Combined Effects of Oligopeptides Isolated from Panax ginseng C.A. Meyer and Ostrea gigas Thunberg on Sexual Function in Male Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Identification of GOPs and OOPs
2.2. Chemicals and Reagents
2.3. Animals
2.4. Groups and Treatment
2.5. Sexual Behavior Study
2.6. Determination of Serum NO and Testosterone
2.7. Determination of Sex Organ Indexes
2.8. Examination of NO, cGMP, and PDE5 in Corpus Cavernosum Tissue
2.9. Statistical Analysis
3. Results
3.1. Analysis of GOPs and OOPs
3.2. Effects of GOPs on Sexual Function in Male Mice
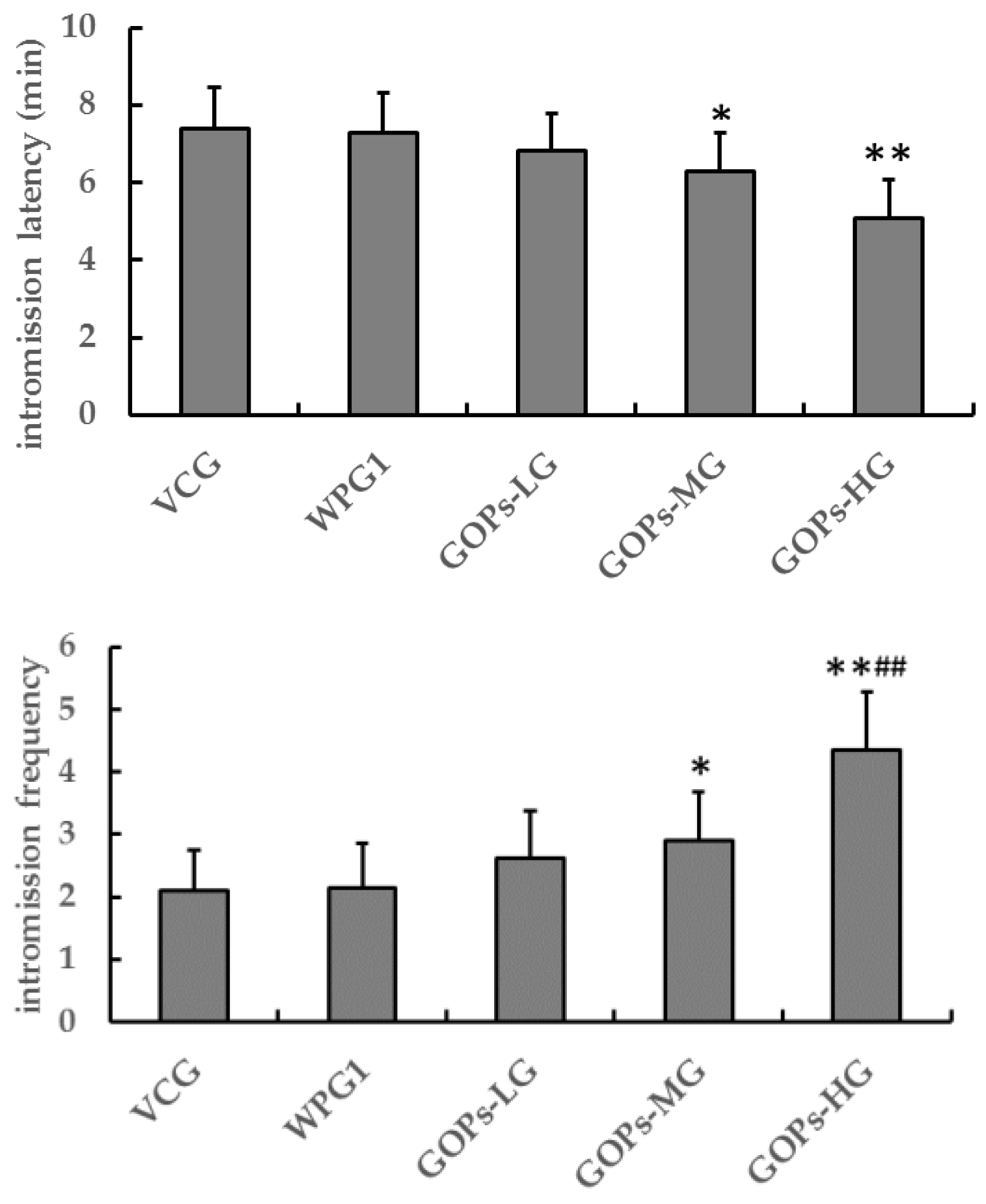
3.2.1. Effects of GOPs on Sexual Behavior in Male Mice
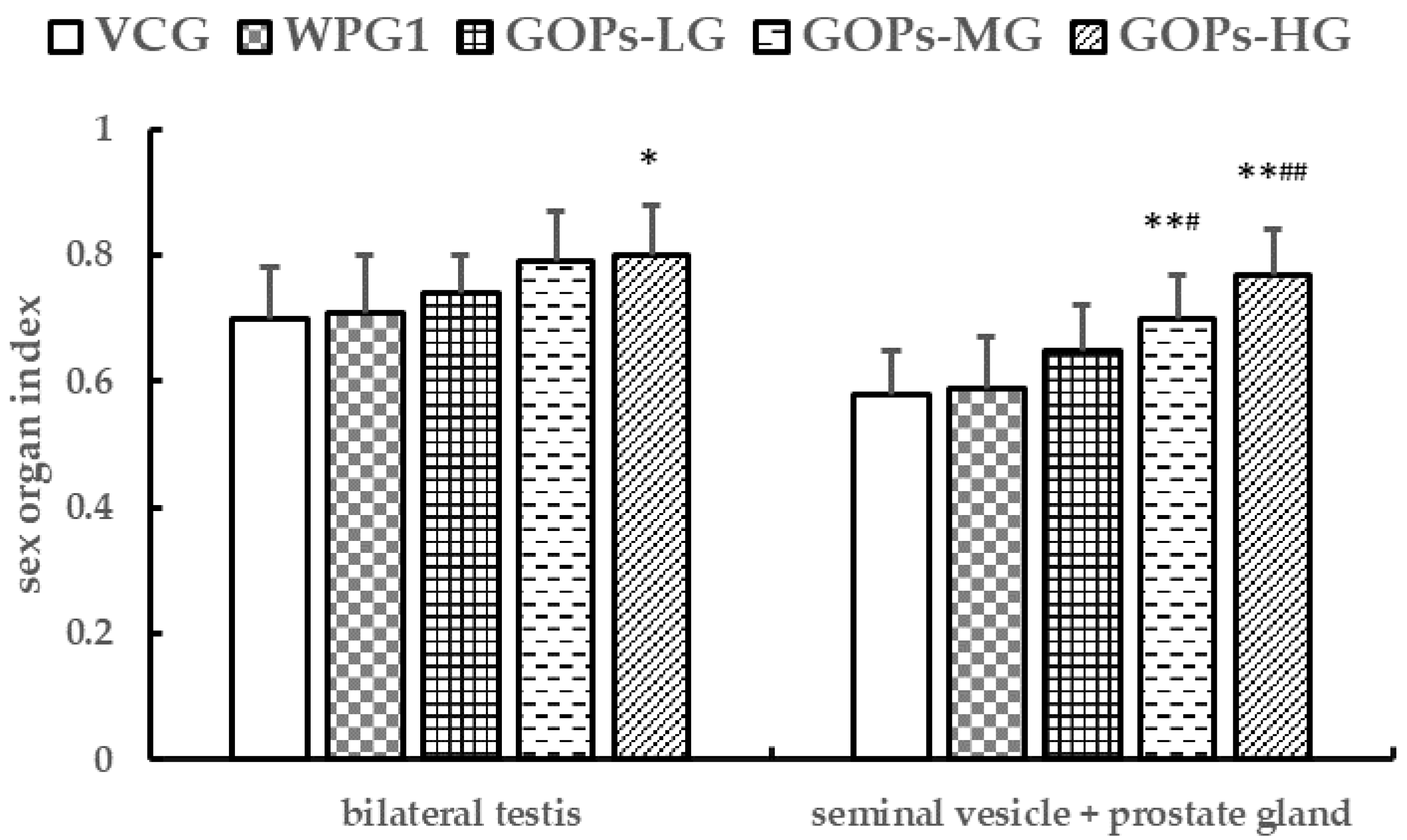
3.2.2. Effects of GOPs on Sex Organ Indexes in Male Mice
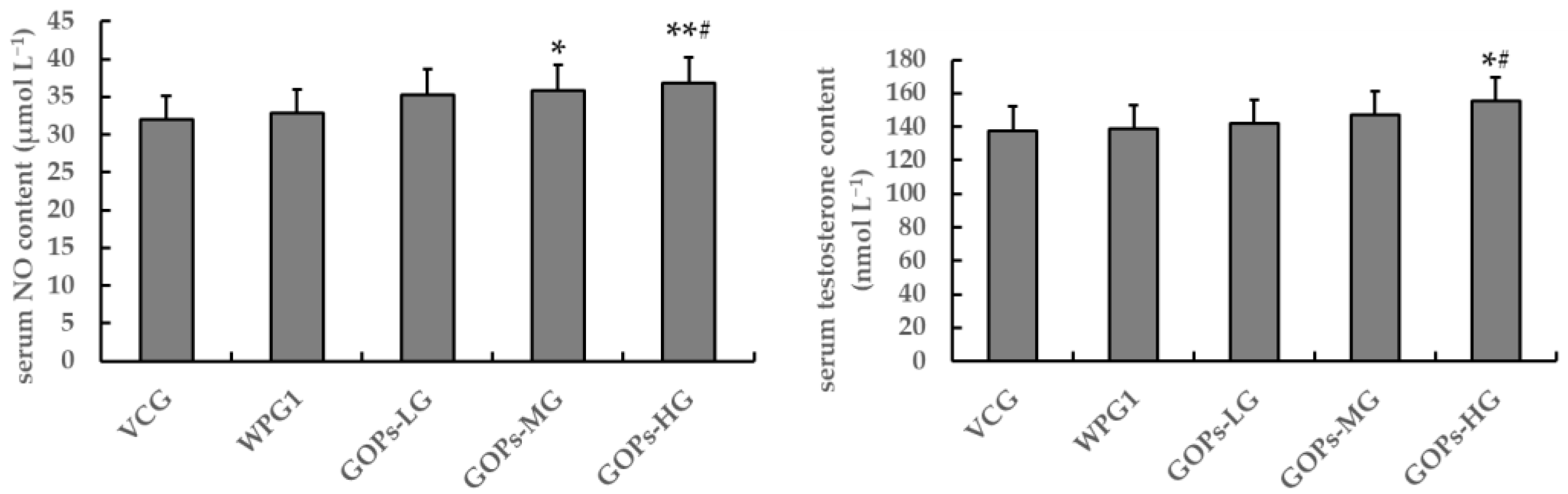
3.2.3. Effects of GOPs on Serum NO and Testosterone Contents in Male Mice
3.3. Effects of OOPs on Sexual Function in Male Mice
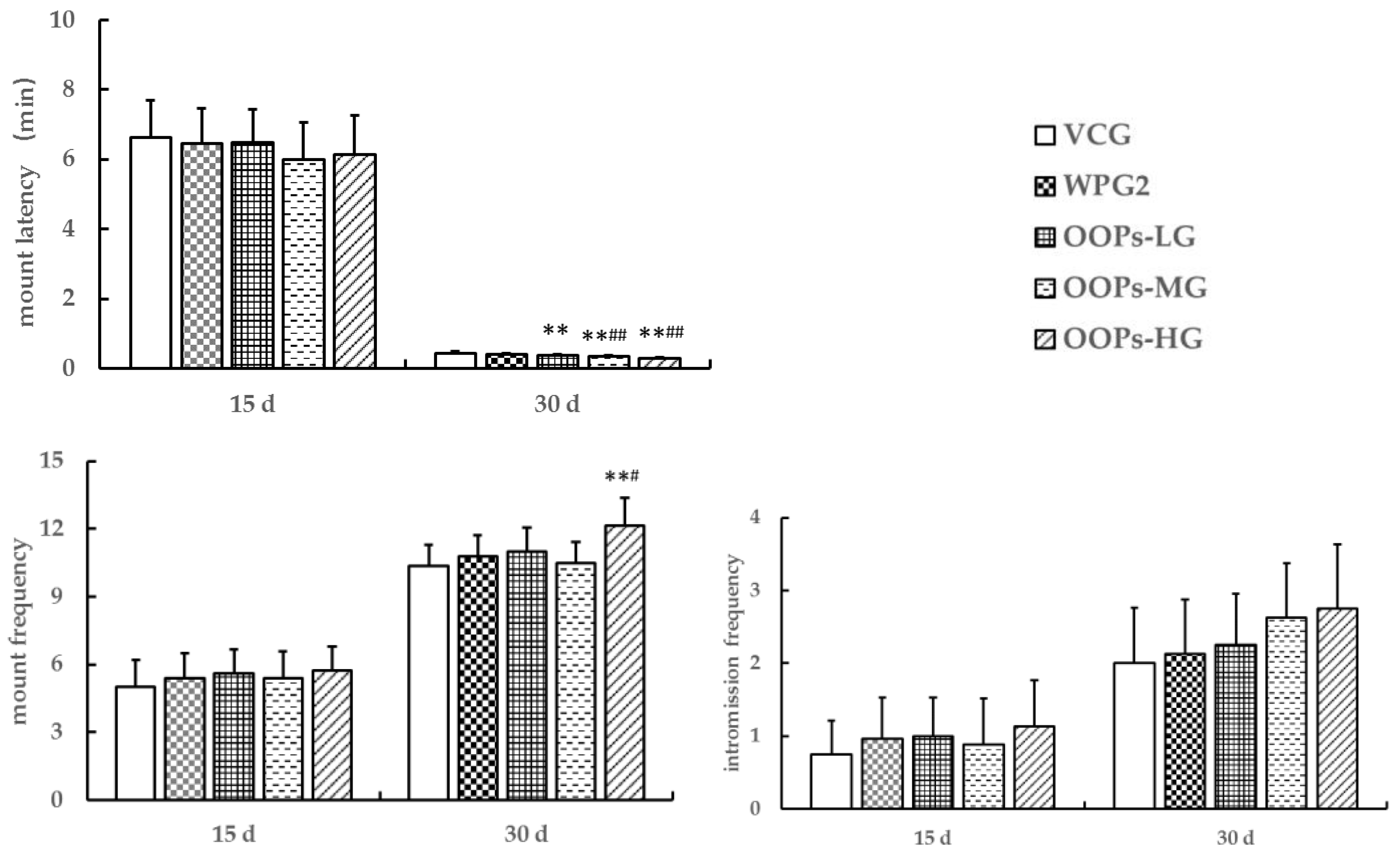
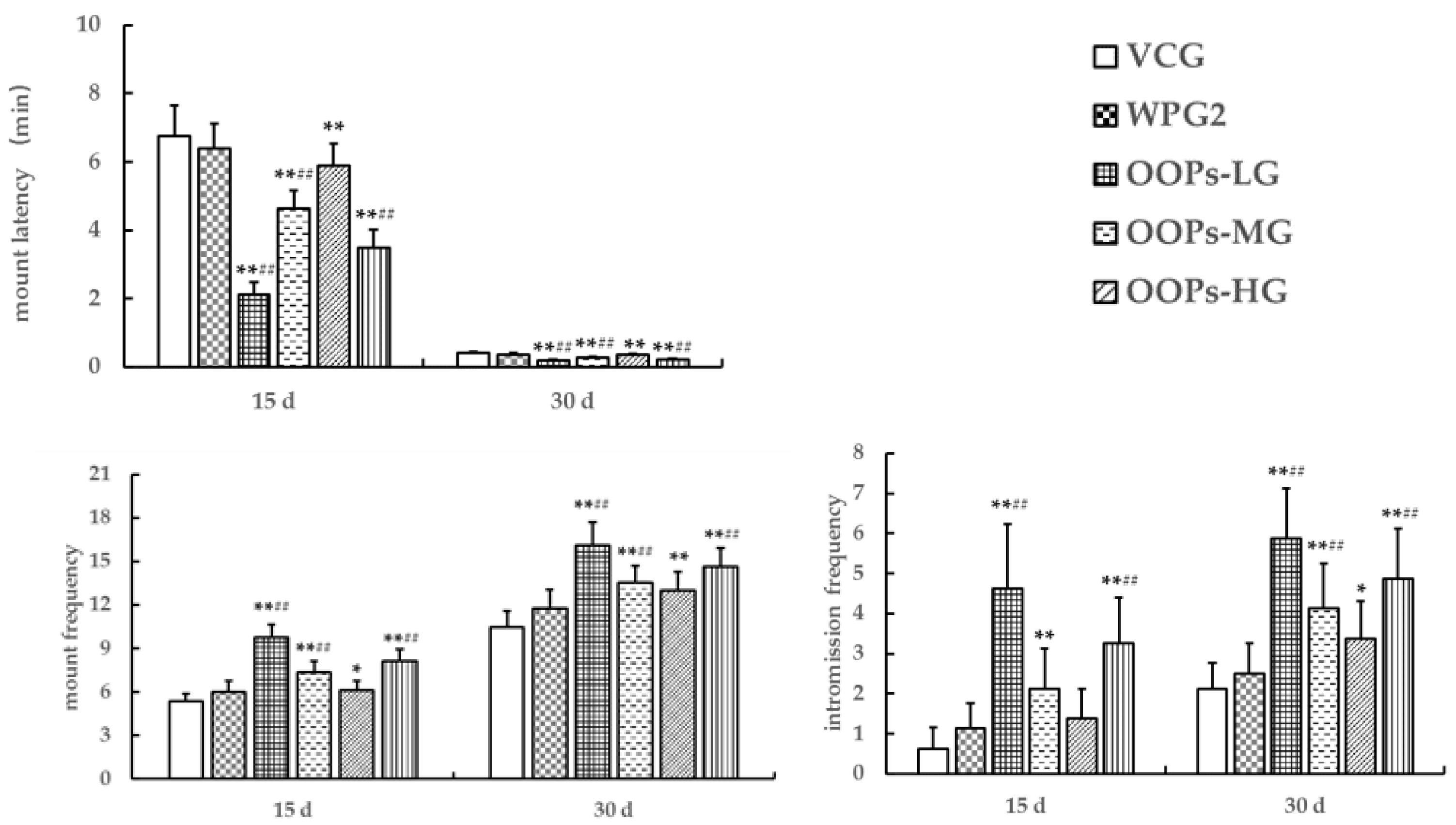
3.3.1. Effects of OOPs on Sexual Behavior in Male Mice
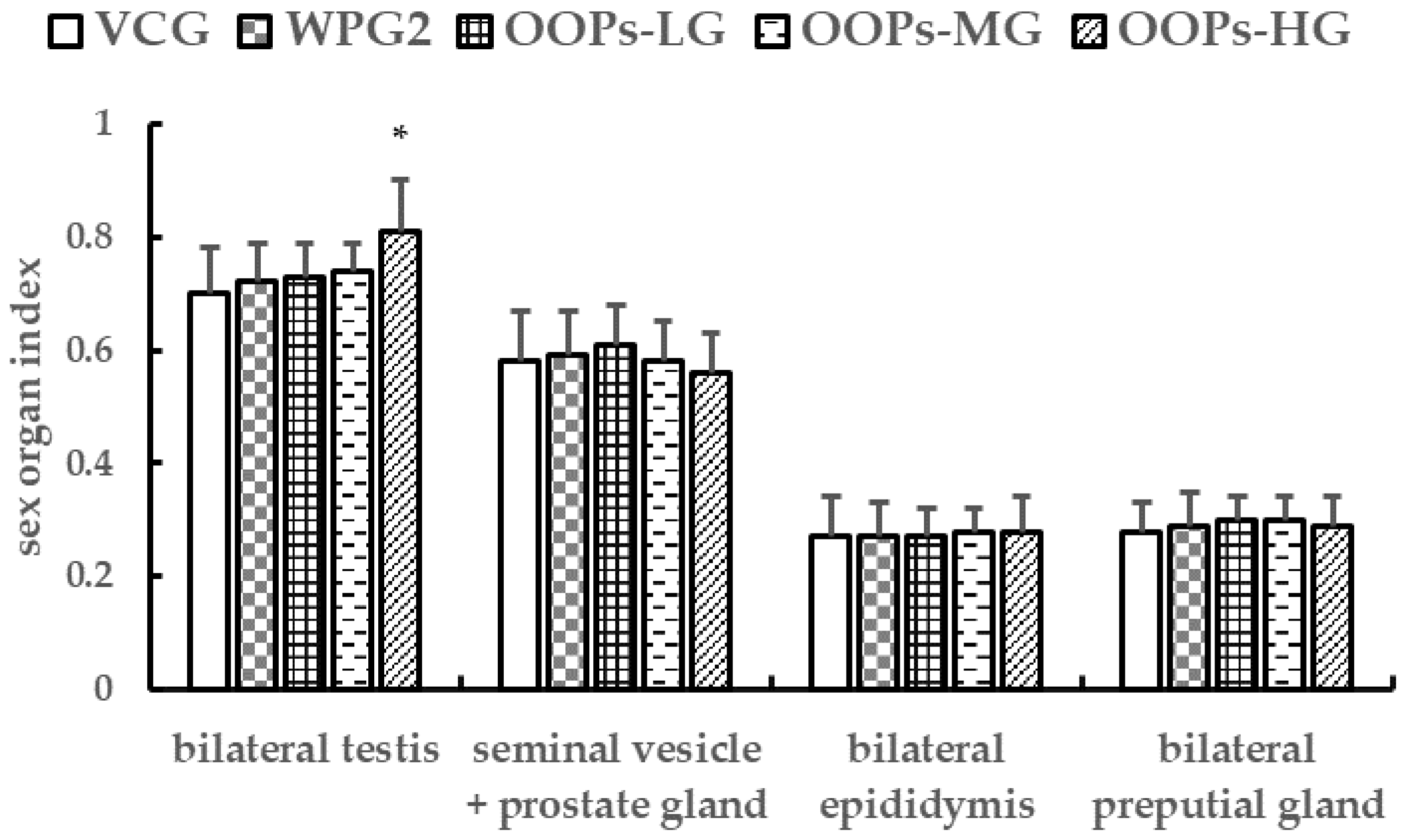
3.3.2. Effects of OOPs on Sex Organ Indexes in Male Mice
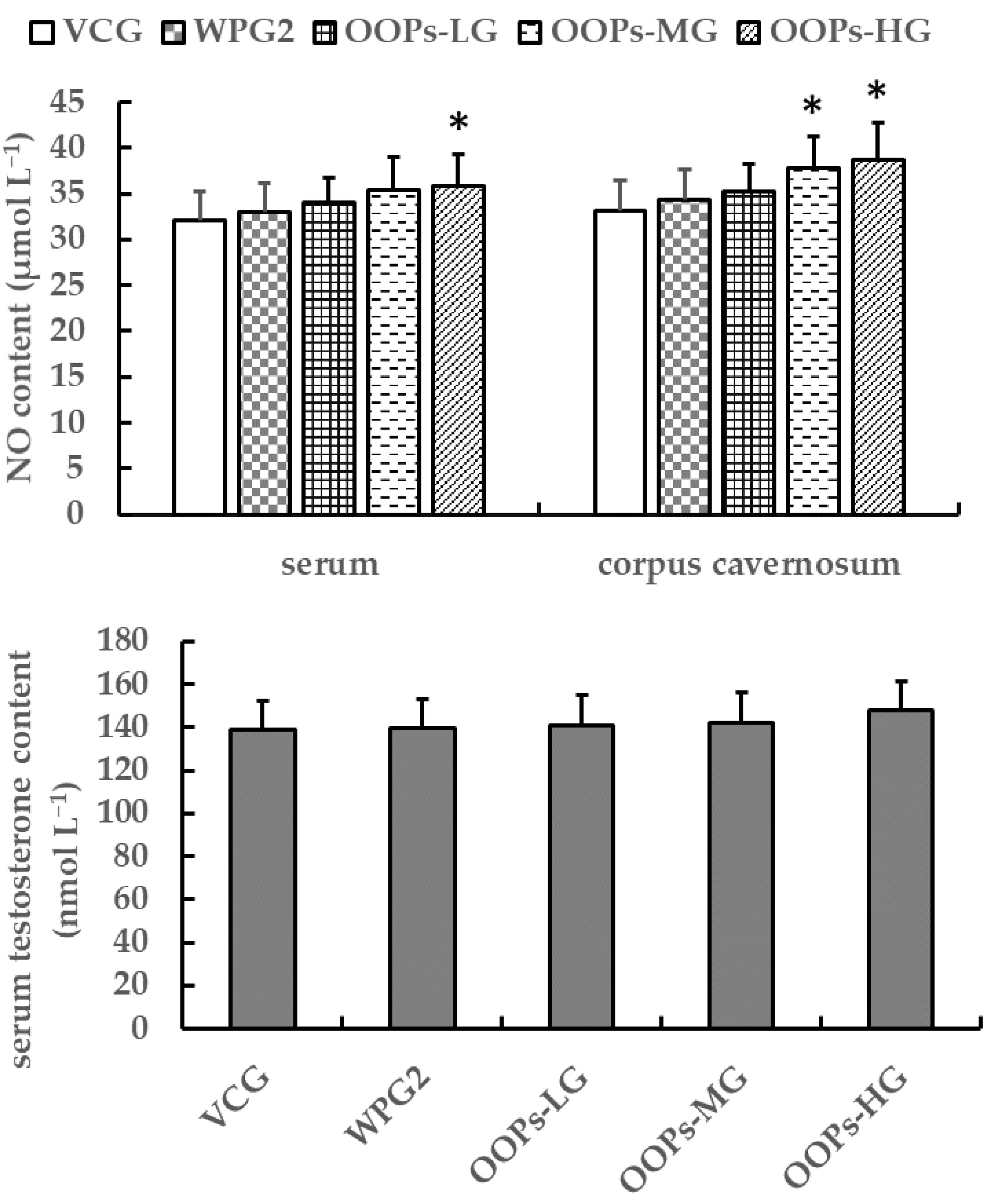
3.3.3. Effects of OOPs on NO and Testosterone Contents in Male Mice
3.4. Combined Effects of GOPs and OOPs on Sexual Function in Male Mice
3.4.1. Combined Effects of GOPs and OOPs on Sexual Behavior in Male Mice
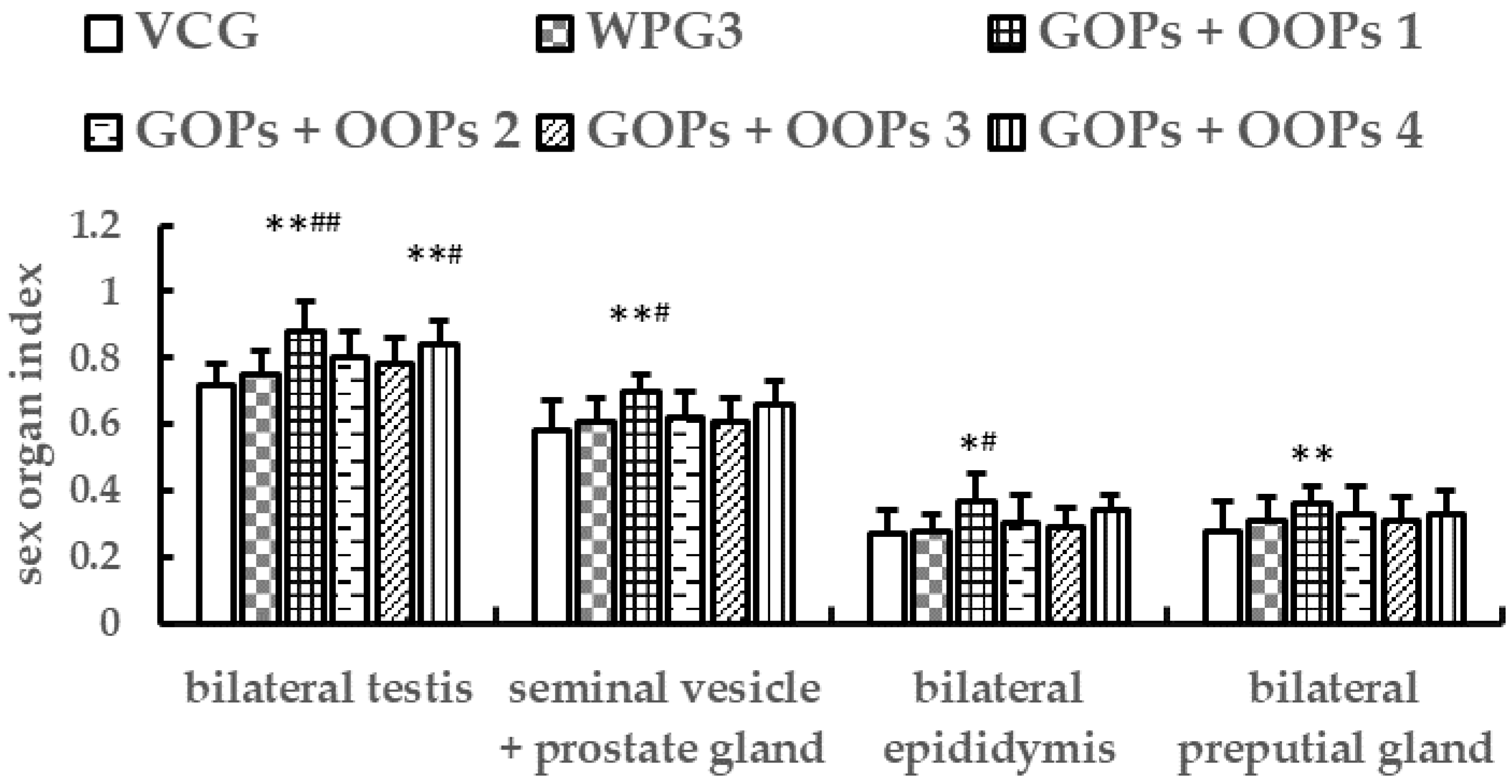
3.4.2. Combined Effects of GOPs and OOPs on Sex Organ Indexes in Male Mice
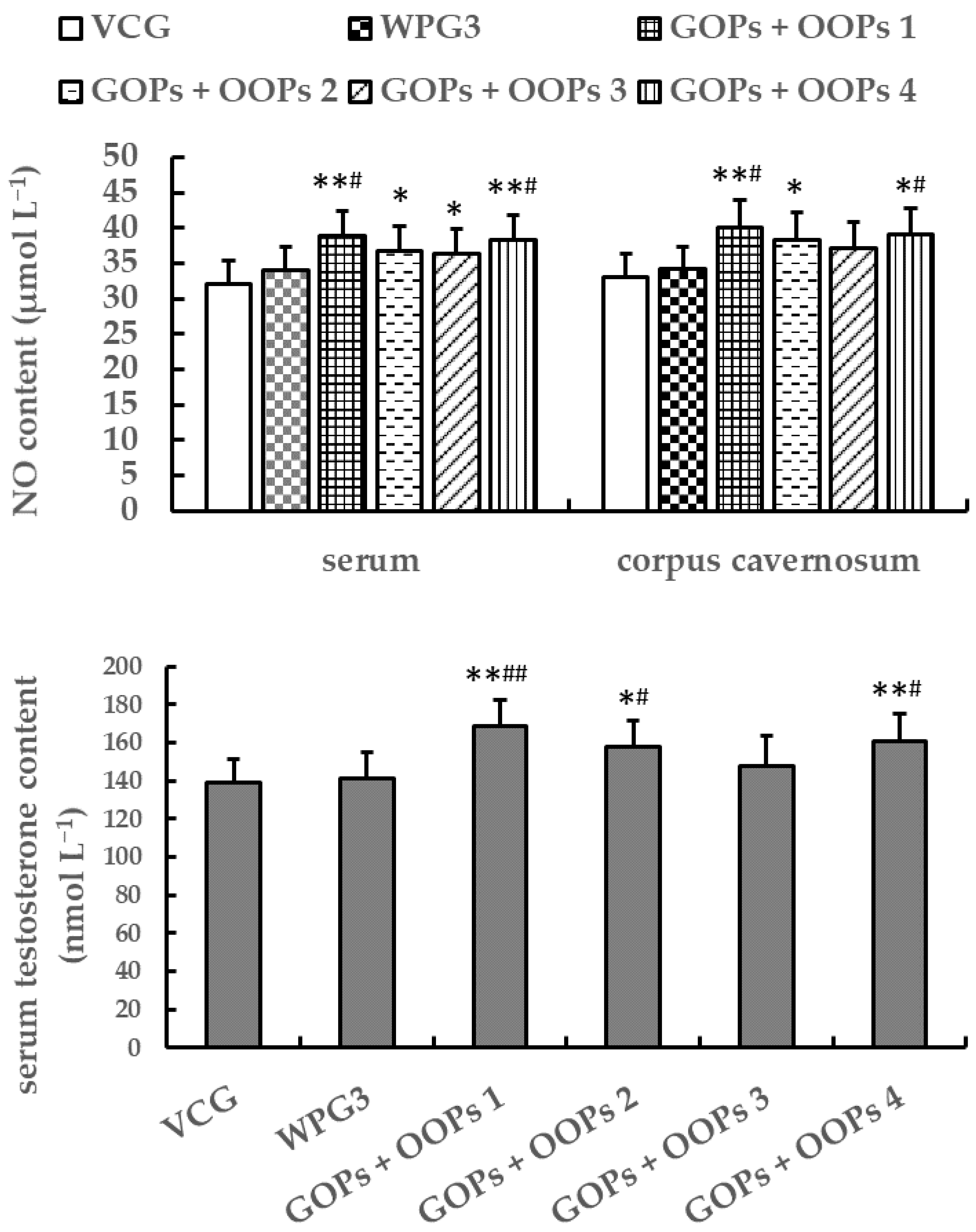
3.4.3. Combined Effects of GOPs and OOPs on NO and Testosterone Contents in Male Mice
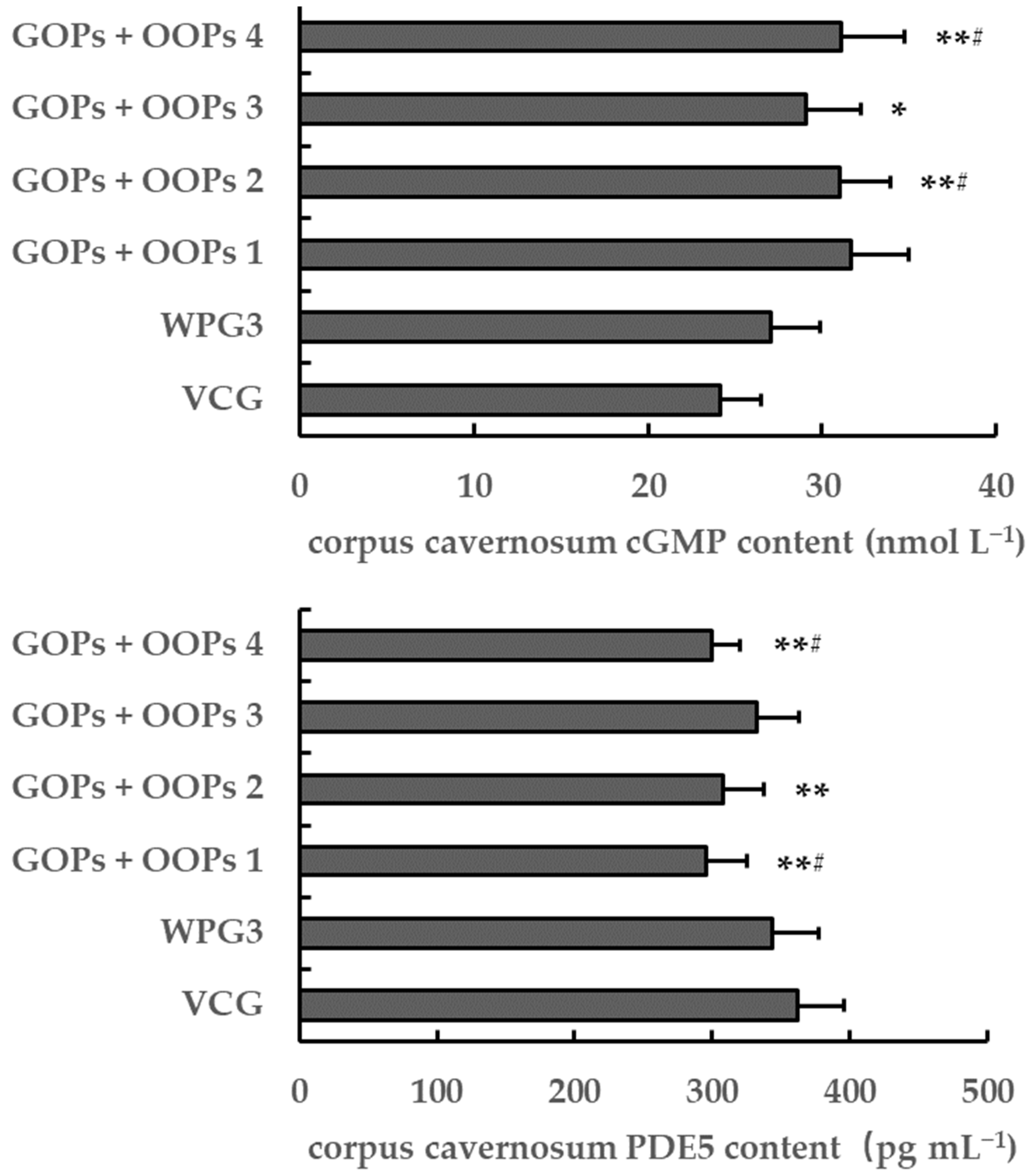
