Metabolic Syndrome: Prevalence and Risk Factors among Adolescent Female Intermediate and Secondary Students in Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeBoer, M.D. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Available online: www.idf.org/webdata/docs/Metabolic_syndrome_definition.pdf/ (accessed on 17 November 2019).
- Roberts, C.K.; Hevener ALBarnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [PubMed]
- Malik, S.; Wong, N.D.; Franklin, S.S.; Kamath, T.V.; L’Italien, G.J.; Pio, J.R.; Williams, G.R. Impact of the metabolic syndrome on mortali-ty from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004, 110, 1245–1250. [Google Scholar] [CrossRef]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Baur, L.; Uauy, R. Obesity in children and young people: A crisis in public health. Obes. Rev. 2004, 5, 4–85. [Google Scholar] [CrossRef]
- Friend, A.; Craig, L.; Turner, S. The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Preventing Suicide: A Global Imperative; WHO Press: Geneva, Switzerland, 2014; Available online: http://apps.who.int/iris/bitstream/10665/131056/1/9789241564779_eng.pdf?ua (accessed on 17 November 2019).
- Al-Enazy, W.H.; Al Dahi, S.K.; Al Hariri, I.M. Prevalence of overweight and obesity among Saudi primary school students in Tabuk, Saudi Arabia. SJO 2014, 2, 13. [Google Scholar]
- Al-Hussaini, A.; Bashir, M.S.; Khormi, M.; Alturaiki, M.; Alkhamis, W.; Alrajhi, M.; Halal, T. Overweight and obesity among Saudi children and adolescents: Where do we stand today? Saudi J. Gastroenterol. 2019, 25, 229–235. [Google Scholar] [CrossRef]
- Al-Rubeaan, K.; Bawazeer, N.; Al Farsi, Y.; Youssef, A.M.; Al-Yahya, A.A.; AlQumaidi, H.; Al-Malki, B.M.; Naji, K.A.; Al-Shehri, K.; Al Rumaih, F.I. Prevalence of metabolic syndrome in Saudi Arabia—A cross sectional study. BMC Endocr. Disord. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, N.J. Metabolic syndrome: Risk factors among adults in Kingdom of Saudi Arabia. J. Fam. Community Med. 2014, 21, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.A. Prevalence of Metabolic Syndrome and Its Components among Saudi Young Adults 18–30 Years of Age. Open J. Endocr. Metab. Dis. 2019, 9, 49–59. [Google Scholar] [CrossRef]
- Shin, S. Prevalence of Metabolic Syndrome in Saudi Arabia: A Meta-Analysis of Cross-sectional Studies. Ann. Med. Health Sci. Res. 2020, 10, 1148–1152. [Google Scholar]
- World Health Organization. The STEPS Instrument and Support Materials. 2020. Available online: https://www.who.int/ncds/surveillance/steps/instrument/en/ (accessed on 22 April 2020).
- El Mouzan, M.I.; Al Salloum, A.A.; Al Herbish, A.S.; Foster, P.J.; Qurashi, M.M.; Al Omar, A.A. The 2005 Growth Charts for Saudi Children and Adolescents (No. AR-20-63). King Abdulaziz City for Science and Technology 2009, Riyadh, KSA. Available online: https://www.moh.gov.sa/HealthAwareness/EducationalContent/BabyHealth/Documents/Intermediate%202%20Compatibility%20Mode.pdf (accessed on 19 November 2019).
