The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research
Abstract
:1. Introduction
- Glossary of terms: (source: Oxford Languages dictionary)
- Nutrition: Science that interprets the nutrients and other substances in food in relation to maintenance, growth, reproduction, health and disease of an organism. It includes ingestion, absorption, assimilation, biosynthesis, catabolism and excretion.
- Diet: In nutrition, it is the sum of food consumed by a person.
- Food: Any nutritious substance that people or animals eat or drink or that plants absorb in order to maintain life and growth.
- Exercise: Any bodily activity that enhances or maintains physical fitness and overall health and wellness.
- Physical activity: Any voluntary bodily movement produced by skeletal muscles that requires energy expenditure. It includes exercise and incidental activity integrated into daily activity.
- Body composition: In physical fitness, it is used to describe the percentages of fat, bone, water and muscle in human bodies.
- Obesity: Medical condition in which excess body fat has accumulated to an extent that it may have a negative effect on health.
- Asthma: Long-term inflammatory disease of the airways of the lungs, characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms.
1.1. Assessment of Asthma and Asthma Control in Epidemiological Studies
1.2. Assessment of Nutritional Factors in Epidemiological Studies
2. The Role of Nutritional Factors in Asthma and Asthma Control: State of the Art
2.1. Diet and Asthma and Its Control
2.1.1. Asthma
2.1.2. Asthma Control
2.2. Physical Activity and Asthma and Its Control
2.2.1. Asthma
2.2.2. Asthma Control
2.3. Body Composition and Asthma and Its Control
2.3.1. Asthma
2.3.2. Asthma Control
2.4. The Complex Interrelations between Nutritional Factors and Asthma and Its Control
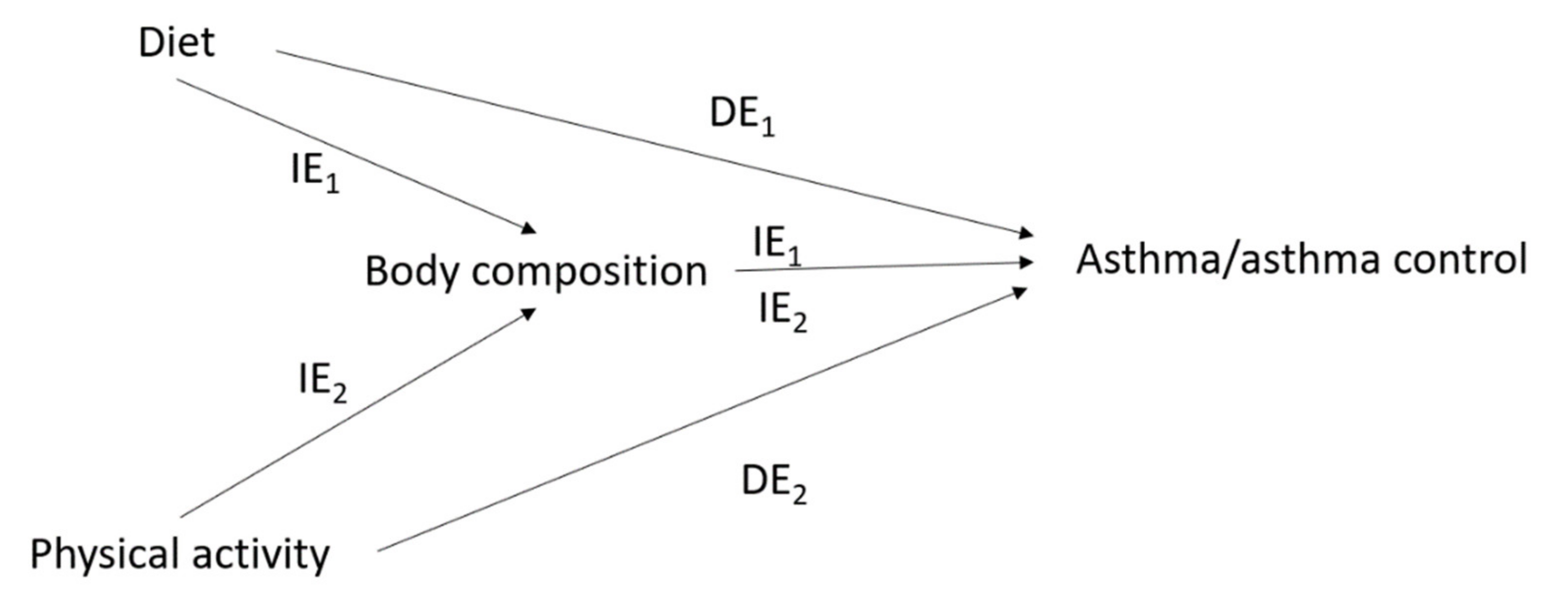
2.4.1. The Issue of Mediation in the Interrelations between Diet, Physical Activity, Body Composition and Asthma
2.4.2. The Issue of Time-Dependent Confounding in the Interrelations between Nutritional Factors and Asthma
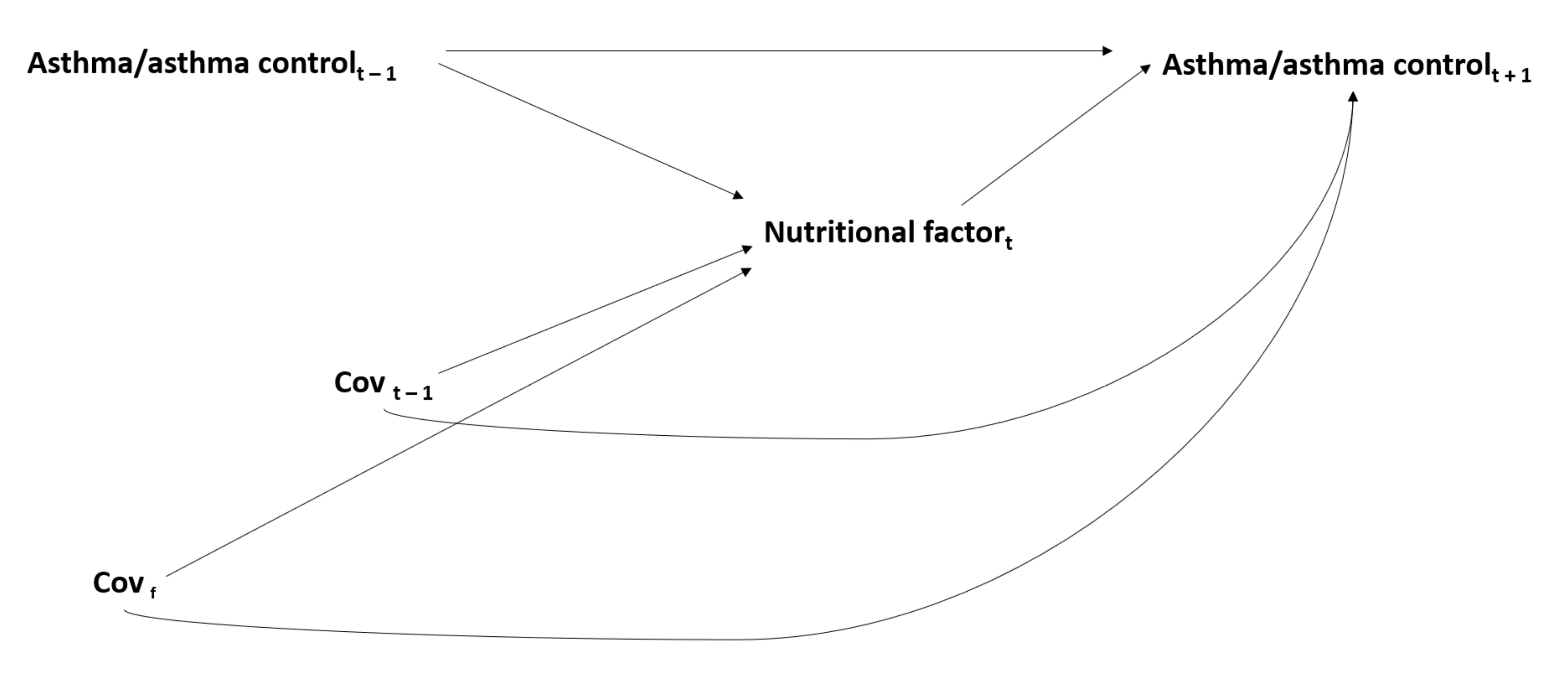
3. The Association between Nutritional Factors and Asthma: An Epiphenomenon of the Complex Interrelations between Genetic, Environmental, Lifestyle and Social Determinants in Asthma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, G.; La Grutta, S. The burden of pediatric asthma. Front. Pediatr. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [Green Version]
- Beasley, R.; Semprini, A.; Mitchell, E.A. Risk factors for asthma: Is prevention possible? Lancet 2015, 386, 1075–1085. [Google Scholar] [CrossRef]
- Braido, F.; Brusselle, G.; Guastalla, D.; Ingrassia, E.; Nicolini, G.; Price, D.; Roche, N.; Soriano, J.B.; Worth, H. Determinants and impact of suboptimal asthma control in Europe: The INTERNATIONAL CROSS-SECTIONAL AND LONGITUDINAL ASSESSMENT ON ASTHMA CONTROL (LIAISON) study. Respir. Res. 2016, 17, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2020. Available online: http://www.ginasthma.org/ (accessed on 11 March 2021).
- Health Promotion Glossary. Available online: https://www.who.int/healthpromotion/about/HPG/en/ (accessed on 28 September 2020).
- von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef]
