Characterization of the Skin Cultivable Microbiota Composition of the Frog Pelophylax perezi Inhabiting Different Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Collection of Skin Cultivable Microbiota
2.3. Bacterial Isolates and Phylogenetic Analysis
2.4. Tolerance of Bacterial Isolates to AMD
2.5. Exopolysaccharide (EPS) Production by Core Cultivable Microbiota Bacteria
2.6. Nucleotide Sequence Accession Numbers
2.7. Ethic Statement
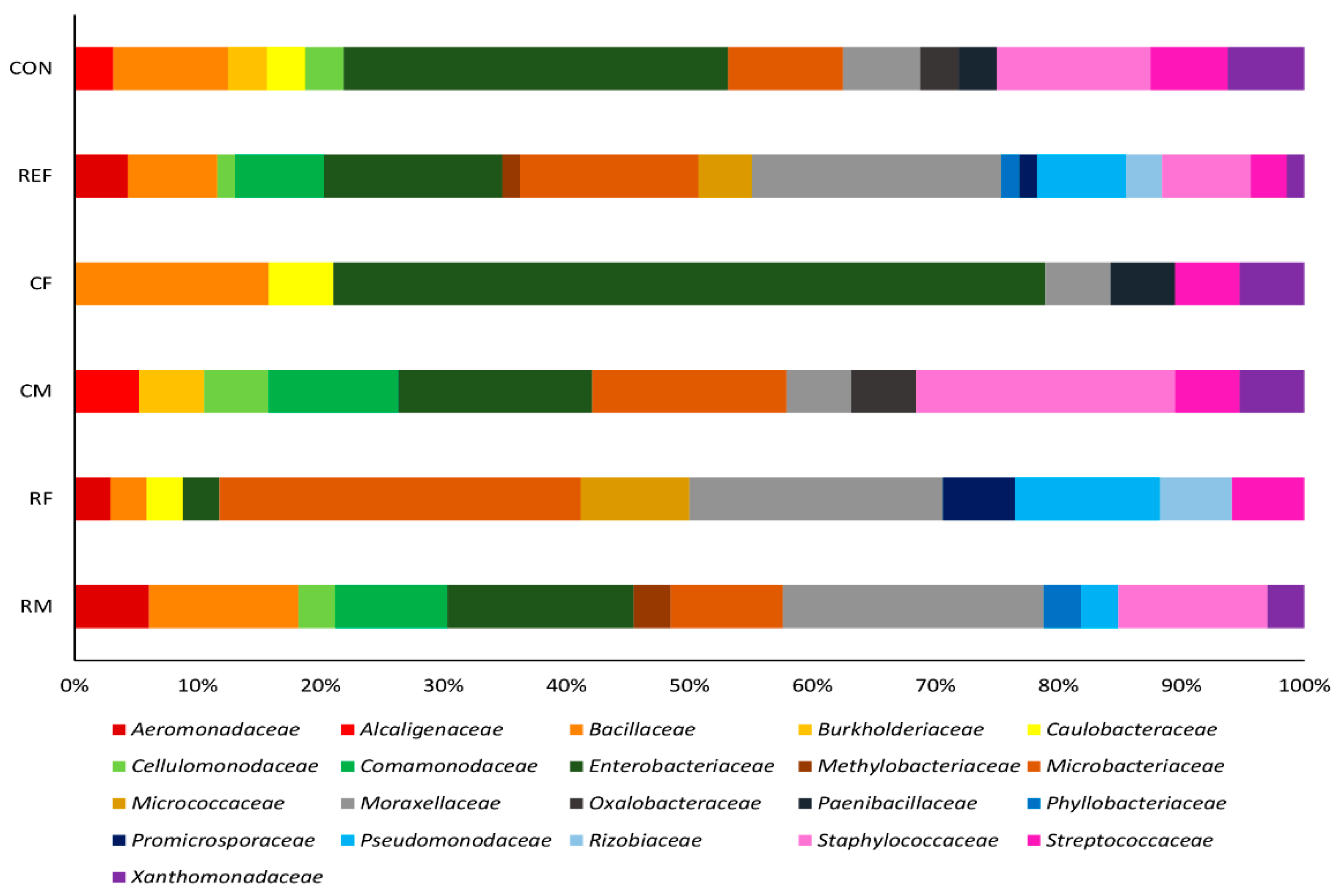
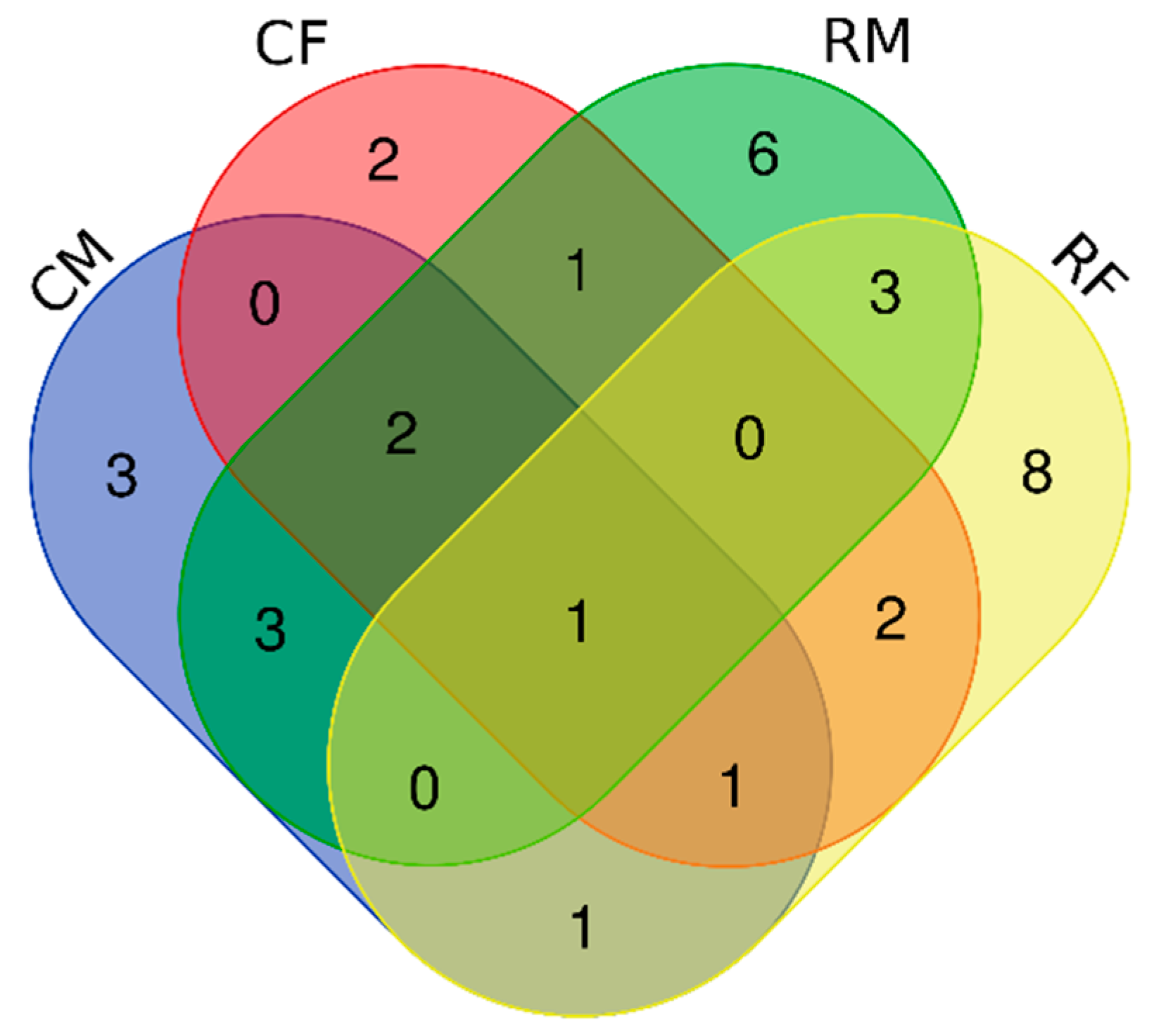
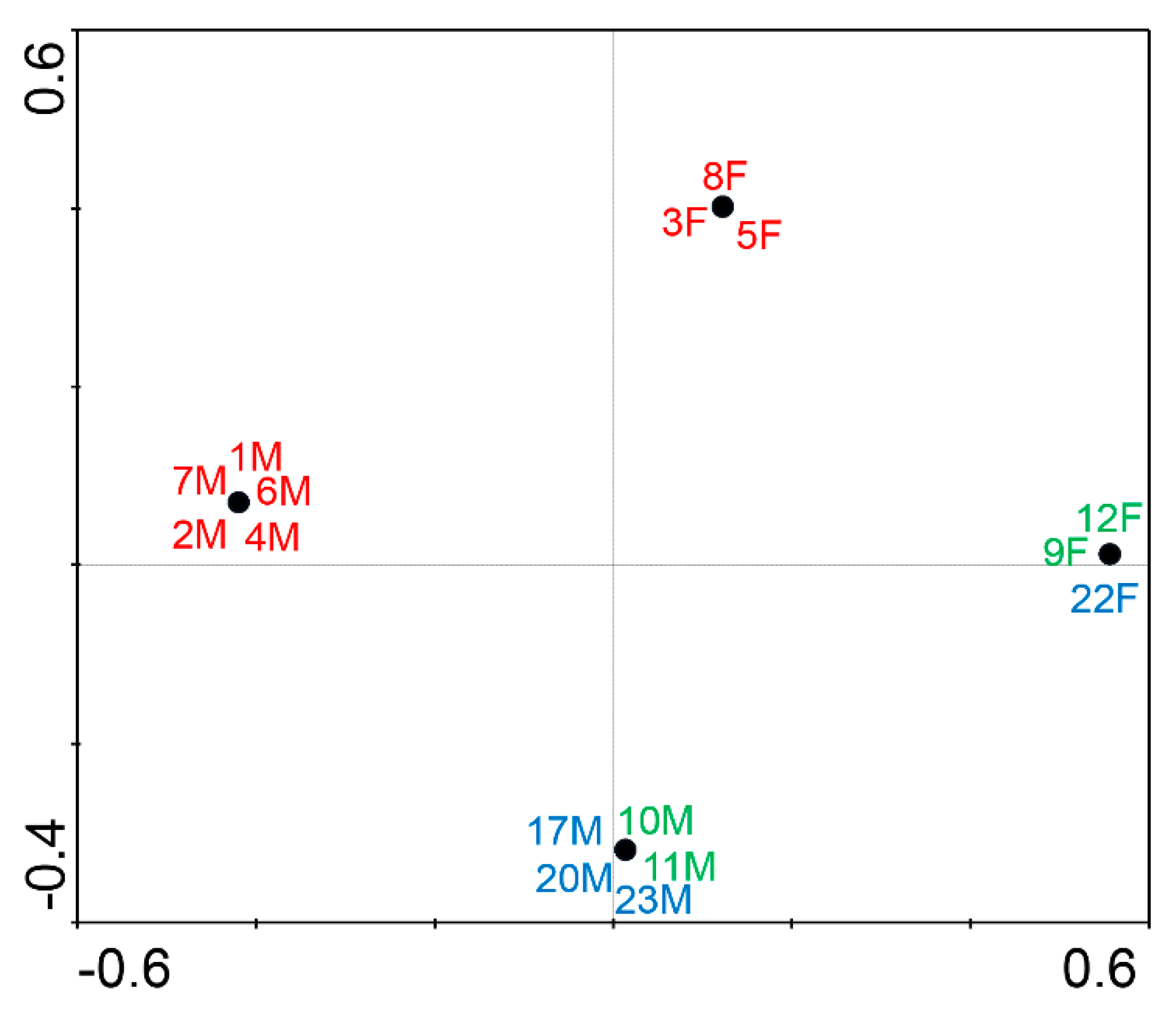
3. Results
3.1. Bacterial Isolates of the Skin Cultivable Microbiota of P. perezi
3.2. Tolerance of Bacterial Isolates to AMD
3.3. Exopolysaccharide (EPS) Production by Acinetobacter Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belden, L.K.; Hughey, M.C.; Rebollar, E.A.; Umile, T.P.; Loftus, S.C.; Burzynski, E.A.; Minbiole, K.P.C.; House, L.L.; Jensen, R.V.; Becker, M.H.; et al. Panamanian frog species host unique skin bacterial communities. Front. Microbiol. 2015, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kueneman, J.C.; Parfrey, L.W.; Woodhams, D.C.; Archer, H.M.; McKenzie, R.; Knight, V.J. The amphibian skin-associated microbiome across species, space and life history stages. Mol. Ecol. 2014, 23, 1238–1250. [Google Scholar] [CrossRef]
- Rebollar, E.A.; Hughey, M.C.; Medina, D.; Harris, R.N.; Ibáñez, R.; Belden, L.K. Skin bacterial diversity of Panamanian frogs is associated with host susceptibility and presence of Batrachochytrium dendrobatidis. ISME J. 2016, 1–14. [Google Scholar] [CrossRef]
- Assis, A.B.; Bevier, C.R.; Chaves Barreto, C.; Arturo Navas, C. Environmental influences on and antimicrobial activity of the skin microbiota of Proceratophrys boiei (Amphibia, Anura) across forest fragments. Ecol. Evol. 2020, 10, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Miele, R.; Renda, T.G.; Barra, D.; Simmaco, M. The synthesis of antimicrobial peptides in the skin of Rana esculenta is stimulated by microorganisms. FASEB J. 2001, 15, 1431–1432. [Google Scholar] [CrossRef] [PubMed]