- Al Herbish, A.S.; El Mouzan, M.I.; Al Salloum, A.A.; Al Qureshi, M.M.; Al Omar, A.A.; Foster, P.J.; Kecojevic, T. Body mass index in Saudi Arabian children and adolescents: A national reference and comparison with international standards. Ann. Saudi Med. 2009, 29, 342–347. [Google Scholar] [CrossRef][Green Version]
- Xavier, H.T.; Ruiz, R.M.; Kencis Jr, L.; Melone, G.; Costa, W.; Fraga, R.F.; Wajman, L.; Krakauer, M.; Scartezini, M. Clinical correlation between the Point-of-care testing method and the traditional clinical laboratory diagnosis in the measure of the lipid profile in patients seen in medical offices. J. Bras. Patol. Med. Lab. 2016, 52, 387–390. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Al-Nozha, M.; Al-Khadra, A.; Arafah, M.R.; A Al-Maatouq, M.; Khalil, M.Z.; Khan, N.B.; Al-Mazrou, Y.Y.; Al-Marzouki, K.; Al-Harthi, S.S.; Abdullah, M.; et al. Metabolic syndrome in Saudi Arabia. Saudi Med. J. 2005, 26, 1918–1925. [Google Scholar]
- Bahathiq, A. Metabolic syndrome in young Saudi females. J. Diabetes Metab. 2018, 9, 17. [Google Scholar]
- Al-Hussein, F.A.; Tamimi, W.; Al Banyan, E.; Al-Twaijri, Y.A.; Tamim, H. Cardiometabolic risk among Saudi children and adoles-cents: Saudi childrens overweight, obesity, and lifestyles (S.Ch.O.O.Ls) study. Ann. Saudi Med. 2014, 34, 46–53. [Google Scholar] [CrossRef]
- Haroun, D.; Mechli, R.; Sahuri, R.; Alkhatib, S.; Obeid, O.; El Mallah, C.; Wood, L.; Alsuwaidi, K. Metabolic syndrome among adolescents in Dubai, United Arab Emirates, is attributable to the high prevalence of low HDL levels: A cross-sectional study. BMC Public Health 2018, 18, 1284. [Google Scholar] [CrossRef]
- Burrows, R.; Correa-Burrows, P.; Reyes, M.; Blanco, E.; Albala, C.; Gahagan, S. High cardiometabolic risk in healthy Chilean ado-lescents: Associations with anthropometric, biological and lifestyle factors. Public Health Nutr. 2016, 19, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, J.H.; Kang, J.W.; Park, H.Y.; Park, J.; Shin, K.J. Prevalence of the metabolic syndrome and abnormal lipid levels among Korean adolescents. J. Paediatr. Child Health 2013, 49, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Tandon, N.; Garg, M.; Singh, Y.; Marwaha, R.K. Prevalence of metabolic syndrome among urban Indian adolescents and its relation with insulin resistance (HOMA-IR). J. Pediatr. Endocrinol. Metab. 2013, 26, 1123–1130. [Google Scholar] [CrossRef]
- Abolfotouh, M.A.; Al-Alwan, I.A.; Al-Rowaily, M.A. Prevalence of Metabolic Abnormalities and Association with Obesity among Saudi College Students. Int. J. Hypertens. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El Mouzan, M.I.; Al Herbish, A.S.; Al Salloum, A.A.; Al Omar, A.A.; Qurachi, M.M. Regional variation in prevalence of overweight and obesity in Saudi children and adolescents. Saudi J. Gastroenterol. 2012, 18, 129–132. [Google Scholar] [CrossRef]
