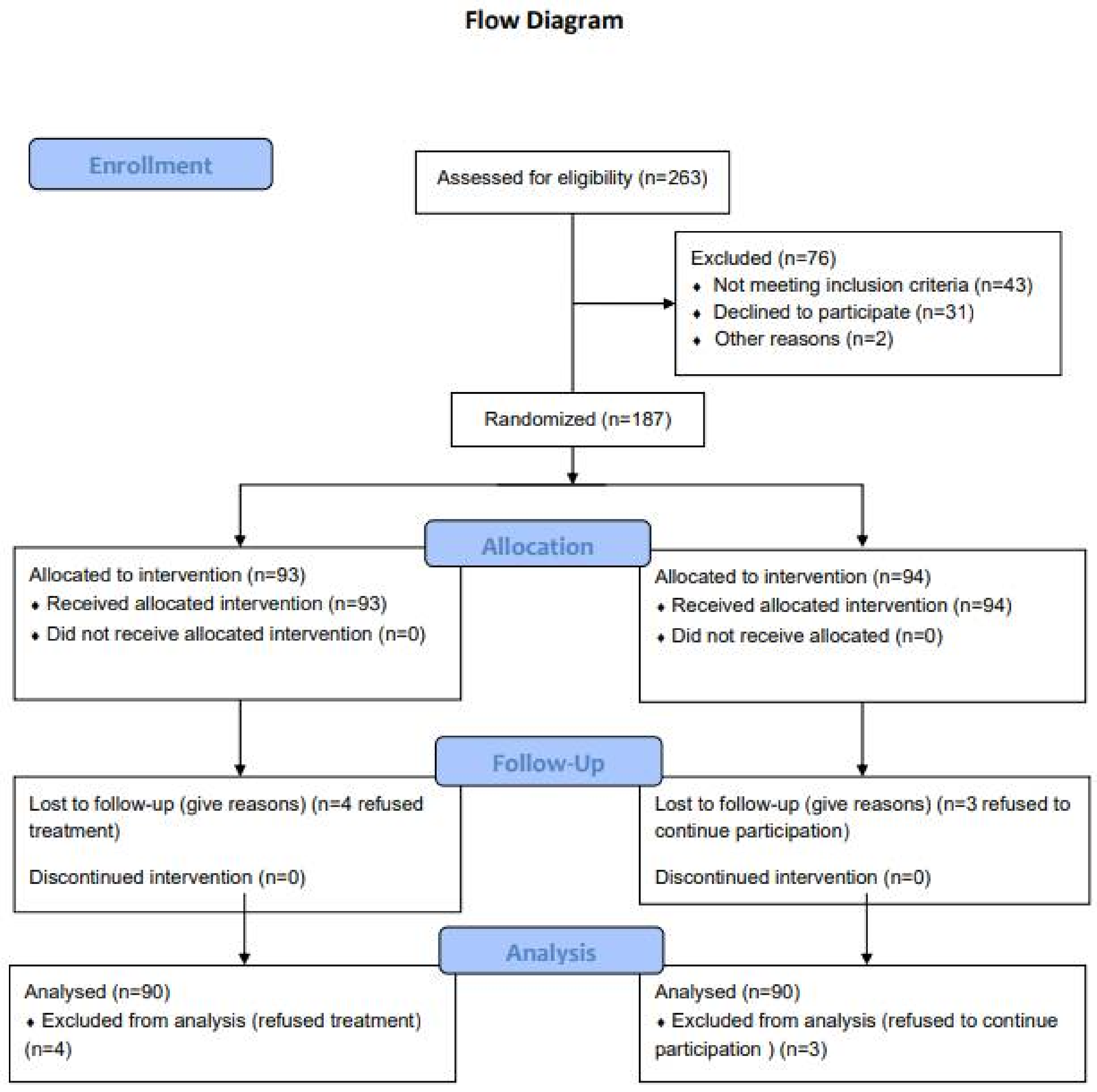
2.2. Recruitment, Randomization and Blinding Status
2.3. Assessment of Clinical Periodontal Parameters
2.4. Non-Surgical Periodontal Treatment
2.5. Study Outcomes
2.6. Governance and Ethics
2.7. Statistics
3. Results
3.1. Correlation Analysis between the Variables of Intervention Group at Baseline
3.2. Comparison of Each Parameter between Groups at Baseline, 3 and 6 Months
3.3. Difference of HbA1c Values Within the Intervention Group Over the 6 Months
3.4. Difference of HbA1c Values Within the Control Group Over the 6 Months
3.5. Difference of CRP Values Within the Intervention Group Over the 6 Months
3.6. Difference of CRP Values Within the Control Group Over the 6 Months
3.7. Effect on HbA1c
3.8. Effect on CRP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S171–S189. [Google Scholar] [CrossRef] [PubMed]
- Hegde, R.; Awan, K.H. Effects of periodontal disease on systemic health. Dis. Mon. 2019, 65, 185–192. [Google Scholar] [CrossRef]
- Yeo, B.K.; Lim, L.P.; Paquette, D.W.; Williams, R.C. Periodontal disease—The emergence of a risk for systemic conditions: Pre-term low birth weight. Ann. Acad Med. Singap. 2005, 34, 111–116. [Google Scholar]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotising periodontal diseases) and endo-periodontal lesions. J. Clin. Periodontol. 2018, 45, S78–S94. [Google Scholar] [CrossRef]
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and diagnosis of aggressive periodontitis. J. Clin. Periodontol. 2018, 45, S95–S111. [Google Scholar] [CrossRef] [PubMed]
