Blood Parasites in Sympatric Vultures: Role of Nesting Habits and Effects on Body Condition
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Areas and Species
2.3. Fieldwork
2.4. Molecular Analyses
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortés-Avizanda, A.; Blanco, G.; DeVault, T.L.; Markandya, A.; Virani, M.Z.; Brandt, J.; Donázar, J.A. Supplementary feeding and endangered avian scavengers: Benefits, caveats, and controversies. Front. Ecol. Environ. 2016, 14, 191–199. [Google Scholar] [CrossRef]
- Morales-Reyes, Z.; Martín-López, B.; Moleón, M.; Mateo-Tomás, P.; Botella, F.; Margalida, A.; Donázar, J.A.; Blanco, G.; Pérez, I.; Sánchez-Zapata, J.A. Farmer perceptions of the eco-system services provided by scavengers: What, who, and to whom. Conserv. Lett. 2018, 11, e12392. [Google Scholar] [CrossRef]
- Blanco, G.; Díaz de Tuesta, J.A. Seasonal and spatial occurrence of zoonotic Salmonella serotypes in griffon vultures at farm-land environments: Implications in pathogen pollution and ecosystem services and disservices. Sci. Total Environ. 2020, 758, 143681. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G. Avian Malarial Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Scheuerlein, A.; Ricklefs, R.E. Prevalence of blood parasites in European passeriform birds. Proc. R Soc. Lond. Ser. B Biol. Sci. 2004, 271, 1363–1370. [Google Scholar] [CrossRef]
- Ferraguti, M.; La Puente, J.M.-D.; Bensch, S.; Roiz, D.; Ruiz, S.; Viana, D.S.; Soriguer, R.C.; Figuerola, J. Ecological determinants of avian malaria infections: An integrative analysis at landscape, mosquito and vertebrate community levels. J. Anim. Ecol. 2018, 87, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ellis, V.A.; Jönsson, J.; Bensch, S. Generalist haemosporidian parasites are better adapted to a subset of host species in a multiple host community. Mol. Ecol. 2018, 27, 4336–4346. [Google Scholar] [CrossRef]
- Roggenbuck, M.; Schnell, I.B.; Blom, N.; Bælum, J.; Bertelsen, M.F.; Sicheritz-Pontén, T.; Sørensen, S.J.; Gilbert, M.T.P.; Graves, G.R.; Hansen, L.H. The microbiome of New World vultures. Nat. Commun. 2014, 5, 5498. [Google Scholar] [CrossRef] [PubMed]
- Blumstein, D.T.; Rangchi, T.N.; Briggs, T.; De Andrade, F.S.; Natterson-Horowitz, B. A Systematic Review of Carrion Eaters’ Adaptations to Avoid Sickness. J. Wildl. Dis. 2017, 53, 577–581. [Google Scholar] [CrossRef]
- Plaza, P.I.; Blanco, G.; Lambertucci, S.A. Implications of bacterial, viral and mycotic microorganisms in vultures for wildlife conservation, ecosystem services and public health. Ibis 2020, 162, 1109–1124. [Google Scholar] [CrossRef]
- Mendoza, M.L.Z.; Roggenbuck, M.; Vargas, K.M.; Hansen, L.H.; Brunak, S.; Gilbert, M.T.P.; Sicheritz-Pontén, T. Protective role of the vul-ture facial skin and gut microbiomes aid adaptation to scavenging. Acta Vet. Scand. 2018, 60, 61. [Google Scholar] [CrossRef]
- Blanco, G.; Junza, A.; Segarra, D.; Barbosa, J.; Barrón, D. Wildlife contamination with fluoroquinolones fromlivestock: Wide-spread prevalence of enrofloxacin and marbofloxacin in vultures. Chemosphere 2016, 144, 1536–1543. [Google Scholar] [CrossRef]
- Blanco, G.; Junza, A.; Barrón, D. Food safety in scavenger conservation: Diet-associated exposure to livestock pharmaceuti-cals and opportunist mycoses in threatened cinereous and Egyptian vultures. Ecotoxicol. Environ. Saf. 2017, 135, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, P.; Blanco, G.; García-Fernández, A.J. Validation of Multi-Residue Method for Quantification of Antibiotics and NSAIDs in Avian Scavengers by Using Small Amounts of Plasma in HPLC-MS-TOF. Int. J. Environ. Res. Public Health 2020, 17, 4058. [Google Scholar] [CrossRef]
- Pitarch, A.; Gil, C.; Blanco, G. Oral mycoses in avian scavengers exposed to antimicrobials from livestock farming. Sci. Total Environ. 2017, 605, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Gajon, A.; Doval, G.; Martínez, F. Absence of Blood Parasites in Griffon Vultures from Spain. J. Wildl. Dis. 1998, 34, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Tella, J.L.; Blanco, G.; Forero, M.G.; Gajón, Á.; Donázar, J.A.; Hiraldo, F. Habitat, world geographic range, and embryonic development of hosts explain the prevalence of avian hematozoa at small spatial and phylogenetic scales. Proc. Natl. Acad. Sci. USA 1999, 96, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Donázar, J.A. Los Buitres Lbéricos: Biología y Conservación; JM Reyero: Madrid, Spain, 1993. [Google Scholar]