- Varraso, R.; Camargo, C.A. Diet and asthma: Need to account for asthma type and level of prevention. Expert Rev. Respir. Med. 2016, 10, 1147–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novosad, S.; Khan, S.; Wolfe, B.; Khan, A. Role of obesity in asthma control, the obesity-asthma phenotype. J. Allergy 2013, 2013, 538642. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Wenzel, S.E. A global perspective in asthma: From phenotype to endotype. Chin. Med. J. 2013, 126, 166–174. [Google Scholar]
- de Marco, R.; Locatelli, F.; Sunyer, J.; Burney, P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am. J. Respir. Crit. Care Med. 2000, 162, 68–74. [Google Scholar] [CrossRef]
- Olivenstein, R.; Hamid, Q. Asthma in the elderly … Their time is right now. Clin. Exp. Allergy 2011, 41, 457–458. [Google Scholar] [CrossRef]
- Orie, N.; Sluiter, H.; De Vries, K.; Tammeling, G.; Witkop, J. The host factor in bronchitis. In Bronchitis; Orie, N., Sluiter, H., Eds.; Royal Van Gorcum: Assen, The Netherlands, 1961; pp. 43–59. [Google Scholar]
- Taylor, D.R.; Cowan, J.O.; Greene, J.M.; Willan, A.R.; Sears, M.R. Asthma in remission: Can relapse in early adulthood be predicted at 18 years of age? Chest 2005, 127, 845–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.D.; Wright, A.L.; Taussig, L.M.; Holberg, C.J.; Halonen, M.; Morgan, W.J. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 1995, 332, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, B.G. Epidemiology Standardization Project (American Thoracic Society). Am. Rev. Respir. Dis. 1978, 118, 1–120. [Google Scholar] [PubMed]
- Brille, D.; Casula, D.; van der Lende, R.; Smidt, U.; Minette, A. Commentaires relatifs au questionnaire pour l’étude de la bronchite chronique et de l’emphysème pulmonaire. In British Medical Research Council/Communauté Européenne du Charbon et de l’Acier; Collection D’hygiène et de Médecine et du Travail, n°14; CEE-CECA: Luxembourg, 1971. [Google Scholar]
- Sunyer, J.; Pekkanen, J.; Garcia-Esteban, R.; Svanes, C.; Künzli, N.; Janson, C.; de Marco, R.; Antó, J.M.; Burney, P. Asthma score: Predictive ability and risk factors. Allergy 2007, 62, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Pekkanen, J.; Sunyer, J.; Anto, J.M.; Burney, P. Operational definitions of asthma in studies on its aetiology. Eur. Respir. J. 2005, 26, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.H.; Zeiger, R.; Sorkness, C.; Mahr, T.; Ostrom, N.; Burgess, S.; Rosenzweig, J.C.; Manjunath, R. Development and cross-sectional validation of the Childhood Asthma Control Test. J. Allergy Clin. Immunol. 2007, 119, 817–825. [Google Scholar] [CrossRef]
- Cloutier, M.M.; Schatz, M.; Castro, M.; Clark, N.; Kelly, H.W.; Mangione-Smith, R.; Sheller, J.; Sorkness, C.; Stoloff, S.; Gergen, P. Asthma outcomes: Composite scores of asthma control. J. Allergy Clin. Immunol. 2012, 129, S24–S33. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; Mccullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hebert, J.R. A New Dietary Inflammatory Index Predicts Interval Changes in Serum High-Sensitivity C-Reactive Protein. J. Nutr. 2009, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. 24-hour recall and diet record methods. In Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 2012; pp. 49–69. [Google Scholar]
- Willett, W.C. Reproducibility and validity of food-frequency questionnaires. In Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 2012; pp. 96–141. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- IPAQ Scoring Protocol. Available online: https://sites.google.com/site/theipaq/scoring-protocol (accessed on 11 March 2021).