- Needleman, I.; Garcia, R.; Gkranias, N.; Kirkwood, K.L.; Kocher, T.; Iorio, A.D.; Moreno, F.; Petrie, A. Mean annual attachment, bone level, and tooth loss: A systematic review. J. Clin. Periodontol. 2018, 45, S112–S129. [Google Scholar] [CrossRef]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Clin. Periodontol. 2018, 45, S130–S148. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, S149–S161. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Sanz, M. Implementation of the New Classification of Periodontal Diseases: Decision-making Algorithms for Clinical Practice and Education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, E.; Moscufo, L.; Corsalini, M.; Coscia, D.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Rapone, B.; Carossa, M.; Carossa, S. Polyamide vs silk sutures in the healing of postextraction sockets: A split mouth study. Oral Implantol. 2018, 11, 115–120. [Google Scholar]
- Rapone, B.; Corsalini, M.; Converti, I.; Loverro, M.T.; Gnoni, A.; Trerotoli, P.; Ferrara, E. Does Periodontal Inflammation Affect Type 1 Diabetes in Childhood and Adolescence? A Meta-Analysis. Front. Endocrinol. 2020, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Montemurro, N.; Perrini, P.; Rapone, B. Clinical Risk and Overall Survival in Patients with Diabetes Mellitus, Hyperglycemia and Glioblastoma Multiforme. A Review of the Current Literature. Int. J. Environ. Res. Public Health 2020, 17, 8501. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Consensus Report: Chronic Periodontitis International Workshop for a Classification of Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 38.