3.4.4. Combined Effects of GOPs and OOPs on Corpus Cavernosum cGMP and PDE5 Content in Male Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casulari, L.A.; Caldas, A.D.; Domingues, C.M.L.; Lofrano-Porto, A. Effects of metformin and short-term lifestyle modification on the improvement of male hypogonadism associated with metabolic syndrome. Minerva Endocrinol. 2010, 35, 145–151. [Google Scholar]
- Lasaite, L.; Ceponis, J.; Preiksa, R.T.; Zilaitiene, B. Effects of two-year testosterone replacement therapy on cognition, emotions and quality of life in young and middle-aged hypogonadal men. Andrologia 2017, 49, e12633. [Google Scholar] [CrossRef] [PubMed]
- Nian, Y.; Ding, M.; Hu, S.; He, H.; Cheng, S.; Yi, L.; Li, Y.; Wang, Y. Testosterone replacement therapy improves health-related quality of life for patients with late-onset hypogonadism: A meta-analysis of randomized controlled trials. Andrologia 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Tirabassi, G.; Delli, M.N.; Corona, G.; Maggi, M.; Balercia, G. Androgen receptor gene CAG repeat polymorphism independently influences recovery of male sexual function after testosterone replacement therapy in postsurgical hypogonadotropic hypogonadism. J. Sex Med. 2014, 11, 1302–1308. [Google Scholar] [CrossRef]
- Dimopoulou, C.; Ceausu, I.; Depypere, H.; Lambrinoudaki, I.; Mueck, A.; Pérez-López, F.R.; Rees, M.; Yt, V.D.S.; Senturk, L.M.; Simonsini, T. EMAS position statement: Testosterone replacement therapy in the aging male. Maturitas 2016, 84, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.M.; Munder, T.; Gerger, H.; Fruhauf, S.; Barth, J. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: A narrative review and meta-analysis. J. Sex Med. 2014, 11, 1376–1391. [Google Scholar] [CrossRef]
