Telerehabilitation Intervention in Patients with COVID-19 after Hospital Discharge to Improve Functional Capacity and Quality of Life. Study Protocol for a Multicenter Randomized Clinical Trial
Abstract
1. Introduction
1.1. Physiotherapy in Patients with COVID-19
1.2. Telerehabilitation
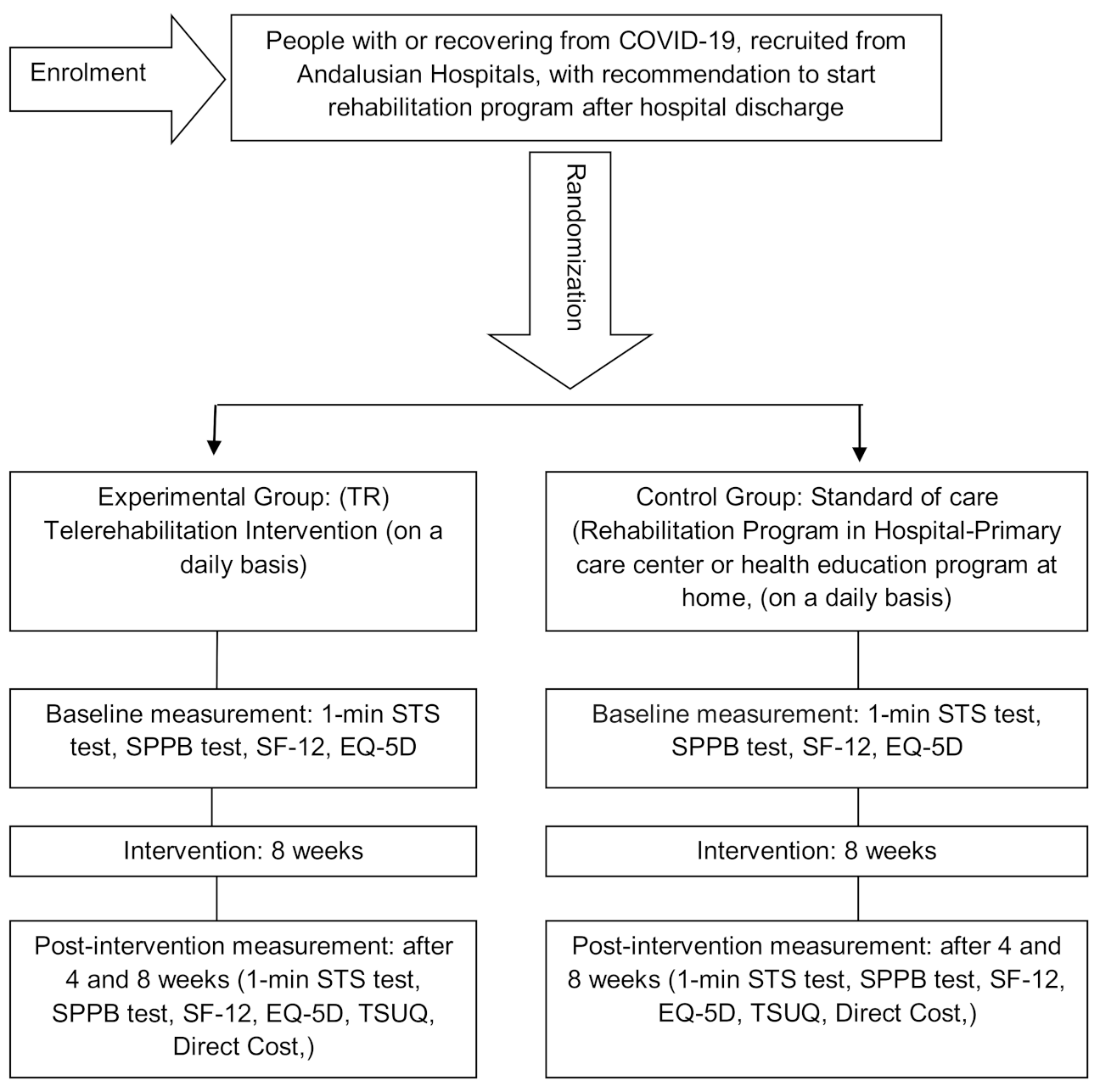
2. Material and Methods
2.1. Patients
2.2. Randomization and Single Blinding
2.3. Intervention
2.4. Outcome Measures
2.4.1. Affiliation Data and Socio-Demographic Questionnaire
2.4.2. Main Explanatory Variable
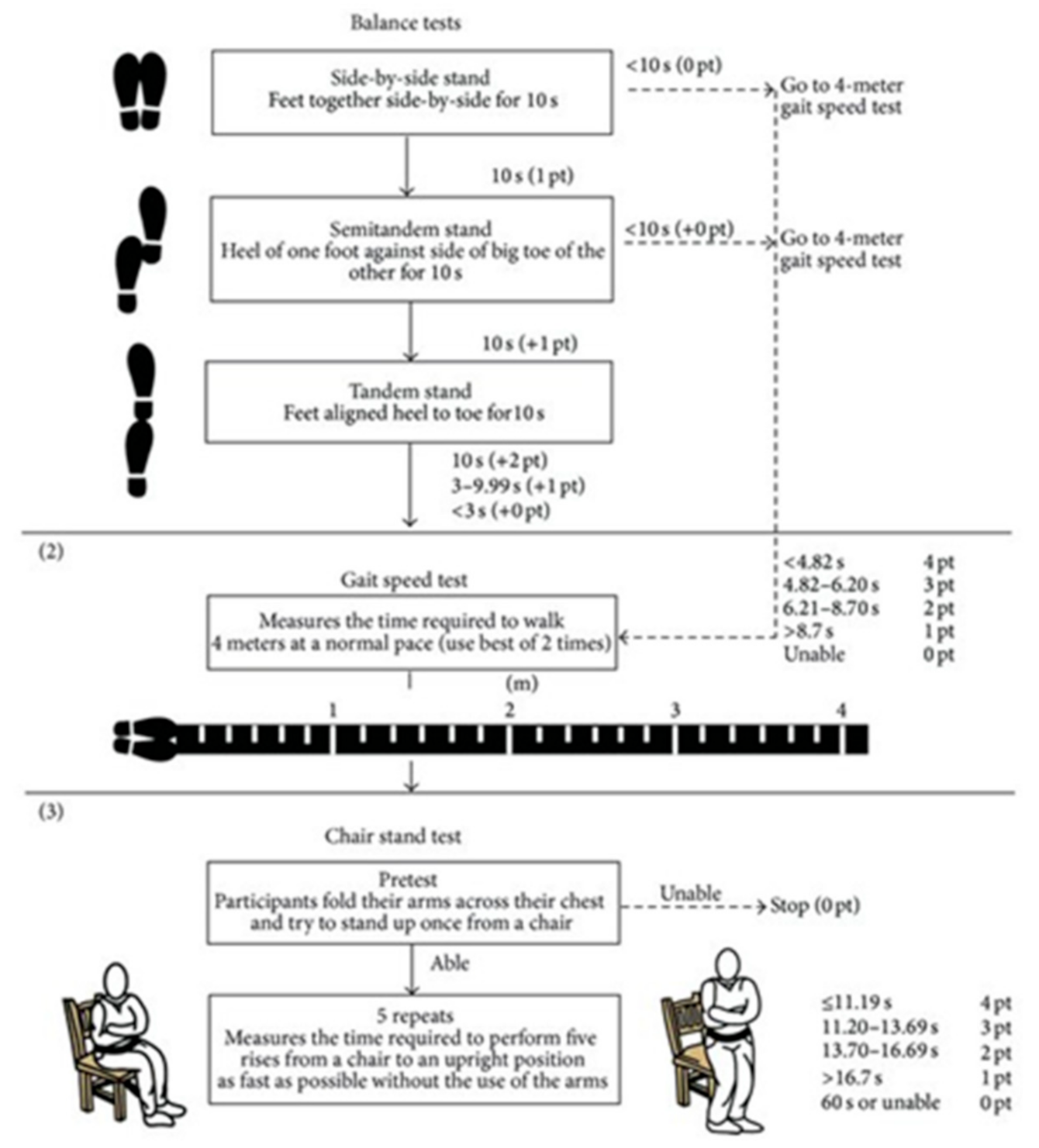
Functional Capacity: For the Evaluation of the Functional Capacity and Fragility of the Patients the Following Tests Will Be Performed
Quality of Life
2.4.3. Secondary Explanatory Variable
Satisfaction and Usability
Analysis of the Cost-Effectiveness
2.5. Data Collection Procedure and Statistical Analysis
Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Del Rio, C.; Malani, P.N. 2019 Novel Coronavirus—Important Information for Clinicians. JAMA J. Am. Med. Assoc. 2020, 323, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Clinical Management of Severe Acute Respiratory Infection When COVID-19 Is Suspected. Available online: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed on 20 May 2020).