- Blanco, G.; Cortés-Avizanda, A.; Frías, Ó.; Arrondo, E.; Donázar, J.A. Livestock farming practices modulate vulture diet-disease interactions. Glob. Ecol. Conserv. 2019, 17, e00518. [Google Scholar] [CrossRef]
- Crespo-Ginés, R.; López, D.S.; Berriatua, E.; Blanco, G.; Candela, M.G.; Pérez-García, J.M. Coccidian Prevalence and Intensity in Free-Ranging and Rehabilitating Wild Raptors. Ardeola 2019, 66, 65–76. [Google Scholar] [CrossRef]
- Van Overveld, T.; Blanco, G.; Moleón, M.; Margalida, A.; Sánchez-Zapata, J.A.; De La Riva, M.; Donázar, J.A. Integrating vulture social behavior into conservation practice. Condor 2020, 122, 1–20. [Google Scholar] [CrossRef]
- Tella, J.L. The evolutionary transition to coloniality promotes higher blood parasitism in birds. J. Evol. Biol. 2002, 15, 32–41. [Google Scholar] [CrossRef]
- Jovani, R.; Tella, J.; Forero, M.G.; Bertellotti, M.; Blanco, G.; Ceballos, O.; Donázar, J.A. Apparent Absence of Blood Parasites in the Patagonian Seabird Community: Is It Related to the Marine Environment? Waterbirds 2001, 24, 430. [Google Scholar] [CrossRef]
- Knowles, S.C.L.; Wood, M.J.; Alves, R.; Wilkin, T.A.; Bensch, S.; Sheldon, B.C. Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol. Ecol. 2010, 20, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Chakarov, N.; Kampen, H.; Wiegmann, A.; Werner, D.; Bensch, S. Blood parasites in vectors reveal a united blackfly com-munity in the upper canopy. Parasit. Vectors 2020, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.D.; Bortolotti, G.R. Effects of hematozoan parasites on condition and return rates of American kestrels. Auk 2000, 117, 373–380. [Google Scholar] [CrossRef]
- Blanco, G.; Rodríguez-Estrella, R.; Merino, S.; Bertellotti, M. Effects of spatial and host variables on hematozoa in white-crowned sparrows wintering in Baja California. J. Wildl. Dis. 2001, 37, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Fargallo, J.A.; Blanco, G.; Soto-Largo, E. Forest management effects on nesting habitat selected by Eurasian Black Vultures Aegypius monachus in central Spain. J. Raptor Res. 1998, 32, 202–207. [Google Scholar]
- Donázar, J.A.; Blanco, G.; Hiraldo, F.; Soto-Largo, E.; Oria, J. Effects of forestry and other land-use practices on the conservation of cinereous vultures. Ecol. Appl. 2002, 12, 1445–1456. [Google Scholar] [CrossRef]
- Martínez, F.; Rodríguez, R.F.; Blanco, G. Effects of Monitoring Frequency on Estimates of Abundance, Age Distribution, and Productivity of Colonial Griffon Vultures. J. Field Ornithol. 1997, 68, 392–399. [Google Scholar]
- Sanz-Aguilar, A.; Cortés-Avizanda, A.; Serrano, D.; Blanco, G.; Ceballos, O.; Grande, J.M.; Tella, J.L.; Donázar, J.A. Sex- and age-dependent patterns of survival and breeding success in a long-lived endangered avian scavenger. Sci. Rep. 2017, 7, 40204. [Google Scholar] [CrossRef]
- Blanco, G. Can livestock carrion availability influence diet of wintering red kites? Implications of sanitary policies in ecosystem services and conservation. Popul. Ecol. 2014, 56, 593–604. [Google Scholar] [CrossRef]
- Pitarch, A.; Gil, C.; Blanco, G. Vultures from different trophic guilds show distinct oral pathogenic yeast signatures and co-occurrence networks. Sci. Total. Environ. 2020, 723, 138166. [Google Scholar] [CrossRef]
- López-Rull, I.; Hornero-Méndez, D.; Frías, Ó.; Blanco, G. Age-Related Relationships between Innate Immunity and Plasma Carotenoids in an Obligate Avian Scavenger. PLoS ONE 2015, 10, e0141759. [Google Scholar] [CrossRef]
- Wink, M.; Sauer-Gürth, H.; Martínez, F.; Doval, G.; Blanco, G.; Hatzofe, O. The use of (GACA) 4-PCR to sex old world vultures (Aves: Accipitridae). Mol. Ecol. 1998, 7, 779–782. [Google Scholar] [CrossRef]
- Fridolfsson, A.-K.; Ellegren, H. A Simple and Universal Method for Molecular Sexing of Non-Ratite Birds. J. Avian Biol. 1999, 30, 116. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; de la Puente, J.; Onrubia, A.; Pérez-Tris, J. Molecular characterization of haemosporidian parasites from kites of the genus Milvus (Aves: Accipitridae). Int. J. Parasitol. 2013, 43, 381–387. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new pcr assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, E.S.G. Epitools Epidemiological Calculators. 2018. Available online: http://epitools.ausvet.com.au (accessed on 16 December 2020).