- Goswami, S.K.; Inamdar, M.N.; Jamwal, R.; Dethe, S. Effect of Cinnamomum cassia methanol extract and sildenafil on arginase and sexual function of young male Wistar rats. J. Sex Med. 2014, 11, 1475–1483. [Google Scholar] [CrossRef]
- Naccarato, A.M.; Reis, L.O.; Ferreira, U.; Denardi, F. Psychotherapy and phosphodiesterase-5 inhibitor in early rehabilitation after radical prostatectomy: A prospective randomised controlled trial. Andrologia 2016, 48, 1183–1187. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, D.; Yang, L.; Tang, C.; Yang, T.; Hu, X.; Shen, H. Efficacy of Phosphodiesterase-5 Inhibitor in Men With Premature Ejaculation: A New Systematic Review and Meta-analysis. Urology 2015, 86, 947–954. [Google Scholar] [CrossRef]
- Bai, W.J.; Li, H.J.; Dai, Y.T.; He, X.Y.; Huang, Y.R.; Liu, J.H.; Sorsaburu, S.; Ji, C.; Jin, J.J.; Wang, X.F. An open-label, multicenter, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in Chinese men naive to phosphodiesterase 5 inhibitor therapy. Asian J. 2015, 17, 61–67. [Google Scholar]
- Okada, K.; Yamaguchi, K.; Chiba, K.; Miyake, H.; Fujisawa, M. Comprehensive evaluation of androgen replacement therapy in aging Japanese men with late-onset hypogonadism. Aging Male 2014, 17, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Ishikawa, T.; Chiba, K.; Fujisawa, M. Assessment of possible effects for testosterone replacement therapy in men with symptomatic late-onset hypogonadism. Andrologia 2011, 43, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Dai, D.Z.; Zhang, Q.; Cheng, Y.S.; Dai, Y. Upregulated NADPH oxidase contributes to diabetic testicular complication and is relieved by strontium fructose 1,6-diphosphate. Exp. Clin. Endocrinol. Diabetes 2010, 118, 459–465. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.; Wang, J.; Zhang, Y.; Sun, B.; Li, Y. Anti-Fatigue Effects of Small Molecule Oligopeptides Isolated from Panax ginseng C. A. Meyer in Mice. Nutrients 2016, 8, 807. [Google Scholar] [CrossRef]
- He, L.X.; Ren, J.W.; Liu, R.; Chen, Q.H.; Zhao, J.; Wu, X.; Zhang, Z.F.; Wang, J.B.; Pettinato, G.; Li, Y. Ginseng (Panax ginseng Meyer) oligopeptides regulate innate and adaptive immune responses in mice via increased macrophage phagocytosis capacity, NK cell activity and Th cells secretion. Food Funct. 2017, 8, 3523–3532. [Google Scholar] [CrossRef]
- Jiao, L.; Li, B.; Wang, M.; Liu, Z.; Zhang, X.; Liu, S. Antioxidant activities of the oligosaccharides from the roots, flowers and leaves of Panax ginseng C.A. Meyer. Carbohydr. Polym. 2014, 106, 293–298. [Google Scholar] [CrossRef]
- Ramesh, T.; Kim, S.W.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr. Res. 2012, 32, 718–726. [Google Scholar] [CrossRef]
- Kim, H.G.; Yoo, S.R.; Park, H.J.; Lee, N.H.; Shin, J.W.; Sathyanath, R.; Cho, J.H.; Son, C.G. Antioxidant effects of Panax ginseng C.A. Meyer in healthy subjects: A randomized, placebo-controlled clinical trial. Food Chem. Toxicol. 2011, 49, 2229–2235. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.; Ha, E.; Yoon, S.; Kim, M.J.; Hong, M.; Leem, K.H.; Hong, S.J.; Yang, J.; Chung, J.H. Panax ginseng increases hypoxia-induced down-regulated cellular response related genes in human neuroblastoma cells, SK-N-MC. Neurol. Res. 2007, 29 (Suppl. 1), S78–S87. [Google Scholar] [CrossRef]