- World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online: https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (accessed on 21 May 2020).
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Azaibi, T.; James, O.L.-S.; de Emmie, W. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yu, X.; Hu, D.; Bao, M.; Liu, H.; Zhou, J.; Jiang, H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020, 80, 639–645. [Google Scholar] [CrossRef]
- Mundell, E.J. In Some Cases, COVID-19 May Harm the Brain. Available online: https://www.webmd.com/lung/news/20200402/in-some-cases-covid-19-may-harm-the-brain#1 (accessed on 27 May 2020).
- Bangash, M.N.; Patel, J.; Parekh, D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol. Hepatol. 2020, 5, 529–530. [Google Scholar] [CrossRef]
- La Fisioterapia, Clave en la Recuperación de la Función Pulmonar Afectada por el Coronavirus. Available online: https://www.colfisio.org/comunicacion_y_prensa/noticias/1092_La_Fisioterapia_clave_en_la_recuperaci_n_de_la_funci_n_pulmonar_afectada_por_el_Coronavirus.html (accessed on 21 May 2020).
- Milagros Pérez Oliva (Redacción El País). Los Hospitales Crean Unidades Para Atender Secuelas de la Enfermedad/Sociedad/EL PAÍS. Available online: https://elpais.com/sociedad/2020-05-26/los-hospitales-crean-unidades-para-atender-secuelas-de-la-enfermedad.html (accessed on 21 May 2020).
- Lista-Paz, A.; González-Doniz, L.; Souto-Camba, S. Qué papel desempeña la Fisioterapia en la pandemia mundial por COVID-19. Fisioterapia 2020, 42, 167–169. [Google Scholar] [CrossRef]
- Concepción Martín Cortijo, Francisco Javier Zarza Bejarano EGD, Marta Sánchez Cortés VTRFHU 12 de, (Madrid) O. Guía. De Fisioterapia en el Paciente con Sospecha De COVID-19 O COVID-19 Confirmado. Available online: https://imas12.es/wp-content/uploads/2020/Repositorio/01.Guia_Fisioterapia_paciente_sospecha_COVID19_o_COVID_19_confirmado.pdf (accessed on 21 May 2020).
- Thomas, P.; Baldwin, C.; Bissett, B.; Boden, I.; Gosselink, R.; Granger, C.L.; Hodgson, C.; Jones, A.Y.M.; Kho, M.E.; Moses, R.; et al. Physiotherapy management for COVID-19 in the acute hospital setting: Recommendations to guide clinical practice description and objectives: This document outlines recommendations for physiotherapy management for COVID-19 in the acute hospital setting. It includes recommendations for physiotherapy workforce planning and preparation, a screening tool for determining requirement of physiotherapy, recommendations for the selection of physiotherapy treatments and personal protective equipment. J. Physiother. 2020, 31. [Google Scholar] [CrossRef]
- ACPRC. COVID-19 Information—ACPRC. Available online: https://www.acprc.org.uk/resources/covid-19-information/physiotherapy-guidance-for-clinicians-and-managers/ (accessed on 22 May 2020).