- Al-Hazzaa, H.M.; Abahussain, N.A.; Al-Sobayel, H.I.; Qahwaji, D.M.; Alsulaiman, N.A.; Musaiger, A.O. Prevalence of Overweight, Obesity, and Abdominal Obesity among Urban Saudi Adolescents: Gender and Regional Variations. J. Health Popul. Nutr. 2014, 32, 634–645. [Google Scholar] [PubMed]
- Jia, P.; Li, M.; Xue, H.; Lu, L.; Xu, F.; Wang, Y. School environment and policies, child eating behavior and overweight/obesity in urban China: The childhood obesity study in China megacities. Int. J. Obes. 2017, 41, 1310. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; González-Ruíz, K.; Correa-Bautista, J.E.; Meneses-Echávez, J.F.; Martínez-Torres, J. Demographic and socioeco-nomic differences in consumption of sugar-sweetened beverages among Colombian children and adolescents. Nutr Hosp. 2015, 31, 2479–2486. [Google Scholar]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardi-ovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence From Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Bomback, A.S.; Derebail, V.K.; Shoham, D.A.; Anderson, C.A.; Steffen, L.M.; Rosamond, W.D.; Kshirsagar, A.V. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010, 77, 609–616. [Google Scholar] [CrossRef]
- Bernabé, E.; Vehkalahti, M.M.; Sheiham, A.; Aromaa, A.; Suominen, A.L. Sugar-sweetened beverages and dental caries in adults: A 4-year prospective study. J. Dent. 2014, 42, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Fan, H.; Tian, X. Rising food accessibility contributed to the increasing dietary diversity in rural and urban China. Asia Pac. J. Clin. Nutr. 2017, 26, 738–747. [Google Scholar]
- Kerry, S.M.; Emmett, L.; Micah, F.B.; Martin-Peprah, R.; Antwi, S.; Phillips, R.O.; Plange-Rhule, J.; Eastwood, J.B.; Cappuccio, F.P. Rural and semi-urban differences in salt intake, and its dietary sources, in Ashanti, West Africa. Ethn. Dis. 2005, 15, 33–39. [Google Scholar]
- Johnson, J.A., 3rd; Johnson, A.M. Urban-rural differences in childhood and adolescent obesity in the United States: A systematic review and meta-analysis. Child Obes. 2015, 11, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, Y.; Kim, Y. Risk of Metabolic Syndrome among Middle-Aged Koreans from Rural and Urban Areas. Nutrients 2018, 10, 859. [Google Scholar] [CrossRef]
- Lim, S.; Jang, H.C.; Lee, H.K.; Kimm, K.C.; Park, C.; Cho, N.H. A rural-urban comparison of the characteristics of the metabolic syn-drome by gender in Korea: The Korean Health and Genome Study (KHGS). J. Endocrinol. Investig. 2006, 29, 313–319. [Google Scholar] [CrossRef]
- Van Zyl, S.; Van der Merwe, L.J.; Walsh, C.M.; Groenewald, A.J.; Van Rooyen, F.C. Risk-factor profiles for chronic diseases of lifestyle and metabolic syndrome in an urban and rural setting in South Africa. Afr. J. Prim Health Care Fam. Med. 2012, 4, 346. [Google Scholar] [CrossRef]