- IBM Corp. IBM SPSS Statistics for Windows (Version 25); IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- Greiner, E.C.; Mundy, P.J. Hematozoa from Southern African Vultures, with a Description of Haemoproteus janovyi sp. n. J. Parasitol. 1979, 65, 147. [Google Scholar] [CrossRef] [PubMed]
- Mundy, P.J. The Vultures of Africa; Acorn Books: Johannesburg, South Africa, 1992. [Google Scholar]
- Crosskey, R.W. The Natural History of Blackflies; Wiley: Chichester, UK, 1990. [Google Scholar]
- Santiago-Alarcon, D.; Palinauskas, V.; Schaefer, H.M. Diptera vectors of avian Haemosporidian parasites: Untangling para-site life cycles and their taxonomy. Biol. Rev. 2012, 87, 928–964. [Google Scholar] [CrossRef]
- Chakarov, N.; Veiga, J.P.; Ruiz-Arrondo, I.; Valera, F. Atypical behavior of one blackfly species connects cavity-nesting birds and generalist blood parasites in arid areas of Spain. In review.
- Boshoff, A.F.; Currie, M.H. Notes on the cape vulture colony at potberg, bredasdorp. Ostrich 1981, 52, 1–8. [Google Scholar] [CrossRef]
- Robertson, A.S. Notes on the breeding cycle of Cape Vultures (Gyps coprotheres). J. Raptor Res. 1986, 20, 51–60. [Google Scholar]
- Chakarov, N.; Boerner, M.; Krüger, O. Fitness in common buzzards at the cross-point of opposite melanin-parasite interac-tions. Funct Ecol. 2008, 22, 1062–1069. [Google Scholar] [CrossRef]
- Chakarov, N.; Linke, B.; Boerner, M.; Goesmann, A.; Krüger, O.; Hoffman, J.I. Apparent vector-mediated parent-to-offspring transmission in an avian malaria-like parasite. Mol. Ecol. 2015, 24, 1355–1363. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Parasites and Their Social Hosts. Trends Parasitol. 2017, 33, 453–462. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Dusek, R.J.; Woods, K.L.; Iko, W.M. Pathogenicity of avian malaria in experimentally-infected Hawaii amakihi. J. Wildl. Dis. 2000, 36, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; de Lope, F.; Navarro, C.; Møller, A.P. Malarial parasites decrease reproductive success: An experimental study in a passerine bird. Oecologia 2005, 142, 541–545. [Google Scholar] [CrossRef]