- Chinese Association of Rehabilitation Medicine; Respiratory Rehabilitation Committee of Chinese Association of Rehabilitation Medicine; Cardiopulmonary Rehabilitation Group of Chinese Society of Physical Medicine and Rehabilitation. Recommendations for respiratory rehabilitation of coronavirus disease 2019 in adult. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, E029. [Google Scholar]
- Almekhlafi, G.A.; Albarrak, M.M.; Mandourah, Y.; Hassan, S.; Alwan, A.; Abudayah, A.; Altayyar, S.; Mustafa, M.; Aldaghestani, T.; AlGhamedi, A.; et al. Presentation and outcome of Middle East respiratory syndrome in Saudi intensive care unit patients. Crit. Care 2016, 20. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.M.C.; Ng, G.Y.F.; Jones, A.Y.M.; Lee, E.W.C.; Siu, E.H.K.; Hui, D.S.C. A randomised controlled trial of the effectiveness of an exercise training program in patients recovering from severe acute respiratory syndrome. Aust. J. Physiother. 2005, 51, 213–219. [Google Scholar] [CrossRef]
- Russell, T.G. Physical rehabilitation using telemedicine. J. Telemed. Telecare 2007, 13, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J. Telerehabilitation. Telemed. J. Health 2004, 10, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Seelman, K.D.; Hartman, L.M. Telerehabilitation: Policy Issues and Research Tools. Int. J. Telerehabil. 2009, 1, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pastora-Bernal, J.M.; Martín-Valero, R.; Barón-López, F.J.; Estebanez-Pérez, M.J. Evidence of benefit of telerehabitation after orthopedic surgery: A systematic review. J. Med. Internet Res. 2017, 19, e142. [Google Scholar] [CrossRef]
- Goldstein, R.S.; O’Hoski, S. Telemedicine in COPD. Chest 2014, 145, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Hergenroeder, A.L.; Burke, L.; Dabbs, A.D.; Morrell, M.; Saptono, A.; Parmanto, B. Delivering an In-Home Exercise Program via Telerehabilitation: A Pilot Study of Lung Transplant Go (LTGO). Int. J. Telerehabil. 2016, 8, 15–26. [Google Scholar] [CrossRef]
- Piqueras, M.; Marco, E.; Coll, M.; Escalada, F.; Ballester, A.; Cinca, C.; Belmonte, R.; Muniesa, J.M. Effectiveness of an interactive virtual telerehabilitation system in patients after total knee arthoplasty: A randomized controlled trial. J. Rehabil. Med. 2013, 45, 392–396. [Google Scholar] [CrossRef]
- Rogante, M.; Grigioni, M.; Cordella, D.; Giacomozzi, C. Ten years of telerehabilitation: A literature overview of technologies and clinical applications. NeuroRehabil. 2010, 27, 287–304. [Google Scholar] [CrossRef]
- Agostini, M.; Moja, L.; Banzi, R.; Pistotti, V.; Tonin, P.; Venneri, A.; Turolla, A. Telerehabilitation and recovery of motor function: A systematic review and meta-analysis. J. Telemed. Telecare 2015, 21, 202–213. [Google Scholar] [CrossRef]
- Pastora-Bernal, J.-M.; Martín-Valero, R.; Barón-López, F.J.; García-Gómez, O. Effectiveness of telerehabilitation programme following surgery in shoulder impingement syndrome (SIS): Study protocol for a randomized controlled non-inferiority trial. Trials 2017, 18, 82. [Google Scholar] [CrossRef]
- Head, B.A.; Keeney, C.; Studts, J.L.; Khayat, M.; Bumpous, J.; Pfeifer, M. Feasibility and Acceptance of a Telehealth Intervention to Promote Symptom Management during Treatment for Head and Neck Cancer. J. Support Oncol. 2011, 9, e1. [Google Scholar] [CrossRef][Green Version]
- Spruit, M.A.; Holland, A.E.; Singh, S.J.; Tonia, T.; Wilson, K.C.; Troosters, T. COVID-19: Interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society- And American Thoracic Society-coordinated international task force. Eur. Respir. J. 2020, 56, 2002197. [Google Scholar] [CrossRef]