- Chee, B.; Park, B.; Bartold, P.M. Periodontitis and type II diabetes: A two-way relationship. Int. J. Evid. Based Healthc. 2013, 11, 317–329. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yuan, Y.H.; Liu, H.H.; Li, S.S.; Zhang, B.W.; Chen, W.; An, Z.J.; Chen, S.Y.; Wu, Y.Z.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Converti, I.; Grassi, F.R.; Santacroce, L.; Topi, S.; Gnoni, A.; Scacco, S.; Scarano, A.; et al. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 54675. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Bascones-Martinez, A.; Munoz-Corcuera, M.; Bascones-Ilundain, J. Diabetes and periodontitis: A bidirectional relationship. Med. Clin. 2015, 145, 31–35. [Google Scholar] [CrossRef]
- Baynes, H.W.; Mideksa, S.; Ambachew, S. The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action. Adipocyte 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.-Y.; Jun, H.-S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, A.B. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef]
- Kay, A.M.; LaShan Simpson, C.; Stewart, J.A., Jr. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef]
- Van Dyke, T.E. Inflammation and periodontal diseases: A reappraisal. J. Periodontol. 2008, 79, 1501–1502. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P. Destructive and Protective Roles of Cytokines in Periodontitis: A Re-appraisal from Host Defense and Tissue Destruction Viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef]
- Dutzan, N.; Kajikawa, T.; Abusleme, L.; Greenwell-Wild, T.; Zuazo, C.E.; Ikeuchi, T.; Brenchley, L.; Abe, T.; Hurabielle, C.; Martin, D.; et al. A dysbiotic microbiome triggers Th17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 2018, 10, eaat0797. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hemme, C.; Beleno, J.; Shi, Z.J.; Ning, D.; Qin, Y.; Tu, Q.; Jorgensen, M.; He, Z.; Wu, L.; et al. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J. 2018, 12, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sima, C.; Cheng, Q.; Rautava, J.; Levesque, C.; Sherman, P.; Glogauer, M. Identification of quantitative trait loci influencing inflammation-mediated alveolar bone loss: Insights into polygenic inheritance of host-biofilm disequilibria in periodontitis. J. Periodont. Res. 2016, 51, 237–249. [Google Scholar] [CrossRef]
- Gani, D.K.; Lakshmi, D.; Krishnan, R.; Emmadi, P. Evaluation of C-reactive protein and interleukin-6 in the peripheral blood of patients with chronic periodontitis. J. Indian Soc. Periodontol. 2009, 13, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; McPartlin, D.; Coward, P.Y.; Crook, M.; Lumb, P.; Wilson, R.F. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J. Clin. Periodontol. 2003, 30, 334–340. [Google Scholar] [CrossRef]
- Gupta, S.; Suri, P.; Patil, P.B.; Rajguru, J.P.; Gupta, P.; Patel, N. Comparative evaluation of role of hs C-reactive protein as a diagnostic marker in chronic periodontitis patients. J. Fam. Med. Prim. Care 2020, 9, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Converti, I.; Gnoni, A.; Scarano, A.; Scacco, S. Gingival crevicular blood as a potential screening tool: A cross sectional comparative study. Int. J. Environ. Res. Public Health 2020, 17, 7356. [Google Scholar] [CrossRef] [PubMed]