- Opoku, S.; Gan, Y.; Fu, W.; Chen, D.; Addo-Yobo, E.; Trofimovitch, D.; Yue, W.; Yan, F.; Wang, Z.; Lu, Z. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: Findings from the China National Stroke Screening and prevention project (CNSSPP). BMC Public Health 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Trivedi, T.; Liu, J.; Probst, J.C.; Martin, A.B. The Metabolic Syndrome: Are Rural Residents at Increased Risk? J. Rural. Health 2012, 29, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Kusama, K.; Kanha, S.; Yoshiike, N.; the FIDR Research and the FIDR Research Team. Urban-Rural Differences in Nutritional Status and Dietary Intakes of School-Aged Children in Cambodia. Nutrients 2018, 11, 14. [Google Scholar] [CrossRef]
- Le Nguyen, B.K.; Le Thi, H.; Nguyen Do, V.A.; Tran Thuy, N.; Nguyen Huu, C.; Thanh Do, T.; Deurenberg, P.; Khouw, I. Double bur-den of undernutrition and overnutrition in Vietnam in 2011: Results of the SEANUTS study in 0•5–11-year-old children. Br. J. Nutr. 2013, 110 (Suppl. 3), S45–S56. [Google Scholar] [CrossRef]
- Sandjaja, S.; Budiman, B.; Harahap, H.; Ernawati, F.; Soekatri, M.; Widodo, Y.; Sumedi, E.; Rustan, E.; Sofia, G.; Syarief, S.N.; et al. Food consumption and nutritional and biochemical status of 0·5–12-year-old Indonesian children: The SEANUTS study. Br. J. Nutr. 2013, 110, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Nurwanti, E.; Hadi, H.; Chang, J.S.; Chao, J.C.; Paramashanti, B.A.; Gittelsohn, J.; Bai, C.H. Rural-Urban Differences in Dietary Be-havior and Obesity: Results of the Riskesdas Study in 10-18-Year-Old Indonesian Children and Adolescents. Nutrients 2019, 11, 2813. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Liu, Y.; Ma, J.; Wang, W.; Yang, G.; Caballero, B. An urban-rural comparison of the prevalence of the metabolic syn-drome in Eastern China. Public Health Nutr. 2007, 10, 131–136. [Google Scholar] [CrossRef]
- Taha, D.; Ahmed, O.; Bin Sadiq, B. The prevalence of metabolic syndrome and cardiovascular risk factors in a group of obese Saudi children and adolescents: A hospital-based study. Ann. Saudi Med. 2009, 29, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Dalleck, L.C.; Kjelland, E.M. The prevalence of metabolic syndrome and metabolic syndrome risk factors in college-aged stu-dents. Am. J. Health Promot. 2012, 27, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.; Ma, S.; Cutter, J.; Chew, S.; Tan, C.; Tai, E. The metabolic syndrome in Chinese, Malays and Asian IndiansFactor analysis of data from the 1998 Singapore National Health Survey. Diabetes Res. Clin. Pr. 2005, 67, 53–62. [Google Scholar] [CrossRef]
- Kelishadi, R.; Ardalan, G.; Adeli, K.; Motaghian, M.; Majdzadeh, R.; Mahmood-Arabi, M.S.; Delavari, A.; Riazi, M.M.; Namazi, R.; Ramezani, M.A. Factor Analysis of Cardiovascular Risk Clustering in Pediatric Metabolic Syndrome: CASPIAN Study. Ann. Nutr. Metab. 2007, 51, 208–215. [Google Scholar] [CrossRef]