- Kang, S.; Min, H. Ginseng, the ’Immunity Boost’: The Effects of Panax ginseng on Immune System. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef]
- Jang, H.I.; Shin, H.M. Wild Panax ginseng (Panax ginseng C.A. Meyer) protects against methotrexate-induced cell regression by enhancing the immune response in RAW 264.7 macrophages. Am. J. Chin. Med. 2010, 38, 949–960. [Google Scholar] [CrossRef]
- Lee, L.S.; Cho, C.W.; Hong, H.D.; Lee, Y.C.; Choi, U.K.; Kim, Y.C. Hypolipidemic and antioxidant properties of phenolic compound-rich extracts from white ginseng (Panax ginseng) in cholesterol-fed rabbits. Molecules 2013, 18, 12548–12560. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, K.S. Effects of Panax ginseng extract on lipid metabolism in humans. Pharm. Res. 2003, 48, 511–513. [Google Scholar] [CrossRef]
- Bella, A.J.; Shamloul, R. Traditional plant aphrodisiacs and male sexual dysfunction. Phytother. Res. 2014, 28, 831–835. [Google Scholar] [CrossRef]
- Morgante, G.; Scolaro, V.; Tosti, C.; Di Sabatino, A.; Piomboni, P.; De Leo, V. Treatment with carnitine, acetyl carnitine, L-arginine and ginseng improves sperm motility and sexual health in men with asthenopermia. Minerva Urol. Nefrol. 2010, 62, 213–218. [Google Scholar]
- Kim, T.H.; Jeon, S.H.; Hahn, E.J.; Paek, K.Y.; Park, J.K.; Youn, N.Y.; Lee, H.L. Effects of tissue-cultured mountain ginseng (Panax ginseng CA Meyer) extract on male patients with erectile dysfunction. Asian J. 2009, 11, 356–361. [Google Scholar] [CrossRef]
- Murphy, L.L.; Lee, T.J. Ginseng, sex behavior, and nitric oxide. Ann. NY Acad. Sci. 2002, 962, 372–377. [Google Scholar] [CrossRef]