- Alexander Pyron, R.; Wiens, J.J. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 2011, 61, 543–583. [Google Scholar] [CrossRef] [PubMed]
- IUCN. Available online: http://www.iucn.org/ (accessed on 2 February 2021).
- Sparling, D.W.; Linder, G.; Bishop, C.A.; Krest, S.K. Ecotoxicology of Amphibians and Reptiles, 2nd ed.; Sparling, D.W., Linder, G., Bishop, C.A., Krest, S.K., Eds.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-6416-2. [Google Scholar]
- Blaustein, A.R.; Bancroft, B.A. Amphibian population declines: Evolutionary considerations. Bioscience 2007, 57, 437–444. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.M.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The impact of conservation on the status of the world’s vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef]
- Fedorenkova, A.; Vonk, J.A.; Lenders, H.J.R.; Creemers, R.C.M.; Breure, A.M.; Hendriks, A.J. Ranking ecological risks of multiple chemical stressors on amphibians. Environ. Toxicol. Chem. 2012, 31, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D.; et al. Threats from climate change to terrestrial vertebrate hotspots in Europe. PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Marco, A.; Saiz, N.; Lizana, M. Impact of ammonium nitrate on growth and survival of six European amphibians. Arch. Environ. Contam. Toxicol. 2004, 47. [Google Scholar] [CrossRef]
- Pask, J. The ebb and flow of antimicrobial skin peptides defends northern leopard frogs (Rana pipiens) against chytridiomycosis. Glob. Chang. 2012, 1231–1238. [Google Scholar] [CrossRef]
- Harris, R.; Brucker, R.; Walke, J. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME 2009, 3, 818–824. [Google Scholar] [CrossRef]