- Grover, H.S.; Luthra, S. Molecular mechanisms involved in the bidirectional relationship between diabetes mellitus and periodontal disease. J. Indian Soc. Periodontol. 2013, 17, 292–301. [Google Scholar] [PubMed]
- Walmsley, A.D.; Laird, W.R.; Williams, A.R. Dental plaque removal by cavitational activity during ultrasonic scaling. J. Clin. Periodontol. 1988, 15, 539–543. [Google Scholar] [CrossRef]
- Russell, A.L. A system for classification and scoring for prevalence surveys of periodontal disease. J. Dent. Res. 1956, 35, 350–359. [Google Scholar] [CrossRef]
- Boutron, I.; Moher, D.; Altman, D.G.; Schulz, K.F.; Ravaud, P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: Explanation and elaboration. Ann. Intern. Med. 2008, 148, 295–309. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Mariano Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Klingspor, L.; Sundin, U.; Altamash, M.; Klinge, B.; Engström, P.E. Periodontal conditions, oral Candida albicans and salivary proteins in type 2 diabetic subjects with emphasis on gender. BMC Oral Health 2009, 9, 12. [Google Scholar] [CrossRef]
- Löe, H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Cóccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Med. Mycol. 2008, 46, 783–793. [Google Scholar] [CrossRef]
- Scarano, A.; Barros, R.R.; Iezzi, G.; Piattelli, A.; Novaes, A.B., Jr. Acellular dermal matrix graft for gingival augmentation: A preliminary clinical, histologic, and ultrastructural evaluation. J. Periodontol. 2009, 80, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.; Hughes, F.; Preshaw, P. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014, 217, 433–437. [Google Scholar] [CrossRef]
- Jowett, A.K.; Orr, M.T.; Rawlinson, A.; Robinson, P.G. Psychosocial impact of periodontal disease and its treatment with 24 h root surface debridement. J. Clin. Periodontol 2009, 36, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.D.; Ghezzi, E.M.; Heiss, G.; Beck, J.D.; Riche, E.; Offenbacher, S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch. Intern. Med. 2003, 163, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2013, 40, S113–S134. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.N.; Kunzel, C.; Lamster, I.; Lalla, E. Diabetes in the dental office: Using NHANES III to estimate the probability of undiagnosed disease. J. Periodontal Res. 2007, 42, 559–565. [Google Scholar] [CrossRef]
- Lorusso, F.; Postiglione, F.; Delvecchio, M.; Rapone, B.; Scarano, A. The impact of diabetes on implant oral rehabilitations: A bibliometric study and literature review. Acta Med. Mediterr. 2020, 36, 3333. [Google Scholar]
- Katz, J. Elevated blood glucose levels in patients with severe periodontal disease. J. Clin. Periodontol. 2001, 28, 710–712. [Google Scholar] [CrossRef]
- Yilmaz, D.; Caglayan, F.; Buber, E.; Könönen, E.; Aksoy, Y.; Gursoy, U.K.; Guncu, G.N. Gingival crevicular fluid levels of human beta-defensin-1 in type 2 diabetes mellitus and periodontitis. Clin. Oral Investig. 2018, 22, 2135–2140. [Google Scholar] [CrossRef]
