Environmental Exposure of Wild Carnivores to Zoonotic Pathogens: Leptospira Infection in the First Free Living Wolf (Canis lupus Linnaeus, 1758) Found Dead in the Friuli Venezia Giulia Region
Abstract
1. Introduction
2. Materials and Methods
3. Results
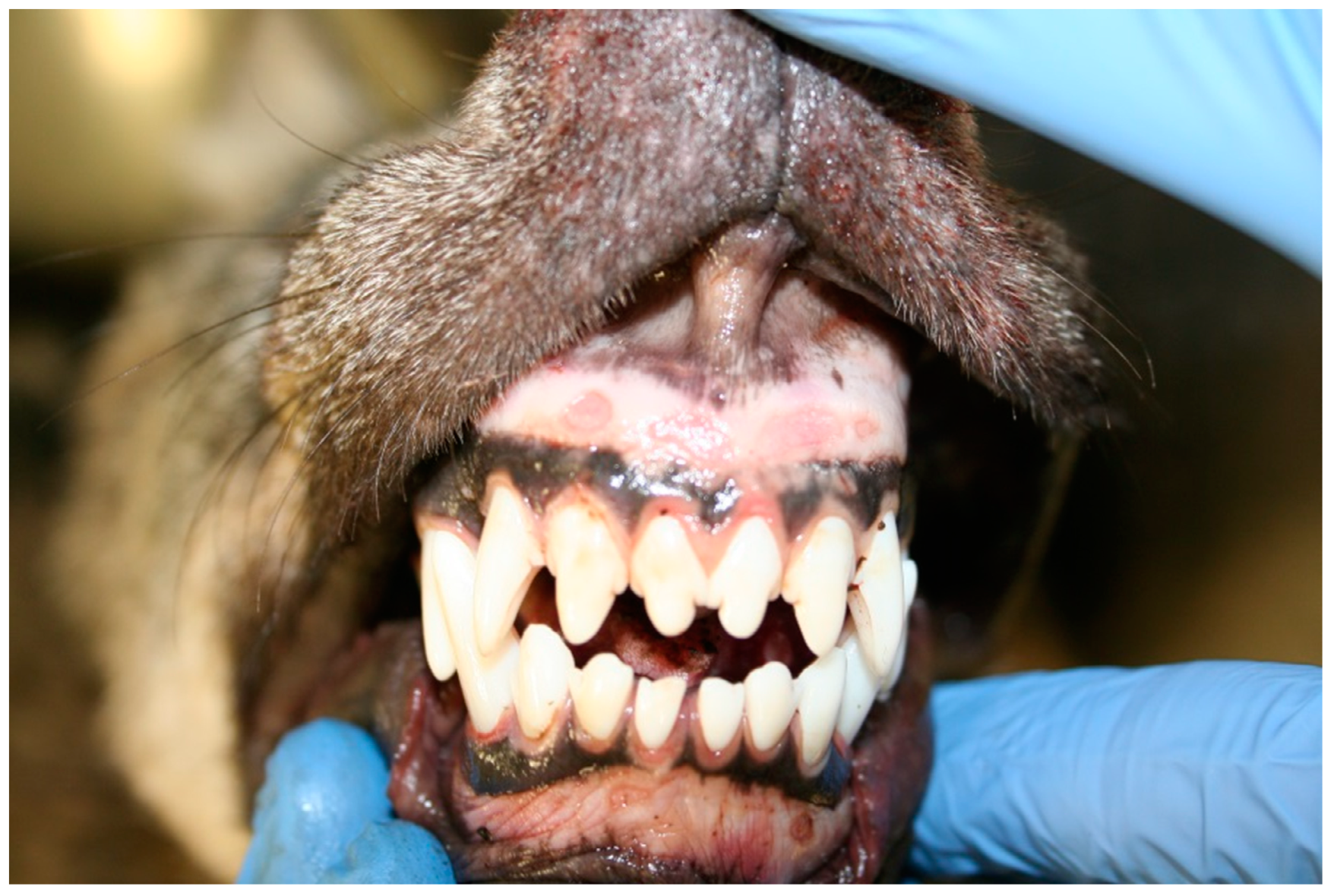
3.1. Gross Pathology
3.2. Diagnostic Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birtles, R. Leptospira infections. In Infectious Diseases of Wild Mammals and Birds in Europe, 1st ed.; Gavier-Widen, D., Duff, J.P., Meredith, A., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 402–408. [Google Scholar]
- Alston, J.M.; Broom, J.C. Leptospirosis in Man and Mammals; F & S Livingstone LTD: Edinburgh, UK, 1958. [Google Scholar]
- Austoni, M. Le Leptospirosi ad uso dei Medici Veterinari; Publisher Tipografia del Seminario di Padova: Padua, Italy, 1953. [Google Scholar]
- Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Bellinati, L.; Mazzucato, M.; Furlanello, T.; D’Incau, M.; Natale, N. Detection of new Leptospira genotypes infecting symptomatic dogs: Is a new vaccine formulation needed? Pathogens 2020, 9, 484. [Google Scholar] [CrossRef]
- Balboni, A.; Zamagni, S.; Bertasio, C.; Boniotti, M.B.; Troìa, R.; Battilani, M.; Dondi, F. Identification of Serogroups Australis and Icterohaemorrhagiae in two dogs with a severe form of acute leptospirosis in Italy. Pathogens 2020, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Lapini, L.; Brugnoli, A.; Krofel, M.; Kranz, A.; Molinari, P. A grey wolf (Canis lupus Linnaeus, 1758) from Fiemme Valley (Mammalia, Canidae; north-eastern Italy). Boll. Mus. Civ. Stor. Nat. Venezia 2010, 61, 117–129. [Google Scholar]
- Carannante, D. Lupo storico e lupo moderno: Presenza ed immaginario collettivo. Atti Mus. Civ. Stor. Nat. Trieste 2014, 57, 63–96. [Google Scholar]
- Razen, N.; Brugnoli, A.; Castagna, C.; Groff, C.; Kaczenski, K.; Knauer, F.; Kos, I.; Krofel, M.; Lustrik, R.; Majic, A.; et al. Long-distance dispersal connects Dinaric-Balkan and Alpine grey wolf (Canis lupus) populations. Eur. J. Wildl. Res. 2016, 62, 137–142. [Google Scholar] [CrossRef]
- Marucco, F.; Avanzinelli, E.; Bassano, B.; Bionda, R.; Bisi, F.; Calderola, S.; Chioso, C.; Fattori, U.; Pedrotti, L.; Righetti, D.; et al. Progetto LIFE 12 NAT/IT/000807 WOLFALPS Wolf in the Alps: Implementation of Coordinated Wolf Conservation Actions in Core Areas and beyond—Azione A4 e D1—Relazione Tecnica LA POPOLAZIONE DI LUPO SULLE ALPI ITALIANE 2014–2018 (CON EVOLUZIONE DAL 1996 AL 2018) Luglio 2018, 1–60. Available online: https://www.lifewolfalps.eu/wp-content/uploads/2020/10/Report_monitoraggio_Alpi_completo.pdf (accessed on 2 March 2021).