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sport. Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, H.J.F.; Brage, S.; Warren, J.; Besson, H.; Ekelund, U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation (WHO). Obesity and Overweight, Fact Sheet No 311 (Updated August 2014). Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 11 March 2021).
- National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. BMI: Body mass index. In Atlanta, GA: Centers for Disease Control and Prevention; 2002. Available online: http://www.cdc.gov/healthyweight/assessing/bmi/index.html (accessed on 11 March 2021).
- Palta, M.; Prineas, R.J.; Berman, R.; Hannan, P. Comparison of self-reported and measured height and weight. Am. J. Epidemiol. 1982, 115, 223–230. [Google Scholar] [CrossRef]
- Willett, W.C. Anthropometric measures and body composition. In Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 2012; pp. 213–240. [Google Scholar]
- Peralta, G.P.; Fuertes, E.; Granell, R.; Mahmoud, O.; Roda, C.; Serra, I.; Jarvis, D.; Henderson, J.; Garcia-Aymerich, J. Childhood body composition trajectories and adolescent lung function findings from the ALSPAC study. Am. J. Respir. Crit. Care Med. 2019, 200, 75–83. [Google Scholar] [CrossRef]
- Brozek, J.; Grande, F.; Anderson, J.T.; Keys, A. Densitometric analysis of body composition: Revision of some quantitative assumptions. Ann. N.Y. Acad. Sci. 1963, 110, 113–140. [Google Scholar] [CrossRef]
- Roubenoff, R.; Kehayias, J.J.; Dawson-Hughes, B.; Heymsfield, S.B. Use of dual-energy x-ray absorptiometry in body-composition studies: Not yet a “gold standard”. Am. J. Clin. Nutr. 1993, 58, 589–591. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Electrical impedance and total body electrical conductivity. In Human Body Composition; Heymsfield, S.B., Wang, Z.M., Baumgartner, R.N., Eds.; Human Kinetics: Champaign, IL, USA, 1996; pp. 79–108. [Google Scholar]
- Willett, K.; Jiang, R.; Lenart, E.; Spiegelman, D.; Willett, W. Comparison of bioelectrical impedance and BMI in predicting obesity-related medical conditions. Obesity 2006, 14, 480–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, T.I.; Stunkard, A.J. Does obesity run in families because of genes? An adoption study using silhouettes as a measure of obesity. Acta Psychiatr. Scand. Suppl. 1993, 370, 67–72. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedón, J.C.; Castro-Rodriguez, J.A. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: A systematic review and meta-analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L. What Are the Effects of a Mediterranean Diet on Allergies and Asthma in Children? Front. Pediatr. 2017, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Hanson, C.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Gold, D.; Camargo, C.A.; Sen, S.; Sordillo, J.E.; Oken, E.; et al. Associations of Prenatal Dietary Inflammatory Potential with Childhood Respiratory Outcomes in Project Viva. J. Allergy Clin. Immunol. Pract. 2020, 8, 945–952e4. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.-M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Fish Oil–Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef]
- Best, K.P.; Sullivan, T.; Palmer, D.; Gold, M.; Kennedy, D.J.; Martin, J.; Makrides, M. Prenatal fish oil supplementation and allergy: 6-Year follow-up of a randomized controlled trial. Pediatrics 2016, 137, e20154443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bédard, A.; Northstone, K.; John Henderson, A.; Shaheen, S.O. Mediterranean diet during pregnancy and childhood respiratory and atopic outcomes: Birth cohort study. Eur. Respir. J. 2020, 55, 1901215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, S.O.; Gissler, M.; Gissler, M.; Devereux, G.; Erkkola, M.; Kinnunen, T.I.; Mcardle, H.; Sheikh, A.; Hemminki, E.; Nwaru, B.I.; et al. Maternal iron supplementation in pregnancy and asthma in the offspring: Follow-up of a randomised trial in Finland. Eur. Respir. J. 2020, 55, 1902335. [Google Scholar] [CrossRef]
- Bédard, A.; Northstone, K.; Henderson, A.J.; Shaheen, S.O. Maternal intake of sugar during pregnancy and childhood respiratory and atopic outcomes. Eur. Respir. J. 2017, 50, 1700073. [Google Scholar] [CrossRef]
- Wright, L.S.; Rifas-Shiman, S.L.; Oken, E.; Litonjua, A.A.; Gold, D.R. Prenatal and early life fructose, fructose-containing beverages, and midchildhood asthma. Ann. Am. Thorac. Soc. 2018, 15, 217–224. [Google Scholar] [CrossRef]
- Chen, L.W.; Lyons, B.; Navarro, P.; Shivappa, N.; Mehegan, J.; Murrin, C.M.; Hébert, J.R.; Kelleher, C.C.; Phillips, C.M. Maternal dietary inflammatory potential and quality are associated with offspring asthma risk over 10-year follow-up: The Lifeways Cross-Generation Cohort Study. Am. J. Clin. Nutr. 2020, 111, 440–447. [Google Scholar] [CrossRef]
- Leynaert, B.; Le Moual, N.; Neukirch, C.; Siroux, V.; Varraso, R. Environmental risk factors for asthma developement. La Presse Médicale 2019, 48, 262–273. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Shrestha, S.K.; Itsiopoulos, C.; Erbas, B. The role of fish intake on asthma in children: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Tromp, I.I.M.; Kiefte-de Jong, J.C.; de Vries, J.H.; Jaddoe, V.W.V.; Raat, H.; Hofman, A.; de Jongste, J.C.; Moll, H.A. Dietary patterns and respiratory symptoms in pre-school children: The Generation R Study. Eur. Respir. J. 2012, 40, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and asthma: Is it time to adapt our message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrianasolo, R.M.; Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Galan, P.; Varraso, R. Association between dietary fibre intake and asthma (symptoms and control): Results from the French national e-cohort NutriNet-Santé. Br. J. Nutr. 2019, 122, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rava, M.; Bédard, A.; Dumas, O.; Garcia-Aymerich, J.; Leynaert, B.; Pison, C.; Le Moual, N.; Romieu, I.; Siroux, V.; et