- Mattila, K.; Vesanen, M.; Valtonen, V.; Nieminen, M.; Palosuo, T.; Rasi, V.; Asikainen, S. Effect of treating periodontitis on C-reactive protein levels: A pilot study. BMC Infect. Dis. 2002, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Teeuw, W.J.; Gerdes, V.E.; Loos, B.G. Effect of periodontal treatment on glycemic control of diabetic patients: A systematic review and meta-analysis. Diabetes Care 2010, 33, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, S.; Nitta, H.; Nagasawa, T.; Uchimura, I.; Izumiyama, H.; Inagaki, K.; Kikuchi, T.; Noguchi, T.; Kanazawa, M.; Matsuo, A.; et al. Multi-center intervention study on glycohemoglobin (HbA1c) and serum, high-sensitivity CRP (hs-CRP) after local anti-infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res. Clin. Pract. 2009, 83, 308–315. [Google Scholar] [CrossRef]
- Buhlin, K.; Gustafsson, A.; Pockley, A.G.; Frostegård, J.; Klinge, B. Risk factors for cardiovascular disease in patients with periodontitis. Eur. Heart J. 2003, 24, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Machen, R.L.; Steffen, M.J.; Willmann, D.E. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin. Exp. Immunol. 1997, 107, 347–352. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.F.; Ding, Y.; Ge, S.; Rao, L.; Tang, H. Serum C-reactive protein levels and lipid profiles concentrations in moderate to severe periodontitis and coronary heart disease: A comparative study. Zhonghua Kou Qiang Yi Xue Za Zhi 2009, 44, 150–154. [Google Scholar] [PubMed]
- Bansal, T.; Pandey, A.; Asthana, A.K. C-Reactive Protein (CRP) and its Association with Periodontal Disease: A Brief Review. J. Clin. Diagn Res. 2014, 8, ZE21–ZE24. [Google Scholar] [PubMed]
- Persson, G.R.; Pettersson, T.; Ohlsson, O.; Renvert, S. High-sensitivity serum C-reactive protein levels in subjects with or without myocardial infarction or periodontitis. J. Clin. Periodontol. 2005, 32, 219–224. [Google Scholar] [CrossRef]
- Salzberg, T.N.; Overstreet, B.T.; Rogers, J.D.; Califano, J.V.; Best, A.M.; Schenkein, H.A. C-reactive protein levels in patients with aggressive periodontitis. J. Periodontol. 2006, 77, 933–939. [Google Scholar] [CrossRef]
| Characteristics | Intervention Group | Control Group |
|---|---|---|
| Sex (N) | ||
| Female Male | 50 40 | 54 36 |
| Age (y) mean ± SD | 53.2 ± 11.2 | 56. ± 6.9 |
| Average *BMI (kg/m2, mean ± SD) | 27.8 ± 6.3 | 22.3 ± 5.1 |
| HbA1c (%) *IG | HbA1c (%) **CG | p Value | CRP mg/L IG | CRP mg/L CG | p Value | PI (mm) IG | PI (mm) CG | p Value | GI (%) IG | GI (%) CG | p Value | PD (mm) IG | PD (mm) CG | p Value | CAL (mm) CG | CAL (mm) IG | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 8.081 | 8.767 | 0.048 | 2.514 | 2.302 | 0.431 | 80.8 | 69.067 | 0.011 | 68.744 | 62.244 | 0.74 | 4.851 | 4.572 | 0.037 | 4.883 | 5.178 | 0.039 |
| Median | 7.7 | 7.5 | - | 2.3 | 2.145 | - | 80.5 | 70.5 | - | 67 | 65 | - | 4.85 | 4.5 | - | 4.8 | 5.3 | - |
| Std. Deviation | 1.965 | 8.514 | - | 1.173 | 1.221 | - | 16.348 | 19.886 | - | 18.757 | 21.439 | - | 0.568 | 0.482 | - | 0.513 | 0.339 | - |
| Variance | 3.861 | 72.484 | - | 1.375 | 1.492 | - | 267.26 | 395.456 | - | 351.83 | 459.647 | - | 0.323 | 0.233 | - | 0.263 | 0.115 | - |