- Becker, M.H.; Brucker, R.M.; Christian, R.; Harris, R.N.; Minbiole, K.P.C.; Schwantes, C.R. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl. Environ. Microbiol. 2009, 75, 6635–6638. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Brandt, H.; Baumgartner, S.; Kielgast, J.; Küpfer, E.; Tobler, U.; Davis, L.R.; Schmidt, B.R.; Bel, C.; Hodel, S.; et al. Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS ONE 2014, 9, e96375. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, V.; Peterson, A. Pathogen pollution and the emergence of a deadly amphibian pathogen. Mol. Ecol. 2012, 5151–5154. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Bletz, M.; Kueneman, J.; McKenzie, V. Managing amphibian disease with skin microbiota. Trends Microbiol. 2016, 24, 161–164. [Google Scholar] [CrossRef]
- Laugen, A.T.; Laurila, A.; Merilä, J. Maternal and genetic contributions to geographical variation in Rana temporaria larval life-history traits. Biol. J. Linn. Soc. 2002, 76, 61–70. [Google Scholar] [CrossRef][Green Version]
- Bresciano, J.; Salvador, C.; Paz-Y-Miño, C.; Parody-Merino, A.; Bosch, J.; Woodhams, D. Variation in the presence of anti-Batrachochytrium dendrobatidis bacteria of amphibians across life stages and elevations in Ecuador. Ecohealth 2015, 12, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, A.R.; Hokit, D.G.; O’Hara, R.K. Pathogenic fungus contributes to amphibian losses in the Pacific Northwest. Biol. Conserv. 1994, 67, 251–254. [Google Scholar] [CrossRef]
- Fernández-Benéitez, M.J.; Ortiz-Santaliestra, M.E.; Lizana, M.; Diéguez-Uribeondo, J. Differences in susceptibility to Saprolegnia infections among embryonic stages of two anuran species. Oecologia 2011, 165, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Baláž, V.; Vörös, J.; Civiš, P.; Vojar, J.; Hettyey, A.; Sós, E.; Dankovics, R.; Jehle, R.; Christiansen, D.G.; Clare, F.; et al. Assessing risk and guidance on monitoring of Batrachochytrium dendrobatidis in Europe through identification of taxonomic selectivity of infection. Conserv. Biol. 2013, 28, 213–223. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Pearman, P.B.; Garner, T.W.J. Susceptibility of Italian agile frog populations to an emerging Ranavirus parallels population genetic diversity. Ecol. Lett. 2005, 8, 401–408. [Google Scholar] [CrossRef]
- Kruger, A. Functional redundancy of Batrachochytrium dendrobatidis inhibition in bacterial communities isolated from Lithobates clamitans skin. Microb. Ecol. 2020, 79, 231–240. [Google Scholar] [CrossRef]
- Muletz, C.; Myers, J.; Domangue, R.; Herrick, J.; Harris, R. Soil bioaugmentation with amphibian cutaneous bacteria protects amphibian hosts from infection by Batrachochytrium dendrobatidis. Biol. Conserv. 2012, 152, 119–126. [Google Scholar] [CrossRef]
- Rebollar, E.A.; Simonetti, S.J.; Shoemaker, W.R.; Harris, R.N. Direct and indirect horizontal transmission of the antifungal probiotic bacterium Janthinobacterium lividum on green frog (Lithobates clamitans) tadpoles. Appl. Environ. Microbiol. 2016, 82, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.H.; Harris, R.N.; Minbiole, K.P.C.; Schwantes, C.R.; Rollins-Smith, L.A.; Reinert, L.K.; Brucker, R.M.; Domangue, R.J.; Gratwicke, B. Towards a better understanding of the use of probiotics for preventing chytridiomycosis in Panamanian golden frogs. Ecohealth 2011, 8, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Bates, K.A.; Clare, F.C.; O’Hanlon, S.; Bosch, J.; Brookes, L.; Hopkins, K.; McLaughlin, E.J.; Daniel, O.; Garner, T.W.J.; Fisher, M.C.; et al. Amphibian chytridiomycosis outbreak dynamics are linked with host skin bacterial community structure. Nat. Commun. 2018, 9, 693. [Google Scholar] [CrossRef]