- Wang, X.; Chu, S.; Qian, T.; Chen, J.; Zhang, J. Ginsenoside Rg1 improves male copulatory behavior via nitric oxide/cyclic guanosine monophosphate pathway. J. Sex Med. 2010, 7, 743–750. [Google Scholar] [CrossRef]
- Liu, T.; Peng, Y.F.; Jia, C.; Yang, B.H.; Tao, X.; Li, J.; Fang, X. Ginsenoside Rg3 improves erectile function in streptozotocin-induced diabetic rats. J. Sex Med. 2015, 12, 611–620. [Google Scholar] [CrossRef]
- Wong, A.S.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef]
- Ge, X.; Low, M.Y.; Zou, P.; Lin, L.; Yin, S.O.; Bloodworth, B.C.; Koh, H.L. Structural elucidation of a PDE-5 inhibitor detected as an adulterant in a health supplement. J. Pharm. Biomed Anal. 2008, 48, 1070–1075. [Google Scholar] [CrossRef]
- Ferraz, M.R.; Ferraz, M.M.; Santos, R. How REM sleep deprivation and amantadine affects male rat sexual behavior. Pharm. Biochem. Behav. 2001, 69, 325–332. [Google Scholar] [CrossRef]
- Allen, D.S.; Martin, K.; Craig, N. Critical update of the 2010 endocrine society clinical practice guidelines for male hypogonadism: A systematic analysis. Mayo Clin. Proc. 2015, 90, 1104–1115. [Google Scholar]
- Ferraz, M.M.; Sab, I.M.; Silva, M.A.; Santos, D.A.; Ferraz, M.R. Prenatal Hypoxia Ischemia Increases Male Rat Sexual Behavior. J. Sex Med. 2015, 12, 2013–2021. [Google Scholar] [CrossRef]
- Gresham, R.; Li, S.; Adekunbi, D.A.; Hu, M.; Li, X.F.; O’Byrne, K.T. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci. Lett. 2016, 627, 13–17. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, A.; Yang, S.; Wang, Y.; Goswami, R.; Zhou, H.; Zhang, Y.; Wang, Z.; Li, R.; Cheng, Q.; et al. Combined effects of sex hormone-binding globulin and sex hormones on risk of incident type 2 diabetes. J. Diabetes 2016, 8, 508–515. [Google Scholar] [CrossRef]