| Minimum | 5.5 | 5.5 | - | 0.26 | 0.26 | - | 45 | 21 | - | 29 | 18 | - | 4 | 3.3 | - | 3.82 | 4.5 | - |
| Maximum | 17 | 88 | - | 5.43 | 5.11 | - | 100 | 100 | - | 100 | 100 | - | 8 | 5.8 | - | 6.5 | 5.9 | - |
| IQR | 1.375 | 1.525 | - | 1.405 | 1.91 | - | 33 | 32.2 | 29.5 | 31..2 | - | 0.7 | 0.8 | - | 0.5 | 0.7 | - | |
| Skew | 2.592 | 9.258 | - | 0.215 | 0.513 | - | −0.314 | −0.2 | - | −0.046 | 0.108 | - | 1.831 | 0.269 | - | 0.107 | −0.162 | - |
| Kurtosis | 8.741 | 87.062 | - | −0.213 | −0.702 | - | −1.166 | −0.712 | - | −0.946 | −0.783 | - | 9.267 | −0.392 | - | 0.082 | −0.784 | - |
| Baseline | HbA1c | PI | GI | PD | CAL | CRP | |
|---|---|---|---|---|---|---|---|
| HbA1c | Spearman correlation | 1 | 0.106 | 0.126 | −0.136 | −0.142 | −0.222 |
| p-value (2-tailed) | 0.322 | 0.237 | 0.201 | 0.181 | 0.093 | ||
| PI | Spearman correlation | 0.106 | 0.09 | 0.123 | 0.091 | 0.938 | 0.224 |
| p-value (2-tailed) | 0.322 | 0 | 0.094 | 0.232 | 0.091 | ||
| GI | Spearman correlation | 0.126 | 0.914 | 1 | 0.12 | 0.112 | −0.205 |
| p-value (2-tailed) | 0.237 | 0 | 0.259 | 0.293 | 0.123 | ||
| PD | Spearman correlation | −0.136 | 0.177 | 0.12 | 1 | 0.718 | −0.224 |
| p-value (2-tailed) | 0.201 | 0.094 | 0.259 | 0 | 0.123 | ||
| CAL | Spearman correlation | −0.142 | 0.127 | 0.112 | 0.718 | 1 | −0.01 |
| p-value (2-tailed) | 0.181 | 0.232 | 0.293 | 0 | 0.938 | ||
| CRP | Spearman correlation | −0.222 | −0.224 | −0.205 | −0.224 | −0.01 | 1 |
| p-value (2-tailed) | 0.093 | 0.091 | 0.123 | 0.091 | 0.938 | 0 |
| 3 Months | HbA1c | GI | PI | PD | CAL | CRP | |
|---|---|---|---|---|---|---|---|
| HbA1c | Spearman correlation | 1 | −0.037 | −0.033 | 0.045 | −0.057 | −0.027 |
| p-value (2-tailed) | 0.728 | 0.757 | 0.677 | 0.59 | 0.799 | ||
| GI | Spearman correlation | −0.037 | 1 | 0.943 | 0.095 | −0.037 | 0.131 |
| p-value (2-tailed) | 0.728 | 0 | 0.372 | 0.727 | 0.217 | ||
| PI | Spearman correlation | −0.033 | 0.943 | 1 | 0.101 | −0.008 | 0.127 |
| p-value (2-tailed) | 0.757 | 0 | 0.342 | 0.942 | 0.233 | ||
| PD | Spearman correlation | 0.045 | 0.095 | 0.101 | 1 | −0.009 | −0.067 |
| p-value (2-tailed) | 0.677 | 0.372 | 0.342 | 0.932 | 0.53 | ||
| CAL | Spearman correlation | −0.057 | −0.037 | −0.008 | −0.009 | 1 | 0.103 |
| p-value (2-tailed) | 0.59 | 0.727 | 0.942 | 0.932 | 0.335 | ||
| CRP | Spearman correlation | −0.027 | 0.131 | 0.127 | −0.067 | 0.103 | 1 |
| p-value (2-tailed) | 0.799 | 0.217 | 0.233 | 0.53 | 0.335 |
| 6 months | HbA1c | PI | GI | PD | CAL | CRP | |
|---|---|---|---|---|---|---|---|
| HbA1c | Spearman correlation | 1 | −0.032 | 0.058 | −0.03 | −0.034 | 0.046 |
| p-value (2-tailed) | 0.767 | 0.587 | 0.779 | 0.753 | 0.669 | ||
| PI | Spearman correlation | −0.032 | 1 | 0.76 | 0.095 | 0.168 | 0.11 |
| p-value (2-tailed) | 0.767 | 0 | 0.375 | 0.113 | 0.301 | ||
| GI | Spearman correlation | 0.058 | 0.76 | 1 | −0.053 | 0.235 | 0.118 |
| p-value (2-tailed) | 0.587 | 0 | 0.62 | 0.025 | 0.268 | ||
| PD | Spearman correlation | −0.03 | 0.095 | −0.053 | −0.152 | −0.152 | −0.05 |
| p-value (2-tailed) | 0.779 | 0.375 | 0.62 | −0.152 | 0.153 | 0.639 | |
| CAL | Spearman correlation | −0.034 | 0.168 | 0.235 | −0.152 | 1 | 0.091 |
| p-value (2-tailed) | 0.753 | 0.113 | 0.025 | 0.153 | 0.396 | ||