- McKenzie, V.J.; Bowers, R.M.; Fierer, N.; Knight, R.; Lauber, C.L. Co-habiting amphibian species harbor unique skin bacterial communities in wild populations. ISME 2012, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.V.; Zamudio, K.R. Environmental fluctuations and host skin bacteria shift survival advantage between frogs and their fungal pathogen. ISME 2016, 1–13. [Google Scholar] [CrossRef]
- Fitzpatrick, B.; Amanda, A. Similarity and differentiation between bacteria associated with skin of salamanders (Plethodon jordani) and free-living assemblages. FEMS 2014. [Google Scholar] [CrossRef] [PubMed]
- Muletz Wolz, C.R.; Yarwood, S.A.; Campbell Grant, E.H.; Fleischer, R.C.; Lips, K.R. Effects of host species and environment on the skin microbiome of Plethodontid salamanders. J. Anim. Ecol. 2018, 87, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Lopes, I.; Proença, D.N.; Ribeiro, R.; Vasconcelo Morais, P. Diversity of cutaneous bacterial community of Pelophylax perezi populations inhabiting different environments. Sci. Total Environ. 2016, 572, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.; Sommers, S. The amphibian microbiome: Natural range of variation, pathogenic dysbiosis, and role in conservation. Biodivers. Conserv. 2017, 26, 763–786. [Google Scholar] [CrossRef]
- Ellison, S.; Rovito, S. The influence of habitat and phylogeny on the skin microbiome of the influence of habitat and phylogeny on the skin microbiome of amphibians in Guatemala and Mexico. Microb. Ecol. 2018. [Google Scholar] [CrossRef]
- Hernández-Gómez, O.; Wuerthner, V.; Hua, J. Amphibian host and skin microbiota response to a common agricultural antimicrobial and internal parasite. Microb. Ecol. 2020, 79, 175–191. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Peng, A. Accumulation of Cu (II) and Pb (II) by biofilms grown on particulate in aquatic systems. J. Environ. Sci. Health Part A 2000, 35, 575–592. [Google Scholar] [CrossRef]