al. Cured meat intake is associated with worsening asthma symptoms. Thorax 2017, 72, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Andrianasolo, R.M.; Hercberg, S.; Touvier, M.; Druesne-Pecollo, N.; Adjibade, M.; Kesse-Guyot, E.; Galan, P.; Varraso, R. Association between processed meat intake and asthma symptoms in the French NutriNet-Santé cohort. Eur. J. Nutr. 2020, 59, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kesse-Guyot, E.; Dumas, O.; Garcia-Aymerich, J.; Leynaert, B.; Pison, C.; Le Moual, N.; Romieu, I.; Siroux, V.; Camargo, C.A.; et al. Longitudinal study of diet quality and change in asthma symptoms in adults, according to smoking status. Br. J. Nutr. 2017, 117, 562–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrianasolo, R.M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Galan, P.; Varraso, R. Associations between dietary scores with asthma symptoms and asthma control in adults. Eur. Respir. J. 2018, 52, 1702572. [Google Scholar] [CrossRef] [Green Version]
- Varraso, R.; Jiang, R.; Barr, R.G.; Willett, W.C.; Camargo, C. a Prospective study of cured meats consumption and risk of chronic obstructive pulmonary disease in men. Am. J. Epidemiol. 2007, 166, 1438–1445. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Camargo, C.A.; Varraso, R.; Paik, D.C.; Willett, W.C.; Barr, R.G. Consumption of cured meats and prospective risk of chronic obstructive pulmonary disease in women. Am. J. Clin. Nutr. 2008, 87, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Varraso, R.; Chiuve, S.E.; Fung, T.T.; Barr, R.G.; Hu, F.B.; Willett, W.C.; Camargo, C.A. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: Prospective study. BMJ 2015, 350, 1–11. [Google Scholar] [CrossRef] [Green Version]
- DeChristopher, L.R.; Tucker, K.L. Excess free fructose, high-fructose corn syrup and adult asthma: The Framingham Offspring Cohort. Br. J. Nutr. 2018, 119, 1157–1167. [Google Scholar] [CrossRef] [Green Version]
- Cassim, R.; Russell, M.A.; Lodge, C.J.; Lowe, A.J.; Koplin, J.J.; Dharmage, S.C. The role of circulating 25 hydroxyvitamin D in asthma: A systematic review. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Kauffmann, F.; Leynaert, B.; Le Moual, N.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Romieu, I. Dietary patterns and asthma in the E3N study. Eur. Respir. J. 2009, 33, 33–41. [Google Scholar] [CrossRef]
- el Bilbeisi, A.H.H.; Albelbeisi, A.; Hosseini, S.; Djafarian, K. Dietary Pattern and Their Association With Level of Asthma Control Among Patients with Asthma at Al-Shifa Medical Complex in Gaza Strip, Palestine. Nutr. Metab. Insights 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Fonseca, J.; de Oliveira, J.F.; Delgado, L.; Barros, R.; Haahtela, T.; Lopes, C.; Castel-Branco, M.G.; Moreira, P. Adherence to the Mediterranean diet and fresh fruit intake are associated with improved asthma control. Allergy 2008, 63, 917–923. [Google Scholar] [CrossRef]
- Sexton, P.; Black, P.; Metcalf, P.; Wall, C.R.; Ley, S.; Wu, L.; Sommerville, F.; Brodie, S.; Kolbe, J. Influence of mediterranean diet on asthma symptoms, lung function, and systemic inflammation: A randomized controlled trial. J. Asthma 2013, 50, 75–81. [Google Scholar] [CrossRef]
- Ma, J.; Strub, P.; Lv, N.; Xiao, L.; Camargo, C.A.; Buist, A.S.; Lavori, P.W.; Wilson, S.R.; Nadeau, K.C.; Rosas, L.G. Pilot randomised trial of a healthy eating behavioural intervention in uncontrolled asthma. Eur. Respir. J. 2016, 47, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Eijkemans, M.; Mommers, M.; Draaisma, J.M.T.; Thijs, C.; Prins, M.H. Physical activity and asthma: A systematic review and meta-analysis. PLoS ONE 2012, 7, e50775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochte, L.; Nielsen, K.G.; Petersen, P.E.; Platts-Mills, T.A.E. Childhood asthma and physical activity: A systematic review with meta-analysis and graphic appraisal tool for epidemiology assessment. BMC Pediatr. 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, A.T.; Staples, K.J.; Wilkinson, T.M.A. Defining a role for exercise training in the management of asthma. Eur. Respir. Rev. 2020, 29, 190106. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.D.; Ferreira, P.G.; Silva, A.G.; Stelmach, R.; Carvalho-Pinto, R.M.; Fernandes, F.L.A.; Mancini, M.C.; Sato, M.N.; Martins, M.A.; Carvalho, C.R.F. The role of exercise in a weight-loss program on clinical control in obese adults with Asthma: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2017, 195, 32–42. [Google Scholar] [CrossRef]
- Türk, Y.; Theel, W.; Van Huisstede, A.; Van De Geijn, G.J.M.; Birnie, E.; Hiemstra, P.S.; Sont, J.K.; Taube, C.; Braunstahl, G.J. Short-term and long-term effect of a high-intensity pulmonary rehabilitation programme in obese patients with asthma: A randomised controlled trial. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Camargo, C.A.; Weiss, S.T.; Zhang, S.; Willett, W.C.; Speizer, F.E. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch. Intern. Med. 1999, 159, 2582–2588. [Google Scholar] [CrossRef] [Green Version]
- Burgess, J.A.; Walters, E.H.; Byrnes, G.B.; Giles, G.G.; Jenkins, M.A.; Abramson, M.J.; Hopper, J.L.; Dharmage, S.C. Childhood adiposity predicts adult-onset current asthma in females: A 25-yr prospective study. Eur. Respir. J. 2007, 29, 668–675. [Google Scholar] [CrossRef]
- Scholtens, S.; Wijga, A.H.; Seidell, J.C.; Brunekreef, B.; de Jongste, J.C.; Gehring, U.; Postma, D.S.; Kerkhof, M.; Smit, H.A. Overweight and changes in weight status during childhood in relation to asthma symptoms at 8 years of age. J. Allergy Clin. Immunol. 2009, 123, 1312–1318.e2. [Google Scholar] [CrossRef]