- Lambert, M.; Paradis, G.; O’Loughlin, J.; E Delvin, E.; A Hanley, J.; Levy, E. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. Int. J. Obes. 2004, 28, 833–841. [Google Scholar] [CrossRef]
- Goodman, E.; Dolan, L.M.; Morrison, J.A.; Daniels, S.R. Factor analysis of clustered cardiovascular risks in adolescence: Obesity is the predominant correlate of risk among youth. Circulation 2005, 111, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.L.; Weigensberg, M.J.; Huang, T.T.-K.; Ball, G.; Shaibi, G.Q.; Goran, M.I. The Metabolic Syndrome in Overweight Hispanic Youth and the Role of Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2004, 89, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Khashayar, P.; Heshmat, R.; Qorbani, M.; Motlagh, M.E.; Aminaee, T.; Ardalan, G.; Farrokhi-Khajeh-Pasha, Y.; Taslimi, M.; Larijani, B.; Kelishadi, R. Metabolic Syndrome and Cardiovascular Risk Factors in a National Sample of Adolescent Population in the Middle East and North Africa: The CASPIAN III Study. Int. J. Endocrinol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Caceres, M.; Teran, C.G.; Rodríguez, S.; Medina, M. Prevalence of insulin resistance and its association with metabolic syndrome criteria among Bolivian children and adolescents with obesity. BMC Pediatr. 2008, 8, 31. [Google Scholar] [CrossRef]
- Agirbasli, M.; Cakir, S.; Özme, S.; Ciliv, G. Metabolic syndrome in Turkish children and adolescents. Metabolism 2006, 55, 1002–1006. [Google Scholar] [CrossRef]
- Ferreira, A.P.; Oliveira, C.E.R.; França, N.M. Metabolic syndrome and risk factors for cardiovascular disease in obese children: The relationship with insulin resistance (HOMA-IR). J. Pediatr. 2007, 83, 21–26. [Google Scholar] [CrossRef]
- Irwin, C.E. Somatic growth and development during adolescent. In Pediatrics, 3rd ed.; Rudolph, A.M., Ed.; Appleton & Lange: East Norwalk, CO, USA, 1991; p. 39. [Google Scholar]
- Damiri, B.; Abualsoud, M.S.; Samara, A.M.; Salameh, S.K. Metabolic syndrome among overweight and obese adults in Palestinian refugee camps. Diabetol. Metab. Syndr. 2018, 10, 34. [Google Scholar] [CrossRef] [PubMed]
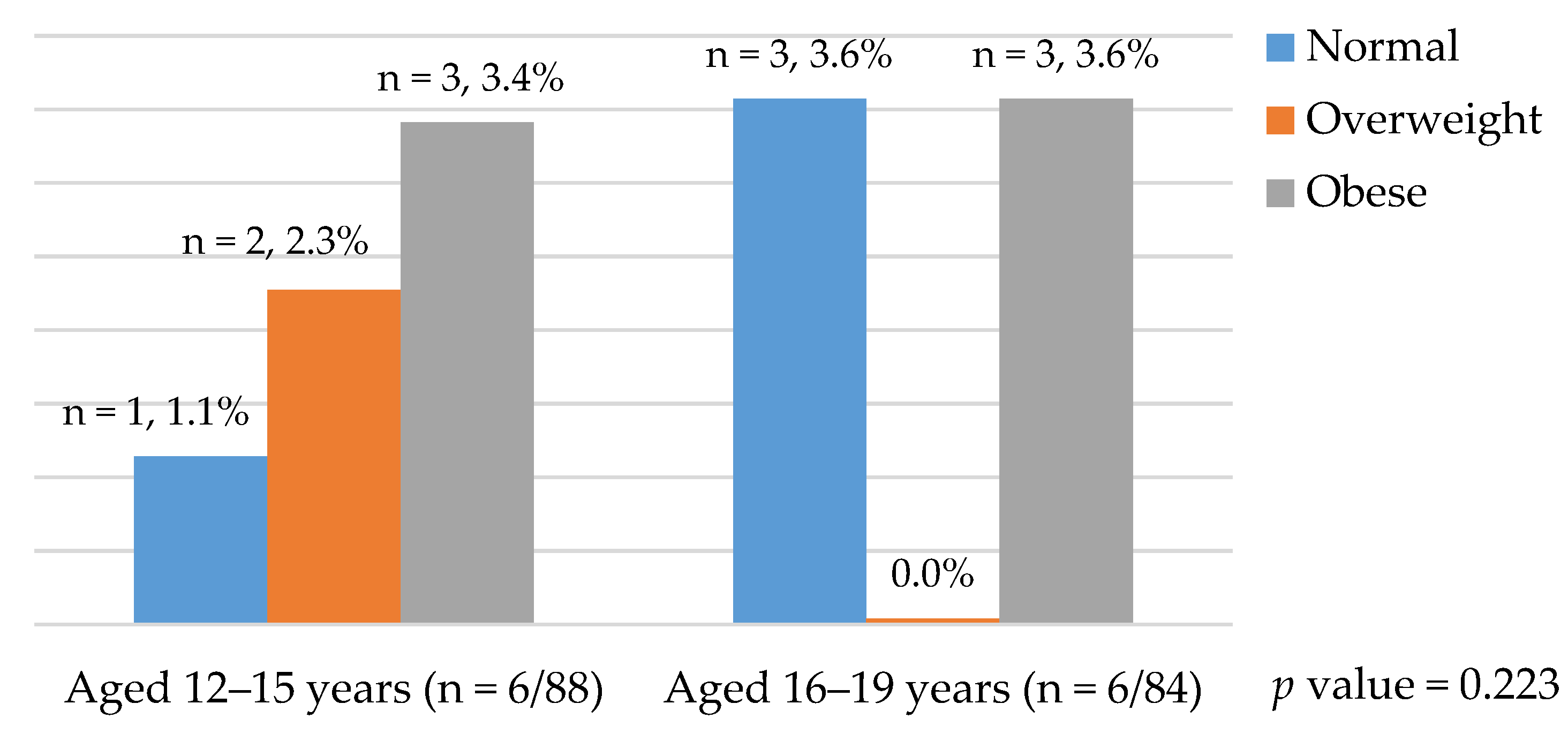
| Variables | Age | BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| 12–15 n = 88 | 16–19 n = 84 | p Value | Underweight n = 3 | Normal n = 125 | Overweight n = 24 | Obese n = 20 | p Value | |
| Waist circumference (cm) | 78.15 (12.71) | 78.92 (12.71) | 0.680 | 68.33 (14.04) | 74.23 (8.43) | 88.79 (8.29) | 94.55 (13.85) | 0.000 * |
| Weight (kg) | 55.40 (11.02) | 55.99 (16.87) | 0.786 | 36.00 (8.00) | 50.47 (7.81) | 65.10 (5.68) | 80.00 (19.55) | 0.000 * |
| Height (cm) | 154.35 (5.69) | 152.21 (14.53) | 0.203 | 152.67 (14.74) | 154.16 (6.88) | 155.04 (5.25) | 145.93 (25.49) | 0.269 |