- Kazy, S.K.; Sar, P.; Singh, S.P.; Sen, A.K.; D’Souza, S.F. Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain: Synthesis, chemical nature and copper binding. World J. Microbiol. Biotechnol. 2002, 18, 583–588. [Google Scholar] [CrossRef]
- Cordeiro, I.F.; Fonseca, N.P.; Felestrino, É.B.; Caneschi, W.L.; Silvério Pires, M.R.; Moreira, L.M. Arsenic resistance in cultured cutaneous microbiota is associated with anuran lifestyles in the Iron Quadrangle, Minas Gerais State, Brazil. Herpetol. Notes 2019, 12, 1083–1093. [Google Scholar]
- Maia, F.; Pinto, C.; Waeremborg, J.; Gonçalves, M.; Prazeres, C.; Carreira, O.; Serio, S. Metal partitioning in sediments and mineralogical controls on the acid mine drainage in Ribeira da Água Forte (Aljustrel, Iberian Pyrite Belt, Southern Portugal). Appl. Geochem. 2012, 27, 1063–1080. [Google Scholar] [CrossRef]
- Luis, A.; Duraes, N.; Pinheiro De Almeida, S.; Ferreira Da Silva, E. Integrating geochemical (surface waters, stream sediments) and biological (diatoms) approaches to assess AMD environmental impact in a pyritic mining area: Aljustrel (Alentejo, Portugal). J. Environ. Sci. 2016, 42, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.; Teixaira, P.; Almeida, S.; Ector, S.; Matos, J.; Ferreira Da Silva, E. Impact of acid mine drainage (AMD) on water quality, stream sediments and periphytic diatom communities in the surrounding streams of Aljustrel mining area (Portugal). Water Air Soil Pollut. 2009, 200, 147–167. [Google Scholar] [CrossRef]
- Lauer, A.; Simon, M.A.; Banning, J.L.; Lam, B.A.; Harris, R.N. Diversity of cutaneous bacteria with antifungal activity isolated from female four-toed salamanders. ISME J. 2008, 145–157. [Google Scholar] [CrossRef]
- Brem, F.; Mendelson, J.R.; Lips, K.R. Field-Sampling Protocol for Batrachochytrium Dendrobatidis from Living Amphibians, Using Alcohol Preserved Swabs; Version 1.0; Conservation International: Arlington, VA, USA, 2007; Available online: http://www.amphibians.org (accessed on 2 February 2021).