- Schoenle, L.A.; Kernbach, M.; Haussmann, M.F.; Bonier, F.; Moore, I.T. An experimental test of the physiological consequences of avian malaria infection. J. Anim. Ecol. 2017, 86, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Cornet, S.; Bichet, C.; Larcombe, S.; Faivre, B.; Sorci, G. Impact of host nutritional status on infection dynamics and parasite virulence in a bird-malaria system. J. Anim. Ecol. 2013, 83, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Laurance, S.G.W.; Jones, D.; Westcott, D.; McKeown, A.; Harrington, G.; Hilbert, D.W. Habitat Fragmentation and Ecological Traits Influence the Prevalence of Avian Blood Parasites in a Tropical Rainforest Landscape. PLoS ONE 2013, 8, e76227. [Google Scholar] [CrossRef]
- Krüger, O. The Evolution of Reversed Sexual Size Dimorphism in Hawks, Falcons and Owls: A Comparative Study. Evol. Ecol. 2005, 19, 467–486. [Google Scholar] [CrossRef]
- Fargallo, J.A.; Martínez, F.; Wakamatsu, K.; Serrano, D.; Blanco, G. Sex-dependent expression and fitness conse-quences of sunlight-derived color phenotypes. Am. Nat. 2018, 191, 726–743. [Google Scholar] [CrossRef] [PubMed]
- Hiraldo, F.; Donázar, J.A. Foraging time in the Cinereous Vulture Aegypius monachus: Seasonal and local variations and in-fluence of weather. Bird Study 1990, 37, 128–132. [Google Scholar] [CrossRef]
- Leggett, H.C.; Buckling, A.; Long, G.H.; Boots, M. Generalism and the evolution of parasite virulence. Trends Ecol. Evol. 2013, 28, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E. Embryonic development period and the prevalence of avian blood parasites. Proc. Natl. Acad. Sci. USA 1992, 89, 4722–4725. [Google Scholar] [CrossRef] [PubMed]
- Shurulinkov, P.; Spasov, L.; Stoyanov, G.; Chakarov, N. Blood parasite infections in a wild population of ravens (Corvus corax) in Bulgaria. Malar. J. 2018, 17, 33. [Google Scholar] [CrossRef]

| A. monachus | G. fulvus | N. percnopterus | |||||
|---|---|---|---|---|---|---|---|
| Year | n | CIAE02 | AEMO02 | n | CIAE02 | n | CIAE02 |
| 2001 | 6 * | 10 | – | ||||
| 2004 | 38 | 4 | 2 | – | 7 | ||
| 2005 | 10 | – | 11 | ||||
| 2006 | – | – | 19 | 1 | |||
| 2007 | 15 | 1 | 8 | 3 | 1 | ||
| 2008 | – | – | 4 | 1 | |||
| 2009 | 7 | 10 | 1 | 8 | |||
| 2010 | 21 | 6 | 8 | 9 | 1 | ||
| 2011 | – | 5 | 7 | ||||
| 2013 | 5 | 12 | 1 | 11 | 1 | ||
| 2014 | 4 | 12 | 10 | ||||