- Anderson, R.A.; Bancroft, J.; Wu, F.C. The effects of exogenous testosterone on sexuality and mood of normal men. J. Clin. Endocrinol. Metab. 1992, 75, 1503–1507. [Google Scholar] [PubMed]
- Andersson, K.E. Pharmacology of penile erection. Pharm. Rev. 2001, 53, 417–450. [Google Scholar] [PubMed]
- Lagoda, G.; Muschamp, J.W.; Vigdorchik, A.; Hull, E.M. A nitric oxide synthesis inhibitor in the medial preoptic area inhibits copulation and stimulus sensitization in male rats. Behav. Neurosci. 2004, 118, 1317–1323. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.B.; Ma, C.G.; Liu, T.; Li, W.R.; Gong, Y.Q.; Xin, Z.C. Icarisid II, a PDE5 inhibitor from Epimedium wanshanense, increases cellular cGMP by enhancing NOS in diabetic ED rats corpus cavernosum tissue. Andrologia 2012, 44 (Suppl. 1), 87–93. [Google Scholar] [CrossRef]
- Taher, A.; Meyer, M.; Stief, C.G.; Jonas, U.; Forssmann, W.G. Cyclic nucleotide phosphodiesterase in human cavernous smooth muscle. World J. Urol. 1997, 15, 32–35. [Google Scholar] [CrossRef] [PubMed]
| OOPs (mg kg−1) | Mount Latency | Mount Frequency | Intromission Frequency | |||
|---|---|---|---|---|---|---|
| 15 days | 30 days | 15 days | 30 days | 15 days | 30 days | |
| 160.0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 320.0 | 0.000 | 0.025 | 0.065 | 0.106 | 0.064 | 0.200 |
| GOPs (mg kg−1) | Mount Latency | Mount Frequency | Intromission Frequency | |||
|---|---|---|---|---|---|---|
| 15 days | 30 days | 15 days | 30 days | 15 days | 30 days | |
| 62.5 | 0.000 | 0.002 | 0.000 | 0.001 | 0.000 | 0.005 |
| 125.0 | 0.000 | 0.000 | 0.000 | 0.023 | 0.003 | 0.014 |