- Smibert, R.M.; Krieg, N.R. Phenotypic Characterization. Methods for General and Molecular Bacteriology; Gerhardt, P., Murray, R.G.E., Wood, W.A., Krieg, N.R., Eds.; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Nielsen, P.; Fritze, D.; Priest, F.G. Phenetic diversity of alkaliphilic Bacillus strains: Proposal for nine new species. Microbiology 1995, 141, 1745–1761. [Google Scholar] [CrossRef]
- Proença, D.N.; Francisco, R.; Kublik, S.; Scholer, A.; Vestergaard, G.; Schloter, M.; Morais, P.V. The microbiome of endophytic, wood colonizing bacteria from pine trees as affected by Pine Wilt Disease. Sci. Rep. 2017, 7, 4205. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- van den Brink, P.J.; Braak, C.J.F.T. Principal response curves: Analysis of time-dependent multivariate responses of biological community to stress. Environ. Toxicol. Chem. 1999, 18, 138–148. [Google Scholar] [CrossRef]
- Kumar, M.A.; Anandapandian, K.T.K.; Parthiban, K. Production and characterization of exopolysaccharides (EPS) from biofilm forming marine bacterium. Braz. Arch. Biol. Technol. 2011, 54, 259–265. [Google Scholar] [CrossRef]
- Edwards, H.A. A novel mechanism for salt and fluid transport across epithelia. J. Exp. Biol. 1979, 83, 335–338. [Google Scholar]
- Boutilier, R.G.; Stiffler, D.F.; Toews, D. Exchange of respiratory gases, ions and water in amphibious and aquatic amphibians. In Environmental Physiology of the Amphibians; Burggren, W.W., Ed.; University of Chicago Press: Chicago, IL, USA; London, UK, 1992; pp. 81–124. [Google Scholar]
- Smith, M.A.; Green, D.M. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populations metapopulations? Ecography 2005, 28, 110–128. [Google Scholar] [CrossRef]
- Bowne, D.; Bowers, M. Interpatch movements in spatially structured populations: A literature review. Landsc. Ecol. 2004, 19, 1–20. [Google Scholar] [CrossRef]
- Bletz, M.C.; Perl, R.G.B.; Vences, M. Skin microbiota differs drastically between co-occurring frogs and newts. R. Soc. Open Sci. 2017, 4, 170107. [Google Scholar] [CrossRef]
- Federici, E.; Rossi, R.; Fidati, L.; Paracucchi, R.; Scargetta, S.; Montalbani, E.; Franzetti, A.; La Porta, G.; Fagotti, A.; Simoncelli, F.; et al. Characterization of the skin microbiota in Italian stream frogs (Rana italica) infected and uninfected by a cutaneous parasitic disease. Microbes Environ. 2015, 30, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Walke, J.B.; Becker, M.H.; Loftus, S.C.; House, L.L.; Cormier, G.; Jensen, R.V.; Belden, L.K. Amphibian skin may select for rare environmental microbes. ISME J. 2014, 8, 2207–2217. [Google Scholar] [CrossRef]
- Skrodenyte-Arbaciauskiene, V.; Sruoga, A.; Butkauskas, D. Assessment of microbial diversity in the river trout Salmo trutta fario L. intestinal tract identified by partial 16S rRNA gene sequence analysis. Fish. Sci. 2006, 72, 597–602. [Google Scholar] [CrossRef]
- Wolf, A.; Fritze, A.; Hagemann, M.; Berg, G. Stenotrophomonas rhizophila sp. nov., a novel plant-associated bacterium with antifungal properties. Int. J. Syst. Evol. Microbiol. 2002, 52, 1937–1944. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Du, C.; Mou, H.; Cui, J.; Guan, H.; Hwang, H.; Wang, P. Characterization of high yield exopolysaccharide produced by Phyllobacterium sp. 921F exhibiting moisture preserving properties. Int. J. Biol. Macromol. 2017, 101, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, C.; Branco, R.; Morais, P.V. Efficient bioaccumulation of tungsten by Escherichia coli cells expressing the Sulfitobacter dubius TupBCA system. Syst. Appl. Microbiol. 2019, 42, 126001. [Google Scholar] [CrossRef] [PubMed]