- Romieu, I.; Avenel, V.; Leynaert, B.; Kauffmann, F.; Clavel-Chapelon, F. Body mass index, change in body silhouette, and risk of asthma in the E3N cohort study. Am. J. Epidemiol. 2003, 158, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, O.; Varraso, R.; Gillman, M.W.; Field, A.E.; Camargo, C.A. Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy 2016. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, B.; Wang, Y.; Wang, K.; Zhang, Z.; Niu, W. Pre-pregnancy maternal weight and gestational weight gain increase the risk for childhood asthma and wheeze: An updated meta-analysis. Front. Pediatr. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Beuther, D.A.; Sutherland, E.R. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Salam, M.T.; Alderete, T.L.; Habre, R.; Bastain, T.M.; Berhane, K.; Gilliland, F.D. Effects of childhood asthma on the development of obesity among school-aged children. Am. J. Respir. Crit. Care Med. 2017, 195, 1181–1188. [Google Scholar] [CrossRef]
- Contreras, Z.A.; Chen, Z.; Roumeliotaki, T.; Annesi-Maesano, I.; Baïz, N.; von Berg, A.; Bergström, A.; Crozier, S.; Duijts, L.; Ekström, S.; et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef] [Green Version]
- Juel, C.T.B.; Ali, Z.; Nilas, L.; Ulrik, C.S. Asthma and obesity: Does weight loss improve asthma control? A systematic review. J. Asthma Allergy 2012, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirsch, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–168. [Google Scholar] [CrossRef]
- Denou, E.; Marcinko, K.; Surette, M.G.; Steinberg, G.R.; Schertzer, J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, 982–993. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Alemao, C.A.; Budden, K.F.; Gomez, H.M.; Rehman, S.F.; Marshall, J.E.; Shukla, S.D.; Donovan, C.; Forster, S.C.; Yang, I.A.; Keely, S.; et al. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy Eur. J. Allergy Clin. Immunol. 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. The infant gut microbiota and risk of asthma: The effect of maternal nutrition during pregnancy and lactation. Microorganisms 2020, 8, 1119. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.-P. Asthma and obesity. Clin. Exp. Allergy 2013, 43, 8–21. [Google Scholar] [CrossRef]
- Chapman, D.G.; Salome, C.M. Lifestyles of the fat and lazy. Clin. Exp. Allergy 2013, 43, 2–4. [Google Scholar] [CrossRef]
- Lucas, S.R.; Platts-Mills, T.A.E. Physical activity and exercise in asthma: Relevance to etiology and treatment. J. Allergy Clin. Immunol. 2005, 115, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Litonjua, A. Asthma, allergy, and responses to methyl donor supplements and nutrients. J. Allergy Clin. Immunol. 2014, 133, 1246–1254. [Google Scholar] [CrossRef] [Green Version]
- Camargo, C.A.J. Vitamin D, acute respiratory infection, and asthma/chronic obstructive pulmonary disease. In Vitamin D, 4th ed.; Feldman, D., Pike, J.W., Bouillon, R., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2018; pp. 1096–1120. [Google Scholar]
- Nyenhuis, S.M.; Dixon, A.E.; Ma, J. Impact of Lifestyle Interventions Targeting Healthy Diet, Physical Activity, and Weight Loss on Asthma in Adults: What Is the Evidence? J. Allergy Clin. Immunol. Pract. 2018, 6, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Strub, P.; Xiao, L.; Lavori, P.W.; Camargo, C.A.; Wilson, S.R.; Gardner, C.D.; Buist, A.S.; Haskell, W.L.; Lv, N. Behavioral weight loss and physical activity intervention in obese adults with asthma: A randomized trial. Ann. Am. Thorac. Soc. 2015, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Toennesen, L.L.; Meteran, H.; Hostrup, M.; Wium Geiker, N.R.; Jensen, C.B.; Porsbjerg, C.; Astrup, A.; Bangsbo, J.; Parker, D.; Backer, V. Effects of Exercise and Diet in Nonobese Asthma Patients—A Randomized Controlled Trial. J. Allergy Clin. Immunol. Pract. 2018, 6, 803–811. [Google Scholar] [CrossRef]
- Garcia-Marcos, L.; Canflanca, I.M.; Garrido, J.B.; Varela, A.L.-S.; Garcia-Hernandez, G.; Guillen Grima, F.; Gonzalez-Diaz, C.; Carvajal-Urueña, I.; Arnedo-Pena, A.; Busquets-Monge, R.M.; et al. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax 2007, 62, 503–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romieu, I.; Mannino, D.M.; Redd, S.C.; McGeehin, M.A. Dietary intake, physical activity, body mass index, and childhood asthma in the Third National Health and Nutrition Survey (NHANES III). Pediatr. Pulmonol. 2004, 38, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Corbo, G.M.; Forastiere, F.; De Sario, M.; Brunetti, L.; Bonci, E.; Bugiani, M.; Chellini, E.; La Grutta, S.; Migliore, E.; Pistelli, R.; et al. Wheeze and asthma in children: Associations with body mass index, sports, television viewing, and diet. Epidemiology 2008, 19, 747–755. [Google Scholar] [CrossRef]
- Mitchell, E.A.; Beasley, R.; Björkstén, B.; Crane, J.; García-Marcos, L.; Keil, U. The association between BMI, vigorous physical activity and television viewing and the risk of symptoms of asthma, rhinoconjunctivitis and eczema in children and adolescents: ISAAC Phase Three. Clin. Exp. Allergy 2013, 43, 73–84. [Google Scholar] [CrossRef]
- Lawson, J.A.; Rennie, D.C.; Dosman, J.A.; Cammer, A.L.; Senthilselvan, A. Obesity, diet, and activity in relation to asthma and wheeze among rural dwelling children and adolescents. J. Obes. 2013, 2013, 315096. [Google Scholar] [CrossRef]
- Beckett, W.S.; Jacobs, D.R.; Yu, X.; Iribarren, C.; Williams, O.D. Asthma is associated with weight gain in females but not males, independent of physical activity. Am. J. Respir. Crit. Care Med. 2001, 164, 2045–2050. [Google Scholar] [CrossRef]