| OOPs (mg kg−1) | Bilateral Testes Index | Seminal Vesicle + Prostate Gland Index |
|---|---|---|
| 160.0 | 0.045 | 0.027 |
| 320.0 | 0.382 | 0.404 |
| GOPs (mg kg−1) | Bilateral Testes Index | Seminal Vesicle + Prostate Gland Index |
|---|---|---|
| 62.5 | 0.098 | 0.048 |
| 125.0 | 0.209 | 0.270 |
| OOPs (mg kg−1) | p |
|---|---|
| 160.0 | 0.016 |
| 320.0 | 0.739 |
| GOPs (mg kg−1) | p |
|---|---|
| 62.5 | 0.189 |
| 125.0 | 0.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Ren, J.; He, L.; Sun, J.; Liu, P.; Li, Y. Combined Effects of Oligopeptides Isolated from Panax ginseng C.A. Meyer and Ostrea gigas Thunberg on Sexual Function in Male Mice. Int. J. Environ. Res. Public Health 2021, 18, 2349. https://doi.org/10.3390/ijerph18052349
Li D, Ren J, He L, Sun J, Liu P, Li Y. Combined Effects of Oligopeptides Isolated from Panax ginseng C.A. Meyer and Ostrea gigas Thunberg on Sexual Function in Male Mice. International Journal of Environmental Research and Public Health. 2021; 18(5):2349. https://doi.org/10.3390/ijerph18052349
Chicago/Turabian StyleLi, Di, Jinwei Ren, Lixia He, Jingqin Sun, Peng Liu, and Yong Li. 2021. "Combined Effects of Oligopeptides Isolated from Panax ginseng C.A. Meyer and Ostrea gigas Thunberg on Sexual Function in Male Mice" International Journal of Environmental Research and Public Health 18, no. 5: 2349. https://doi.org/10.3390/ijerph18052349
APA StyleLi, D., Ren, J., He, L., Sun, J., Liu, P., & Li, Y. (2021). Combined Effects of Oligopeptides Isolated from Panax ginseng C.A. Meyer and Ostrea gigas Thunberg on Sexual Function in Male Mice. International Journal of Environmental Research and Public Health, 18(5), 2349. https://doi.org/10.3390/ijerph18052349