| CRP | Spearman correlation | 0.046 | 0.11 | 0.118 | −0.05 | 0.091 | 1 |
| p-value (2-tailed) | 0.669 | 0.301 | 0.268 | 0.639 | 0.396 |
| Indices | Mann-Whitney U | W of Wilcoxon | Z | Asymptotic Significance (2-tailed) | Exact Significance (2-tailed) |
|---|---|---|---|---|---|
| HbA1c | |||||
| Baseline | 3.93 | 1.56 | −0.33 | 0.73 | 0.73 |
| 3 months | 3.08 | 2.26 | −2.77 | 0.006 | 0.006 |
| 6 months | 3.75 | 1.49 | −0.83 | 0.4 | 0.4 |
| PI | |||||
| Baseline | 2.64 | 2.46 | −4.04 | 0 | 0 |
| 3 months | 57.5 | 3.00 | −11.4 | 0 | 0 |
| 6 months | 15 | 0 | −11.5 | 0 | 0 |
| GI | |||||
| Baseline | 3.33 | 2.44 | −2.06 | 0.039 | 0.04 |
| 3 months | 70 | 12.0 | −11.39 | 0 | 0 |
| 6 months | 33 | 1.00 | 11.49 | 0 | 0 |
| PD | |||||
| Baseline | 2.88 | 2.15 | −3.3 | 0.001 | 0.001 |
| 3 months | 42.5 | 0 | −11.4 | 0 | 0 |
| 6 months | 2 | 0 | −11.5 | 0 | 0 |
| CAL | |||||
| Baseline | 2.62 | 2.32 | −4.08 | 0 | 0 |
| 3 months | 1.30 | 6.25 | −7.85 | 0 | 0 |
| 6 months | 9.48 | 2.84 | −8.8 | 0 | 0 |
| CRP | |||||
| Baseline | 3.62 | 4.48 | 1.22 | 0.222 | 0.221 |
| 3 months | 2.62 | 2.12 | −4.07 | 0 | 0 |
| 6 months | 1.83 | 1.83 | −6.32 | 0 | 0 |
| 3 Months | Unstandardized Coefficients | Standardized Coefficients | |||
|---|---|---|---|---|---|
| Model | B | β | Standard Error | t | p-Value |
| (Constant) | 13.9 | 3.23 | 4.32 | 0 | |
| PI | −0.03 | −0.32 | 0.02 | −1.3 | 0.18 |
| GI | 0.03 | 0.32 | 0.02 | 1.36 | 0.17 |
| PD | 0.27 | 0.08 | 0.45 | 0.61 | 0.5 |
| CAL | −1.24 | −0.21 | 0.74 | −1.66 | 0.1 |
| Unstandardized Coefficients | Standardized Coefficients | ||||
|---|---|---|---|---|---|
| Model | B | Beta | Standard error | t | p-Value |
| (Constant) | 1.37 | 1.01 | 1.348 | 0.181 | |
| HbA1c | 0.01 | 0.01 | 0.07 | 0.16 | 0.873 |
| PI | −0.006 | −0.05 | 0.05 | −0.125 | 0.901 |
| GI | 0.02 | 0.21 | 0.05 | 0.455 | 0.651 |
| PD | −0.08 | −0.05 | 0.1 | −0.468 | 0.641 |
| CAL | 0.11 | 0.07 | 0.16 | 0.67 | 0.504 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapone, B.; Ferrara, E.; Corsalini, M.; Qorri, E.; Converti, I.; Lorusso, F.; Delvecchio, M.; Gnoni, A.; Scacco, S.; Scarano, A. Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 3018. https://doi.org/10.3390/ijerph18063018
Rapone B, Ferrara E, Corsalini M, Qorri E, Converti I, Lorusso F, Delvecchio M, Gnoni A, Scacco S, Scarano A. Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2021; 18(6):3018. https://doi.org/10.3390/ijerph18063018
Chicago/Turabian StyleRapone, Biagio, Elisabetta Ferrara, Massimo Corsalini, Erda Qorri, Ilaria Converti, Felice Lorusso, Maurizio Delvecchio, Antonio Gnoni, Salvatore Scacco, and Antonio Scarano. 2021. "Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial" International Journal of Environmental Research and Public Health 18, no. 6: 3018. https://doi.org/10.3390/ijerph18063018
APA StyleRapone, B., Ferrara, E., Corsalini, M., Qorri, E., Converti, I., Lorusso, F., Delvecchio, M., Gnoni, A., Scacco, S., & Scarano, A. (2021). Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial. International Journal of Environmental Research and Public Health, 18(6), 3018. https://doi.org/10.3390/ijerph18063018