- Morais, P.V.; Branco, R.; Francisco, R. Chromium resistance strategies and toxicity: What makes Ochrobactrum tritici 5bvl1 a strain highly resistant. BioMetals 2011, 24, 401–410. [Google Scholar] [CrossRef]
- Proença, D.N.; Heine, T.; Senges, C.H.R.; Bandow, J.E.; Morais, P.V.; Tischler, D. Bacterial metabolites produced under iron limitation kill pinewood nematode and attract Caenorhabditis elegans. Front. Microbiol. 2019, 10, 2166. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, J.B.; Morais, P.V.; Branco, R. Exploiting the biological response of two Serratia fonticola strains to the critical metals, gallium and indium. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Shenoy, A.T.; Orihuela, C.J.; González-Juarbe, N. Killing of Serratia marcescens biofilms with chloramphenicol. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 19. [Google Scholar] [CrossRef]

| Strain | Bacterial Species | Identity (%) | Resistance to [AMD] (%) | Growth on TSB + 5% Sucrose (OD600nm) | EPS Production (g) | EPS.OD−1 (g) |
|---|---|---|---|---|---|---|
| 22F13B | Acinetobacter antiviralis KNF2022T | 97.6 | 75 | 1.01 | 0.05 | 0.05 |
| 12F14 | Acinetobacter beijerinckii NIPH 838T | 98.7 | 50 | 1.05 | 0.03 | 0.03 |
| 9F7 | Acinetobacter indicus A648T | 98.9 | 50 | 1.2 | 0.34 | 0.28 |
| 11M7 | Acinetobacter johnsonii DSM 6963T | 99.8 | 25 | 1.5 | 0.03 | 0.02 |
| 23M19 | Acinetobacter junii DSM 6964T | 100 | 75 | 1.09 | 0.45 | 0.41 |
| 10M2B | Acinetobacter oryzae B23T/Acinetobacter johnsonii DSM 6963T | 98.6 | 50 | 1.28 | 0.05 | 0.04 |
| 8F3 | Acinetobacter pragensis ANC 4149T/Acinetobacter bohemicus ANC 3994T | 100 | 75 | 3.02 | 0.15 | 0.05 |
| 6M5 | Acinetobacter soli B1T | 99.2 | 25 | 2.27 | 0.11 | 0.05 |
| 10M6B | Acinetobacter wuhouensis WCHA60T/Acinetobacter guillouiae DSM 590T | 98.4 | 75 | 1.17 | 0.19 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proença, D.N.; Fasola, E.; Lopes, I.; Morais, P.V. Characterization of the Skin Cultivable Microbiota Composition of the Frog Pelophylax perezi Inhabiting Different Environments. Int. J. Environ. Res. Public Health 2021, 18, 2585. https://doi.org/10.3390/ijerph18052585
Proença DN, Fasola E, Lopes I, Morais PV. Characterization of the Skin Cultivable Microbiota Composition of the Frog Pelophylax perezi Inhabiting Different Environments. International Journal of Environmental Research and Public Health. 2021; 18(5):2585. https://doi.org/10.3390/ijerph18052585
Chicago/Turabian StyleProença, Diogo Neves, Emanuele Fasola, Isabel Lopes, and Paula V. Morais. 2021. "Characterization of the Skin Cultivable Microbiota Composition of the Frog Pelophylax perezi Inhabiting Different Environments" International Journal of Environmental Research and Public Health 18, no. 5: 2585. https://doi.org/10.3390/ijerph18052585
APA StyleProença, D. N., Fasola, E., Lopes, I., & Morais, P. V. (2021). Characterization of the Skin Cultivable Microbiota Composition of the Frog Pelophylax perezi Inhabiting Different Environments. International Journal of Environmental Research and Public Health, 18(5), 2585. https://doi.org/10.3390/ijerph18052585