- Vitacca, M.; Carone, M.; Clini, E.M.; Paneroni, M.; Lazzeri, M.; Lanza, A.; Privitera, E.; Pasqua, F.; Gigliotti, F.; Castellana, G.; et al. Joint statement on the role of respiratory rehabilitation in the COVID-19 crisis: The Italian position paper. Respiration 2020, 99, 493–499. [Google Scholar] [CrossRef]
- Zhao, H.M.; Xie, Y.X.; Wang, C. Recommendations for respiratory rehabilitation in adults with COVID-19. Chin. Med. J. 2020, 133, 1595–1602. [Google Scholar] [CrossRef]
- Davidoff, F.; Batalden, P.; Stevens, D.; Ogrinc, G.; Mooney, S.; SQUIRE Development Group. Publication guidelines for quality improvement in health care: Evolution of the SQUIRE project. Qual. Saf. Health Care 2008, 17, 3–9. [Google Scholar] [CrossRef]
- Piaggio, G.; Elbourne, D.R.; Altman, D.G.; Pocock, S.J.; Evans, S.J.W.; CONSORT Group. Reporting of Noninferiority and Equivalence Randomized Trials. JAMA 2006, 295, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, Á.; Álvarez, G.; Russo, F.; San-José, B.; Sánchez-Tomero, J.A.; Barril, G. Es útil el SPPB como método de screening de capacidad funcional en pacientes con enfermedad renal crónica avanzada. Nefrología 2019, 39, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Arbillaga, A.; Pardàs, M.; Escudero, R.; Rodríguez, R.; Alcaraz, V.; Llanes, S.; Herrero, B.; Gimeno, H.; Rios, A. Fisioterapia Respiratoria en el Manejo del Paciente con Covid-19: Recomendaciones Generales área de Fisioterapia Respiratoria Sociedad Española de Neumología y Cirugía Torácica-Separ-Versión 1.0-26 de Marzo 2020. Available online: https://www.cofpv.org/doc/cajita/FISIOTERAPIA_RESPIRATORIA_PACIENTE_COVID-19.pdf (accessed on 20 May 2020).
- Crook, S.; Büsching, G.; Schultz, K.; Lehbert, N.; Jelusic, D.; Keusch, S.; Wittmann, M.; Schuler, M.; Radtke, T.; Frey, M.; et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur. Respir. J. 2017, 49, 1601871. [Google Scholar] [CrossRef] [PubMed]
- Andalucía J de. Informe COVID-19 Coronavirus en Andalucía. Consejería de Salud y Familias. Junta de Andalucía. Available online: http://www.juntadeandalucia.es/institutodeestadisticaycartografia/salud/COVID19.html (accessed on 27 May 2020).
- Jenkinson, C.; Layte, R.; Jenkinson, D.; Lawrence, K.; Petersen, S.; Paice, C.; Stradling, J. A shorter form health survey: Can the sf-12 replicate results from the sf-36 in longitudinal studies? J. Public Health Med. 1997, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bakken, S.; Grullon-Figueroa, L.; Izquierdo, R.; Lee, N.-J.; Morin, P.; Palmas, W.; Teresi, J.; Weinstock, R.S.; Shea, S.; Starren, J.; et al. Development, Validation, and Use of English and Spanish Versions of the Telemedicine Satisfaction and Usefulness Questionnaire. J. Am. Med. Inform. Assoc. 2006, 13, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Demiris, G.; Speedie, S.F.S. An instrument for the assessment of patients’ impressions of the risks and benefits of home telecare. J. Telemed. Telecare 2000, 6, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Glick, H.A.; Doshi, J.A.; Sonnad, S.S.P.D. Economic Evaluation in Clinical Trials, 2nd ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Tousignant, M.; Moffet, H.; Nadeau, S.; Mérette, C.; Boissy, P.; Corriveau, H.; Marquis, F.; Cabana, F.; Ranger, P.; Belzile, É.L.; et al. Cost Analysis of In-Home Telerehabilitation for Post-Knee Arthroplasty. J. Med. Internet Res. 2015, 17, e83. [Google Scholar] [CrossRef] [PubMed]