| 2015 | 10 | 1 | 14 | 1 | 12 | 1 | |
| 2016 | 14 | 1 | 24 | – | |||
| 2017 | 16 | 24 | 1 | 13 | |||
| 2018 | – | 1 | – | ||||
| Total | 146 | 13 | 2 | 128 | 4 | 114 | 6 |
| Parameter Estimate | |||||
|---|---|---|---|---|---|
| Species, Variable | B | SE | F | df | p |
| Aegypius monachus | |||||
| Sex (male) | −533.619 | 115.171 | 21.467 | 1,98 | <0.0001 |
| Infection status (absence) | 441.546 | 200.912 | 4.830 | 1,98 | 0.030 |
| Wing length | 10.895 | 0.688 | 250.508 | 1,98 | <0.0001 |
| Pinewood type (P. pinaster) | 406.540 | 168.109 | 5.848 | 1,98 | 0.017 |
| Gyps fulvus | |||||
| Sex (male) | −109.712 | 175.577 | 0.390 | 1,94 | 0.534 |
| Infection status (absence) | 468.791 | 479.693 | 0.955 | 1,94 | 0.331 |
| Wing length | 10.743 | 0.858 | 156.647 | 1,94 | <0.0001 |
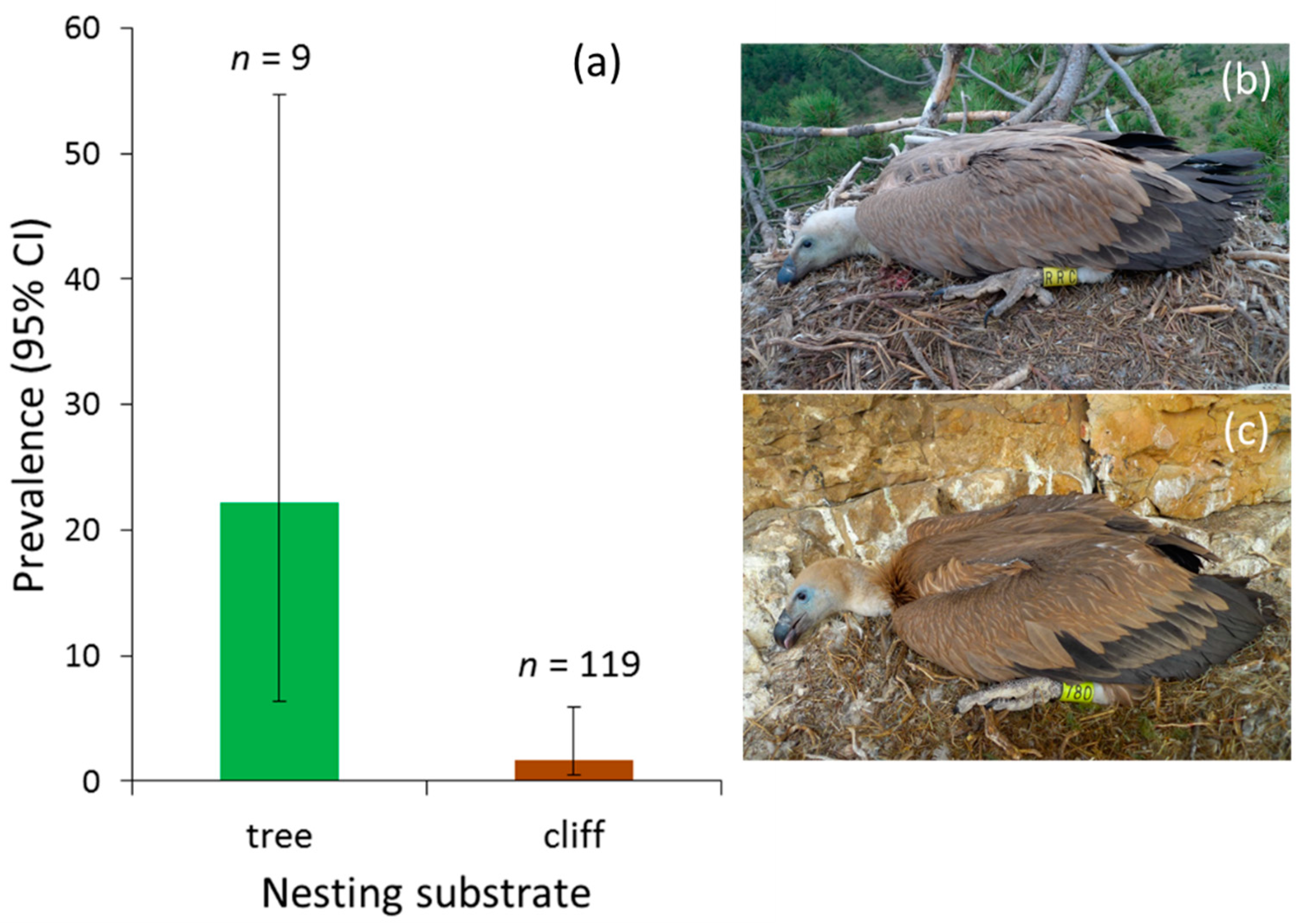
| Nesting substrate (cliff) | 814.339 | 374.061 | 4.739 | 1,94 | 0.032 |
| Neophron percnopterus | |||||
| Sex (male) | −65.645 | 32.327 | 4.124 | 1,93 | 0.045 |
| Infection status (absence) | 60.032 | 70.838 | 0.718 | 1,93 | 0.399 |
| Wing length | 2.519 | 0.292 | 74.378 | 1,93 | <0.0001 |
| (Brood size = 1) (hatching order = 0) | 6.866 | 41.758 | 0.015 | 1,93 | 0.985 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakarov, N.; Blanco, G. Blood Parasites in Sympatric Vultures: Role of Nesting Habits and Effects on Body Condition. Int. J. Environ. Res. Public Health 2021, 18, 2431. https://doi.org/10.3390/ijerph18052431
Chakarov N, Blanco G. Blood Parasites in Sympatric Vultures: Role of Nesting Habits and Effects on Body Condition. International Journal of Environmental Research and Public Health. 2021; 18(5):2431. https://doi.org/10.3390/ijerph18052431
Chicago/Turabian StyleChakarov, Nayden, and Guillermo Blanco. 2021. "Blood Parasites in Sympatric Vultures: Role of Nesting Habits and Effects on Body Condition" International Journal of Environmental Research and Public Health 18, no. 5: 2431. https://doi.org/10.3390/ijerph18052431
APA StyleChakarov, N., & Blanco, G. (2021). Blood Parasites in Sympatric Vultures: Role of Nesting Habits and Effects on Body Condition. International Journal of Environmental Research and Public Health, 18(5), 2431. https://doi.org/10.3390/ijerph18052431