- Kilpeläinen, M.; Terho, E.O.; Helenius, H.; Koskenvuo, M. Body mass index and physical activity in relation to asthma and atopic diseases in young adults. Respir. Med. 2006, 100, 1518–1525. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Tu, Y.-K.; Huang, K.-C.; Chen, P.-C.; Chu, D.-C.; Lee, Y.L. Pathway From Central Obesity to Childhood Asthma: Physical Fitness and Sedentary Time Are Leading Factors. Am. J. Respir. Crit. Care Med. 2014, 189, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Strachan, D.; Asher, I.; Ellwood, P.; Pearce, N.; Garcia-Marcos, L. Combined impact of healthy lifestyle factors on risk of asthma, rhinoconjunctivitis and eczema in school children: ISAAC phase III. Thorax 2019, 74, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrier, I.; Platt, R.W. Reducing bias through directed acyclic graphs. BMC Med. Res. Methodol. 2008, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Schisterman, E.F.; Cole, S.R.; Platt, R.W. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009, 20, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Lange, T.; Vansteelandt, S.; Bekaert, M. A Simple Unified Approach for Estimating Natural Direct and Indirect Effects. Am. J. Epidemiol. 2012, 176, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Valeri, L.; VanderWeele, T.J. Mediation analysis allowing for exposure–mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychol. Methods 2013, 18, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Audureau, E.; Pouchot, J.; Coste, J. Gender-related differential effects of obesity on health-related quality of life via obesity-related comorbidities. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 246–256. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Wang, B.; Zhou, M.; Cao, L.; Qiu, W.; Mu, G.; Chen, A.; Yang, S.; Chen, W. Systemic inflammation mediates the associations between abdominal obesity indices and lung function decline in a chinese general population. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuertes, E.; Carsin, A.E.; Garcia-Larsen, V.; Guerra, S.; Pin, I.; Leynaert, B.; Accordini, S.; Martinez-Moratalla, J.; Antó, J.M.; Urrutia, I.; et al. The role of C-reactive protein levels on the association of physical activity with lung function in adults. PLoS ONE 2019, 14, e0222578. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, F.A.; Penders, J.; Stobberingh, E.E.; Postma, D.S.; Koppelman, G.H.; Kerkhof, M.; Reijmerink, N.E.; Dompeling, E.; Van Den Brandt, P.A.; Ferreira, I.; et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol. 2011, 128, 948–955e3. [Google Scholar] [CrossRef]
- Campbell, B.; Simpson, J.A.; Bui, D.S.; Lodge, C.J.; Lowe, A.J.; Matheson, M.C.; Bowatte, G.; Burgess, J.A.; Hamilton, G.S.; Leynaert, B.; et al. Early menarche is associated with lower adult lung function: A longitudinal cohort study from the first to sixth decade of life. Respirology 2020, 25, 289–297. [Google Scholar] [CrossRef]
- Assmann, K.E.; Ruhunuhewa, I.; Adjibade, M.; Li, Z.; Varraso, R.; Hercberg, S.; Galan, P.; Kesse-Guyot, E. The mediating role of overweight and obesity in the prospective association between overall dietary quality and healthy aging. Nutrients 2018, 10, 515. [Google Scholar] [CrossRef] [Green Version]
- Robins, J.M.; Hernán, M.A.; Brumback, B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000, 11, 550–560. [Google Scholar] [CrossRef]
- Hernan, M.A. A definition of causal effect for epidemiological research. J. Epidemiol. Community Heal. 2004, 58, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Sparks, J.A.; Lin, T.C.; Camargo, C.A.; Barbhaiya, M.; Tedeschi, S.K.; Costenbader, K.H.; Raby, B.A.; Choi, H.K.; Karlson, E.W. Rheumatoid arthritis and risk of chronic obstructive pulmonary disease or asthma among women: A marginal structural model analysis in the Nurses’ Health Study. Semin. Arthritis Rheum. 2018, 47, 639–648. [Google Scholar] [CrossRef]
- Dumas, O.; Le Moual, N.; Siroux, V.; Heederik, D.; Garcia-Aymerich, J.; Varraso, R.; Kauffmann, F.; Basagaña, X. Work related asthma. A causal analysis controlling the healthy worker effect. Occup. Environ. Med. 2013, 70, 603–610. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Lange, P.; Serra, I.; Schnohr, P.; Antó, J.M. Time-dependent confounding in the study of the effects of regular physical activity in chronic obstructive pulmonary disease: An application of the marginal structural model. Ann. Epidemiol. 2008, 18, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Serra, I.; Dumas, O.; Basaganã, X.; Clavel-Chapelon, F.; Le Moual, N.; Sanchez, M.; Siroux, V.; Varraso, R.; Garcia-Aymerich, J. Time-Dependent Associations between Body Composition, Physical Activity, and Current Asthma in Women: A Marginal Structural Modeling Analysis. Am. J. Epidemiol. 2017, 186, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Carsin, A.E.; Fuertes, E.; Accordini, S.; Dharmage, S.C.; Garcia-Larsen, V.; Heinrich, J.; Janson, C.; Johannessen, A.; Leynaert, B.; et al. Physical activity and lung function-Cause or consequence? PLoS ONE 2020, 15, e0237769. [Google Scholar] [CrossRef] [PubMed]
- Tager, I.B.; Haight, T.; Sternfeld, B.; Yu, Z.; van Der Laan, M. Effects of Physical Activity and Body Composition on Functional Limitation in the Elderly. Epidemiology 2004, 15, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Robins, J. A new approach to causal inference in mortality studies with a sustained exposure period—Application to control of the healthy worker survivor effect. Math Mod. 1986, 7, 1393–1512. [Google Scholar] [CrossRef] [Green Version]
- Williamson, E.J.; Polak, J.; Simpson, J.A.; Giles, G.G.; English, D.R.; Hodge, A.; Gurrin, L.; Forbes, A.B. Sustained adherence to a Mediterranean diet and physical activity on all-cause mortality in the Melbourne Collaborative Cohort Study: Application of the g-formula. BMC Public Health 2019, 19, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerman, B.A.; Giovannucci, E.; Pernar, C.H.; Mucci, L.A.; Hernán, M.A. Guideline-based physical activity and survival among US men with nonmetastatic prostate cancer. Am. J. Epidemiol. 2019, 188, 579–586. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Peres, M.A.; Mittinty, M.N.; Peres, K.G.; Do, L.G.; Horta, B.L.; Gigante, D.P.; Corrêa, M.B.; Demarco, F.F. Diet-induced overweight and obesity and periodontitis risk: An application of the parametric g-formula in the 1982 pelotas birth cohort. Am. J. Epidemiol. 2017, 185, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Taubman, S.L.; Robins, J.M.; Mittleman, M.A.; Hernán, M.A. Intervening on risk factors for coronary heart disease: An application of the parametric g-formula. Int. J. Epidemiol. 