- Bini, S.A.; Mahajan, J. Clinical outcomes of remote asynchronous telerehabilitation are equivalent to traditional therapy following total knee arthroplasty: A randomized control study. J. Telemed. Telecare 2016, 23, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rosen, K.; Patel, M.; Lawrence, C.; Mooney, B. Delivering Telerehabilitation to COVID-19 Inpatients:A Retrospective Chart Review Suggests It Is a Viable Option. HSS J. 2020, 16, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Taito, S.; Yamauchi, K.; Kataoka, Y. Telerehabilitation in Subjects with Respiratory Disease: A Scoping Review. Respir. Care 2021. [Google Scholar] [CrossRef]
- Moffet, H.; Tousignant, M.; Nadeau, S.; Mérette, C.; Boissy, P.; Corriveau, H.; Marquis, F.; Cabana, F.; Ranger, P.; Belzile, E.L.; et al. In-Home Telerehabilitation Compared with Face-to-Face Rehabilitation after Total Knee Arthroplasty: A Noninferiority Randomized Controlled Trial. J. Bone Jt. Surg. Am. 2015, 97, 1129–1141. [Google Scholar] [CrossRef]
| Enrolment | Allocation | Post Allocation | Close out | ||
|---|---|---|---|---|---|
| Time Point | −T 1 | T 0 [Baseline] | T.1 [4 Weeks] | T.2 [8 Weeks] | |
| ENROLMENT: | |||||
| Eligibility screen | X | ||||
| Informed consent | X | ||||
| Advance info | X | ||||
| Allocation | X | ||||
| INTERVENTION: | |||||
| TR Group |  | ||||
| Control Group |  | ||||
| ASSESMENT: | |||||
| Baseline Variables | X | ||||
| Initial Assesment | X | ||||
| STS Test | X | X | X | X | |
| SPPB test. | X | X | X | X | |
| SF-12 and EQ-5D | X | X | X | X | |
| TSUQ | X | X | X | ||
| Direct Costs | X | X | X | ||
| Data Collection |  | ||||
| Statistical Análisis | X | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastora-Bernal, J.-M.; Estebanez-Pérez, M.-J.; Molina-Torres, G.; García-López, F.-J.; Sobrino-Sánchez, R.; Martín-Valero, R. Telerehabilitation Intervention in Patients with COVID-19 after Hospital Discharge to Improve Functional Capacity and Quality of Life. Study Protocol for a Multicenter Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 2924. https://doi.org/10.3390/ijerph18062924
Pastora-Bernal J-M, Estebanez-Pérez M-J, Molina-Torres G, García-López F-J, Sobrino-Sánchez R, Martín-Valero R. Telerehabilitation Intervention in Patients with COVID-19 after Hospital Discharge to Improve Functional Capacity and Quality of Life. Study Protocol for a Multicenter Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2021; 18(6):2924. https://doi.org/10.3390/ijerph18062924
Chicago/Turabian StylePastora-Bernal, José-Manuel, María-José Estebanez-Pérez, Guadalupe Molina-Torres, Francisco-José García-López, Raquel Sobrino-Sánchez, and Rocío Martín-Valero. 2021. "Telerehabilitation Intervention in Patients with COVID-19 after Hospital Discharge to Improve Functional Capacity and Quality of Life. Study Protocol for a Multicenter Randomized Clinical Trial" International Journal of Environmental Research and Public Health 18, no. 6: 2924. https://doi.org/10.3390/ijerph18062924
APA StylePastora-Bernal, J.-M., Estebanez-Pérez, M.-J., Molina-Torres, G., García-López, F.-J., Sobrino-Sánchez, R., & Martín-Valero, R. (2021). Telerehabilitation Intervention in Patients with COVID-19 after Hospital Discharge to Improve Functional Capacity and Quality of Life. Study Protocol for a Multicenter Randomized Clinical Trial. International Journal of Environmental Research and Public Health, 18(6), 2924. https://doi.org/10.3390/ijerph18062924