- Smythe, L.D.; Smith, I.L.; Smith, G.A.; Dohnt, M.F.; Symonds, M.L.; Barnett, L.J.; McKay, D.B. A quantitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infect. Dis. 2002, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Bertasio, C.; Papetti, A.; Scaltriti, E.; Tagliabue, S.; D’Incau, M.; Boniotti, M.B. Serological Survey and Molecular Typing Reveal New Leptospira Serogroup Pomona Strains among Pigs of Northern Italy. Pathogens 2020, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Maiden, M.C.J. Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, S.C.; Todd, J.N.; Headlam, S.A.; Jeffrey, M. Possible role of leptospires of the Pomona serogroup in sporadic bovine abortion in the south west of England. Vet. Rec. 1984, 115, 623–626. [Google Scholar] [CrossRef]
- Renauld, C.; Andrews, S.; Djelouadji, Z.; Lecheval, S.; Corrao-Revol, N.; Buff, S.; Demont, P.; Kodjo, A. Prevalence of the Leptospira serovars bratislava, grippotyphosa, mozdok and pomona in French dogs. Vet. J. 2013, 196, 126–127. [Google Scholar] [CrossRef]
- Cilia, G.; Bertelloni, F.; Angelini, M.; Cerri, D.; Fratini, F. Leptospira survey in wild boar (Sus scrofa) hunted on Tuscany, central Italy. Pathogens 2020, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Majetic, Z.S.; Galloway, R.; Sabljic, E.R.; Milas, Z.; Perko, V.M.; Habus, J.; Margaletic, J.; Pernar, R.; Turk, N. Epizootiological survey of small mammals as Leptospira spp. reservoirs in Eastern Croatia. Acta Trop. 2014, 131, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Obiegala, A.; Woll, D.; Karnath, C.; Silaghi, C.; Schex, S.; Eßbauer, S.; Pfeffer, M. Prevalence and genotype allocation of pathogenic Leptospira species in small mammals from various habitat types in Germany. PLoS Negl. Trop. Dis. 2016, 10, e0004501. [Google Scholar] [CrossRef] [PubMed]
- Vengust, G.; Lindtner-Knific, R.; Zele, D.; Bidovec, A. Leptospira antibodies in wild boars (Sus scrofa) in Slovenia. Eur. J. Wildl. Res. 2008, 54, 749–752. [Google Scholar] [CrossRef]
- Millan, J.; Garcia, E.J.; Oleaga, A.; Lopez-Bao, J.V.; Llaneza, L.; Palacios, V.; Candela, M.G.; Cevinades, A.; Rodriguez, A.; Leon-Vizcaino, L. Using a top predator as a sentinel for environmental contamination with pathogenic bacteria: The Iberian wolf and leptospires. Memórias Inst. Oswaldo Cruz 2014, 109, 1041–1044. [Google Scholar] [CrossRef]
- Bertelloni, F.; Cilia, G.; Turchi, B.; Pinzauti, P.; Cerri, D.; Fratini, F. Epidemiology of leptospirosis in north-central Italy: Fifteen years of serological data (2002–2016). Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Balassiano, I.T.; Vital-Brazil, J.M.; Ramos, T.M.V.; Timm, L.N.; Pereria, M.M. Molecular and serological characterization of Leptospira kirschneri serogroup Pomona isolated from a human case in a Brazilian rural area. Rev. Soc. Bras. Med. Trop. 2017, 50, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, J.; Bouhry, P.; Schiettekatte, O.; Zinini, F.; Brisse, S.; Picardeau, M. Genus-wide Leptospira core genome multilocus sequence typing for strain taxonomy and global surveillance. PLoS Negl. Trop. Dis. 2019, 13, e0007374. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.E.; Lin, R.C.; Langston, C.E.; Scrivani, P.V.; Erb, H.N.; Barr, S.C. Influence of infecting serogroup on clinical features of leptospirosis in dogs. J. Vet. Intern. Med. 2006, 20, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Millan, J.; Candela, M.G.; Lopez-Bao, J.V.; Pereira, M.; Jimenez, M.A.; Leon-Vizcaino, L. Leptospirosis in wild and domestic carnivores in natural areas in Andalusia, Spain. Vector Borne Zoonotic Dis. 2009, 9, 549–554. [Google Scholar] [CrossRef]
- Andreoli, E.; Radaelli, E.; Bertoletti, I.; Bianchi, A.; Scanziani, E.; Tagliabue, S.; Mattielllo, S. Leptospira spp. infection in wild ruminants: A survey in Central Italian Alps. Vet. Ital. 2014, 50, 285–291. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bregoli, M.; Pesaro, S.; Ustulin, M.; Vio, D.; Beraldo, P.; Galeotti, M.; Cocchi, M.; Lucchese, L.; Bertasio, C.; Boniotti, M.B.; et al. Environmental Exposure of Wild Carnivores to Zoonotic Pathogens: Leptospira Infection in the First Free Living Wolf (Canis lupus Linnaeus, 1758) Found Dead in the Friuli Venezia Giulia Region. Int. J. Environ. Res. Public Health 2021, 18, 2512. https://doi.org/10.3390/ijerph18052512
Bregoli M, Pesaro S, Ustulin M, Vio D, Beraldo P, Galeotti M, Cocchi M, Lucchese L, Bertasio C, Boniotti MB, et al. Environmental Exposure of Wild Carnivores to Zoonotic Pathogens: Leptospira Infection in the First Free Living Wolf (Canis lupus Linnaeus, 1758) Found Dead in the Friuli Venezia Giulia Region. International Journal of Environmental Research and Public Health. 2021; 18(5):2512. https://doi.org/10.3390/ijerph18052512
Chicago/Turabian StyleBregoli, Marco, Stefano Pesaro, Martina Ustulin, Denis Vio, Paola Beraldo, Marco Galeotti, Monia Cocchi, Laura Lucchese, Cristina Bertasio, Maria Beatrice Boniotti, and et al. 2021. "Environmental Exposure of Wild Carnivores to Zoonotic Pathogens: Leptospira Infection in the First Free Living Wolf (Canis lupus Linnaeus, 1758) Found Dead in the Friuli Venezia Giulia Region" International Journal of Environmental Research and Public Health 18, no. 5: 2512. https://doi.org/10.3390/ijerph18052512
APA StyleBregoli, M., Pesaro, S., Ustulin, M., Vio, D., Beraldo, P., Galeotti, M., Cocchi, M., Lucchese, L., Bertasio, C., Boniotti, M. B., Lapini, L., & Natale, A. (2021). Environmental Exposure of Wild Carnivores to Zoonotic Pathogens: Leptospira Infection in the First Free Living Wolf (Canis lupus Linnaeus, 1758) Found Dead in the Friuli Venezia Giulia Region. International Journal of Environmental Research and Public Health, 18(5), 2512. https://doi.org/10.3390/ijerph18052512