2009, 38, 1599–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajous, M.; Willett, W.C.; Robins, J.; Young, J.G.; Rimm, E.; Mozaffarian, D.; Hernán, M.A. Changes in fish consumption in midlife and the risk of coronary heart disease in men and women. Am. J. Epidemiol. 2013, 178, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Danaei, G.; Robins, J.M.; Young, J.; Hu, F.B.; Manson, J.E.; Hernán, M.A. Estimated effect of weight loss on risk of coronary heart disease and mortality in middle-aged or older women: Sensitivity analysis analysis for Unmeasured Confounding By Undiagnosed Disease. Epidemiology 2017, 27, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Aymerich, J.; Varraso, R.; Danaei, G.; Camargo, C.A.; Hernán, M.A. Incidence of adult-onset asthma after hypothetical interventions on body mass index and physical activity: An application of the parametric g-formula. Am. J. Epidemiol. 2014, 179, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.M.; Costello, S.; Elser, H.; Neophytou, A.M.; Picciotto, S.; Silverman, D.T.; Eisen, E.A. Chronic obstructive pulmonary disease mortality: The Diesel Exhaust in Miners Study (DEMS). Environ. Res. 2020, 180, 108876. [Google Scholar] [CrossRef]
- Neophytou, A.M.; Costello, S.; Picciotto, S.; Noth, E.M.; Liu, S.; Lutzker, L.; Balmes, J.R.; Hammond, K.; Cullen, M.R.; Eisen, E.A. Accelerated lung function decline in an aluminium manufacturing industry cohort exposed to pm 2.5: An application of the parametric g-formula. Occup. Environ. Med. 2019, 76, 888–894. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Tchetgen Tchetgen, E.J. Mediation analysis with time varying exposures and mediators. J. R. Stat. Soc. Ser. B Stat. Methodol. 2017, 79, 917–938. [Google Scholar] [CrossRef] [Green Version]
- Pinto Pereira, S.M.; De Stavola, B.L.; Rogers, N.T.; Hardy, R.; Cooper, R.; Power, C. Adult obesity and mid-life physical functioning in two British birth cohorts: Investigating the mediating role of physical inactivity. Int. J. Epidemiol. 2020, 49, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Berhane, K.; McConnell, R.; Gauderman, W.J.; Avol, E.; Peters, J.M.; Gilliland, F.D. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax 2009, 64, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romieu, I.; Sienra-Monge, J.J.; Ramírez-Aguilar, M.; Moreno-Macías, H.; Reyes-Ruiz, N.I.; Estela del Río-Navarro, B.; Hernández-Avila, M.; London, S.J. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax 2004, 59, 8–10. [Google Scholar] [PubMed]
- Gref, A.; Rautiainen, S.; Gruzieva, O.; Håkansson, N.; Kull, I.; Pershagen, G.; Wickman, M.; Wolk, A.; Melén, E.; Bergström, A. Dietary total antioxidant capacity in early school age and subsequent allergic disease. Clin. Exp. Allergy 2017, 47, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Limaye, S.; Salvi, S. Obesity and Asthma: The Role of Environmental Pollutants. Immunol. Allergy Clin. North Am. 2014, 34, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Barraza-Villarreal, A.; Escamilla-Núñez, C.; Texcalac-Sangrador, J.L.; Hernandez-Cadena, L.; Díaz-Sánchez, D.; De Batlle, J.; Del Rio-Navarro, B.E. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir. Res. 2009, 10, 122. [Google Scholar] [CrossRef] [Green Version]
- de Castro Mendes, F.; Paciência, I.; Cavaleiro Rufo, J.; Silva, D.; Cunha, P.; Farraia, M.; Delgado, L.; Garcia-Larsen, V.; Severo, M.; Moreira, A.; et al. The inflammatory potential of diet impacts the association between air pollution and childhood asthma. Pediatr. Allergy Immunol. 2020, 31, 290–296. [Google Scholar] [CrossRef]
- Brigham, E.P.; Woo, H.; McCormack, M.; Rice, J.; Koehler, K.; Vulcain, T.; Wu, T.; Koch, A.; Sharma, S.; Kolahdooz, F.; et al. Omega-3 and omega-6 intake modifies asthma severity and response to indoor air pollution in children. Am. J. Respir. Crit. Care Med. 2019, 199, 1478–1486. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.E.; Loft, S.; Ulrik, C.S.; Raaschou-Nielsen, O.; Hertel, O.; Tjønneland, A.; Overvad, K.; Nieuwenhuijsen, M.J.; Andersen, Z.J. Physical activity, air pollution, and the risk of asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2016, 194, 855–865. [Google Scholar] [CrossRef]
- Chiolero, A.; Faeh, D.; Paccaud, F.; Cornuz, J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am. J. Clin. Nutr. 2008, 87, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEvoy, C.T.; Schilling, D.; Clay, N.; Jackson, K.; Go, M.D.; Spitale, P.; Bunten, C.; Leiva, M.; Gonzales, D.; Hollister-Smith, J.; et al. Vitamin C Supplementation for Pregnant Smoking Women and Pulmonary Function in Their Newborn Infants. JAMA 2014, 311, 2074. [Google Scholar] [CrossRef] [Green Version]
- Varraso, R. Nutrition and asthma. Curr. Allergy Asthma Rep. 2012, 12, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Guillien, A.; Lepeule, J.; Seyve, E.; Le Moual, N.; Pin, I.; Degano, B.; Garcia-Aymerich, J.; Pépin, J.L.; Pison, C.; Dumas, O.; et al. Profile of exposures and lung function in adults with asthma: An exposome approach in the EGEA study. Environ. Res. 2020, 110422. [Google Scholar] [CrossRef] [PubMed]
| Reference | Population | Design | Outcome | Exposures | Results | Comments |
|---|---|---|---|---|---|---|
| Li Z et al., Thorax 2017 | 971 adults from the French prospective EGEA case-control study (baseline: 2003–2007; follow-up: 2011–2013) | Mediation analysis in the counterfactual framework to estimate the direct effect of baseline cured meat intake on change in asthma symptom over follow-up, and the indirect effect mediated by BMI at baseline | Change in asthma symptom score (calculated at each time-point using information as sum of 5 respiratory asthma symptoms in the last 12 months) categorized as ‘stable/improved’ or ‘worsening’ | Cured meat intake (<1, 1–3.9, ≥4 servings/week) estimated using information on average dietary intakes during the previous 12 months of ham, dried sausage and sausage consumption (from a 118-item semi-quantitative food-frequency questionnaire (FFQ) based on a French validated dietary questionnaire) BMI: calculated using measures of height and weight | Positive direct effect of cured meat intake on worsening asthma symptoms: multivariable odds ratio (OR) = 1.76 (95% CI: 1.01, 3.06) for ≥4 vs. <1 serving/week Positive indirect effect mediated by BMI: OR=1.07 (1.01, 1.14) for ≥4 vs. <1 serving/week, accounting for 14% of the total effect | Physical activity at baseline, expressed in metabolic equivalents (METS)/week, was considered as potential confounder and thus adjusted for in the models |