| BMI (kg/m2) | 23.13 (4.30) | 23.96 (8.02) | 0.396 | 15.31 (.98) | 21.12 (2.49) | 27.07 (1.80) | 35.65 (10.14) | 0.000 * |
| Systolic Blood Pressure (mmHg) | 107.35 (11.43) | 107.49 (14.39) | 0.941 | 107.0 (9.54) | 106.35 (13.53) | 107.08 (11.15) | 114.50 (9.34) | 0.075 |
| Diastolic Blood Pressure (mmHg) | 75.22 (10.55) | 75.38 (11.18) | 0.921 | 81.00 (2.65) | 74.05 (11.55) | 77.20 (9.38) | 79.90 (6.01) | 0.076 |
| Fasting Plasma Glucose (mg/dL) | 100.69 (12.35) | 105.77 (39.83) | 0.256 | 96.67 (18.93) | 104.68 (33.29) | 99.00 (13.69) | 99.75 (11.53) | 0.746 |
| Triglycerides (mg/dL) | 89.36 (40.32) | 88.37 (41.52) | 0.874 | 69.67 (4.16) | 88.10 (43.58) | 86.50 (29.09) | 99.45 (36.99) | 0.548 |
| High-Density Lipoprotein (mg/dL) | 55.13 (12.06) | 52.44 (12.96) | 0.161 | 48.00 (3.00) | 54.46 (11.75) | 53.45 (14.93) | 51.05 (14.97) | 0.581 |
| Low-Density Lipoprotein (mg/dL) | 59.90 (23.32) | 61.55 (25.89) | 0.661 | 49.67 (23.80) | 59.58 (24.43) | 66.17 (18.98) | 62.85 (31.00) | 0.533 |
| Variables | Elevated WC n (%) | p | Elevated BP n (%) | p | High FPG n (%) | p | High TG n (%) | p | Low HDL n (%) | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 172) | 74 (43.02%) | 25 (14.53%) | 85 (49.41%) | 9 (5.23%) | 17 (9.88%) | |||||
| Age | ||||||||||
| 12–15 (n = 88) | 37 (42.00%) | 0.791 | 12 (13.64%) | 0.732 | 45 (51.14%) | 0.645 | 5 (5.68%) | 0.787 | 8 (9.09%) | 0.721 |
| 16–19 (n = 84) | 37 (44.04%) | 13 (15.48%) | 40 (47.62%) | 4 (4.76%) | 9 (10.71%) | |||||
| BMI | ||||||||||
| Normal (n = 125) | 34 (27.20%) * | 0.000 | 15 (12.00%) * | 0.046 | 62 (49.60%) | 0.757 | 7 (5.60%) | 0.968 | 9 (7.20%) * | 0.015 |
| Underweight (n = 3) | 1 (33.33%) | 0 (0.00%) | 2 (66.67%) | 0 (0.00%) | 0 (0.00%) | |||||
| Overweight (n = 24) | 21 (87.50%) * | 3 (12.50%) | 10 (41.67%) | 1 (4.16%) | 2 (8.33%) | |||||
| Obese (n = 20) | 18 (90.00%) * | 7 (35.00%) * | 11 (55.00%) | 1 (5.00%) | 6 (20.00%) * |
| Variables | No. of Samples | Number of Adolescents with Metabolic Syndrome n (%) | p-Value |
|---|---|---|---|
| Total | 172 | 12 (7.0%) | |
| Age (years) 12–15 | 88 (51.16%)) | 6 (6.89%) | 0.933 |
| 16–19 | 84 (48.84%) | 6 (7.14%) | |
| BMI underweight (<5th Percentile) | 3 (1.74%) | 0 (0.0%) | 0.000 |
| Normal (5th to <85th percentile) | 125 (72.68%) | 4 (3.20%) * | |
| Overweight (85th to <95th percentile) | 24 (13.95%) | 2 (8.33%) | |
| Obese (≥95th percentile) | 20 (11.63%) | 6 (30.0%) * | |
| Parental history of diabetes mellitus | |||
| Yes | 40 (23.26%) | 5 (12.5%) | 0.118 |
| No | 132 (76.74%) | 7 (5.30%) | |
| Parental history of hypertension | |||
| Yes | 50 (29.07%) | 5 (10%) | 0.319 |
| No | 122 (70.93%) | 7 (5.74%) | |
| Parental history of high cholesterol level | |||
| Yes | 34 (19.77%) | 4 (11.76%) | 0.221 |
| No | 138 (80.23%) | 8 (5.80%) | |
| Parental obesity | |||
| Yes | 26 | 2 (7.69%) | 0.876 |
| No | 146 | 10 (6.85%) | |
| Fast food consumption (daily) | |||
| No | 16 | 1 (6.25%) | 0.609 |
| Sometimes | 115 | 10 (8.70%) | |
| Always | 40 | 1 (2.5%) | |
| Weekly exercise | |||
| Yes | 71 | 4 (5.63%) | 0.562 |
| No | 101 | 8 (7.92%) |
| Variables | 0 Risk Factors | 1 Risk Factor | 2 Risk Factors | 3 Risk Factors | 4 Risk Factors | 5 Risk Factors | p-Value |
|---|---|---|---|---|---|---|---|
| Total (n = 172) | 35 (20.35%) | 82 (4.67%) | 42 (24.41%) | 9 (5.23%) | 4 (2.33%) | 0 (0.00%) | |
| Age 12–15 (n = 88) | 20 (22.73%) | 44 (50.00%) | 23 (26.14%) | 6 (6.82%) | 3 (3.41%) | 0 (0.00%) | 0.487 |
| 16–19 (n = 84) | 15 (17.86%) | 38 (45.24%) | 19 (22.62%) | 3 (3.57%) | 1 (1.19%) | 0 (0.00%) | |
| Waist circumference | |||||||
| Normal (<80 cm) (n = 98) | 35 (35.71%) | 56 (57.14%) * | 7 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0.000 |
| High (≥80 cm) (n = 74) | 0 (0.00%) | 26 (35.14%) | 35 (47.30%) * | 9 (12.16%) * | 4 (5.41%) * | 0 (0.00%) | |
| BMI | |||||||
| Normal (n = 125) | 33 (26.40%) | 63 (50.40%) | 25 (20.00%) | 3 (2.40%) | 1 (0.80%) | 0 (0.00%) | 0.000 |
| Underweight (n = 3) | 0 (0.00%) | 3 (100%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Overweight (n = 24) | 2 (8.33%) | 10 (41.67%) | 9 (37.50%) | 3 (12.50%) | 0 (0.00%) | 0 (0.00%) | |
| Obese (n = 20) | 0 (0.00%) | 6 (30.00%) | 8 (40.00%) | 3 (15.00%) | 3 (15.00%) * | 0 (0.00%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alowfi, A.; Binladen, S.; Irqsous, S.; Khashoggi, A.; Khan, M.A.; Calacattawi, R. Metabolic Syndrome: Prevalence and Risk Factors among Adolescent Female Intermediate and Secondary Students in Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 2142. https://doi.org/10.3390/ijerph18042142
Alowfi A, Binladen S, Irqsous S, Khashoggi A, Khan MA, Calacattawi R. Metabolic Syndrome: Prevalence and Risk Factors among Adolescent Female Intermediate and Secondary Students in Saudi Arabia. International Journal of Environmental Research and Public Health. 2021; 18(4):2142. https://doi.org/10.3390/ijerph18042142
Chicago/Turabian StyleAlowfi, Areej, Sumayah Binladen, Sumaya Irqsous, Alya Khashoggi, Muhammad Anwar Khan, and Ramah Calacattawi. 2021. "Metabolic Syndrome: Prevalence and Risk Factors among Adolescent Female Intermediate and Secondary Students in Saudi Arabia" International Journal of Environmental Research and Public Health 18, no. 4: 2142. https://doi.org/10.3390/ijerph18042142
APA StyleAlowfi, A., Binladen, S., Irqsous, S., Khashoggi, A., Khan, M. A., & Calacattawi, R. (2021). Metabolic Syndrome: Prevalence and Risk Factors among Adolescent Female Intermediate and Secondary Students in Saudi Arabia. International Journal of Environmental Research and Public Health, 18(4), 2142. https://doi.org/10.3390/ijerph18042142