| Li Z et al., Br J Nutr 2017 | 969 adults from the French prospective EGEA case-control study (baseline: 2003–2007; follow-up: 2011–2013) | Mediation analysis in the counterfactual framework to estimate the direct effect of baseline AHEI score on change in asthma symptom over follow-up, and the indirect effect mediated by BMI at baseline | Change in asthma symptom score (calculated at each time-point using information as sum of 5 respiratory asthma symptoms in the last 12 months) categorized as ‘stable/improved’ or ‘worsening’ | The AHEI-2010 dietary score (range 0-10 based on high intake of vegetables, fruits, whole grains, nuts and legumes, long-chain n-3 fatty acids and PUFA; moderate intake of alcohol; and low intake of sugar-sweetened drinks and fruit juice, red/ processed meat, trans-fat and Na), estimated using information from a 118-item semi-quantitative FFQ based on a French validated dietary questionnaire BMI: calculated using measures of height and weight | - Among never smokers: positive total effect (multivariable OR = 1.39 [1.07, 1.80] and positive direct effect (OR = 1.41 [1.09, 1.80] of the AHEI-2010 (per ten-point increment) on improved symptoms; no indirect effect mediated through BMI (OR= 0.99 [0.91, 1.07]). - Among former and current smokers: no statistically significant effect | Physical activity at baseline, expressed in metabolic equivalents (METS)/week, was considered as potential confounder and thus adjusted for in the models |
| Bédard A et al., Am J Epidemiol 2017 | 15,353 adult women from the Asthma-E3N case-control study (nested within the French E3N cohort) with data collected at least 4 times between 1997 and 2011 | Marginal structural models (MSMs) considering three time periods: t − 1/t/t + 1 (1997/2000/2002, 2000/2002/2005, and 2002/2005/2011) with BMI and physical activity at time t, current asthma at time t + 1, and covariates at time t − 1 or baseline | Current asthma: asthma attacks and/or asthma treatment (inhaled bronchodilators or inhaled corticosteroids) in the last 12 months (self-report) | BMI: calculated using self-reported height and weight Physical activity: expressed in metabolic equivalent of task (MET)-hours per week using self-reported amount of time spent doing different activities. All MET-hours/week values were added and categorized in tertiles (low/moderate /high level of physical activity) | - Strong significant and positive dose–response relationship between BMI and current asthma: OR = 0.90 (0.79, 1.03), 1.29 (1.17, 1.42) and 1.87 (1.60, 2.18) for the BMI groups < 20.0, 25.0–29.9, and ≥30.0 respectively, versus the normal-weight group (BMI 20.0–24.9). - No association between physical activity and current asthma | Information on diet was only available once, and thus total daily energy intake (assessed using a validated FFQ) was considered as a time-fixed covariate in the MSMs |
| Garcia-Aymerich J et al., Am J Epidemiol 2014 | 76,470 asthma-free women from the Nurses’ Health Study who were followed between 1988 and 1998 | g-formula analysis to assess the 10-year risk of adult–onset asthma after hypothetical interventions on BMI (i.e., reducing BMI by 5% every 2 years) or/and physical activity (i.e., engaging in at least 2.5 h per week of moderate-to-vigorous physical activity) | Adult-onset asthma: self-reported physician diagnosis of asthma plus the use of an asthma medication in the past 12 months | BMI: calculated using self-reported height and weight Physical activity: total time spent per week at moderate-to-vigorous physical activities (walking at ≥3 miles/hour, hiking outdoors, jogging, running, cycling, swimming, tennis and calisthenics/aerobics/aerobic dance/rowing machine) | Compared with no intervention, the population risk ratios were 0.96 (0.93, 0.99) under the BMI intervention, 0.96 (0.81, 1.10) under the physical activity intervention, and 0.92 (0.78, 1.06) under the joint intervention | Because of a large proportion of missing data, diet (assessed the dietary “prudent pattern” and “Western pattern”) was considered as a time-fixed covariate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bédard, A.; Li, Z.; Ait-hadad, W.; Camargo, C.A., Jr.; Leynaert, B.; Pison, C.; Dumas, O.; Varraso, R. The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research. Int. J. Environ. Res. Public Health 2021, 18, 3013. https://doi.org/10.3390/ijerph18063013
Bédard A, Li Z, Ait-hadad W, Camargo CA Jr., Leynaert B, Pison C, Dumas O, Varraso R. The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research. International Journal of Environmental Research and Public Health. 2021; 18(6):3013. https://doi.org/10.3390/ijerph18063013
Chicago/Turabian StyleBédard, Annabelle, Zhen Li, Wassila Ait-hadad, Carlos A. Camargo, Jr., Bénédicte Leynaert, Christophe Pison, Orianne Dumas, and Raphaëlle Varraso. 2021. "The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research" International Journal of Environmental Research and Public Health 18, no. 6: 3013. https://doi.org/10.3390/ijerph18063013
APA StyleBédard, A., Li, Z., Ait-hadad, W., Camargo, C. A., Jr., Leynaert, B., Pison, C., Dumas, O., & Varraso, R. (2021). The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research. International Journal of Environmental Research and Public Health, 18(6), 3013. https://doi.org/10.3390/ijerph18063013






