The Association between Low Blood Pressure and Attention-Deficit Hyperactivity Disorder (ADHD) Observed in Children/Adolescents Does Not Persist into Young Adulthood. A Population-Based Ten-Year Follow-Up Study
Abstract
1. Introduction
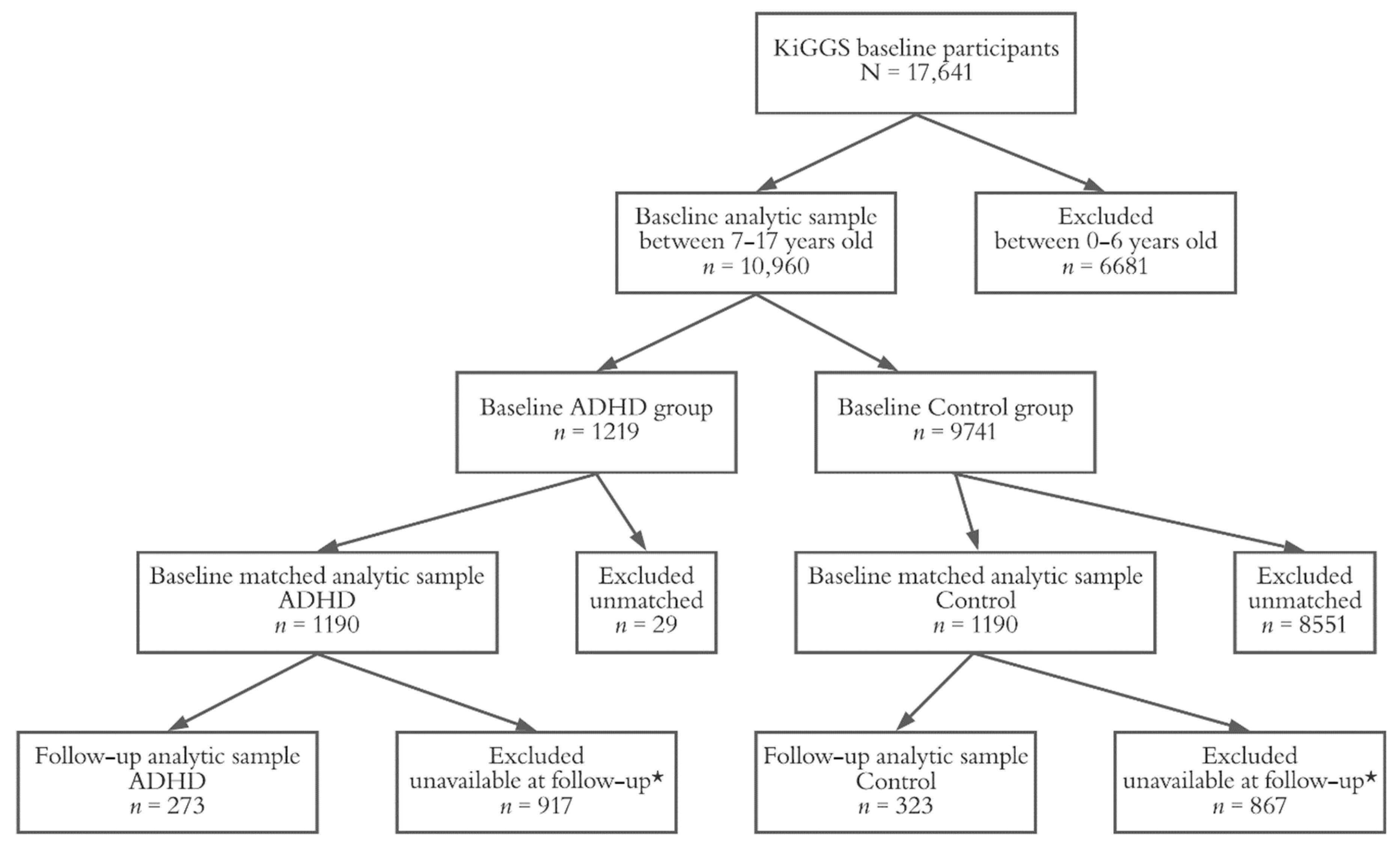
2. Materials and Methods
2.1. KiGGS Study Design
2.2. Assessment of ADHD Symptoms
2.3. Blood Pressure and Heart Rate Measurements
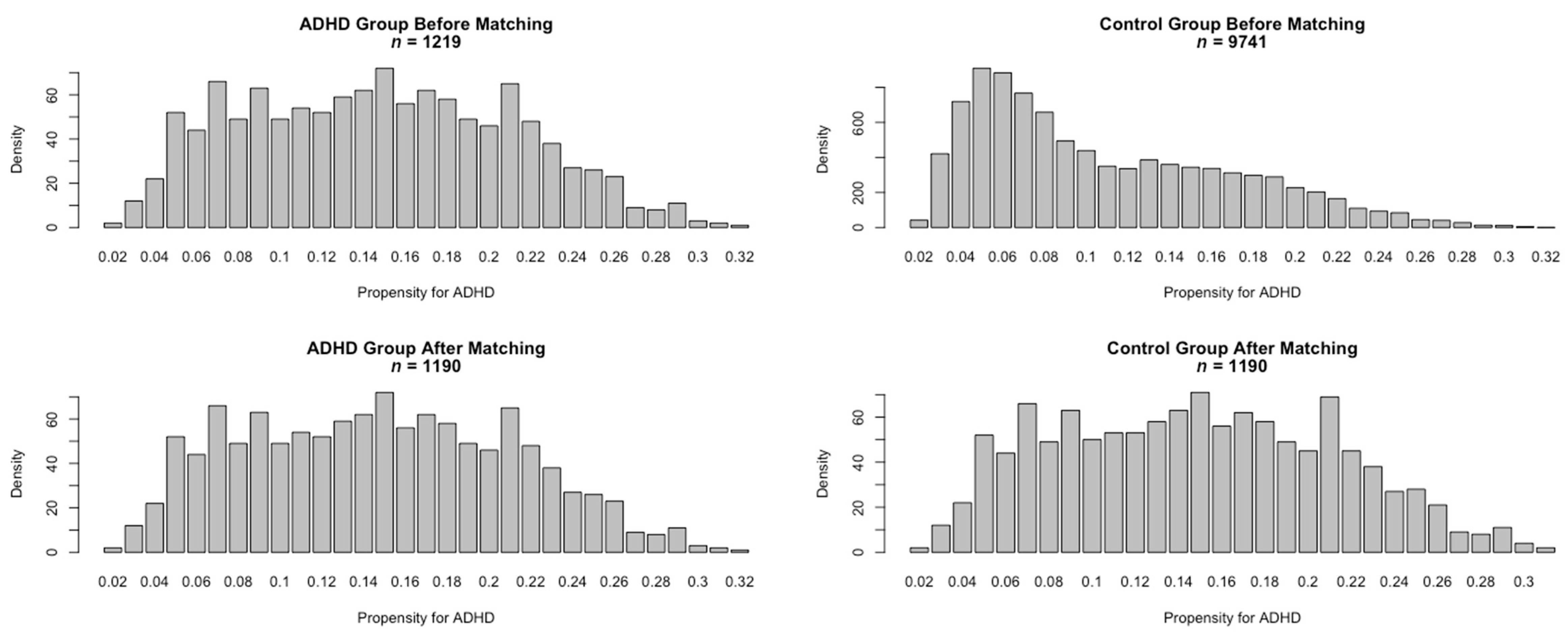
2.4. Statistical Analysis
3. Results
3.1. Characterization of the KiGGS Baseline Cohort
3.2. Blood Pressure in the KiGGS Baseline Cohort
3.3. The Matched Sample at KiGGS Follow-Up
3.4. Sensitivity Analysis with Stringent ADHD Criteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fayyad, J.; Graaf, R.D.; Kessler, R.; Alonso, J.; Angermeyer, M.; Demyttenaere, K.; Girolamo, G.D.; Haro, J.M.; Karam, E.G.; Lara, C.; et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Brit. J. Psychiatry 2007, 190, 402–409. [Google Scholar] [CrossRef]
- Fayyad, J.; Sampson, N.A.; Hwang, I.; Adamowski, T.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Andrade, L.H.S.G.; Borges, G.; de Girolamo, G.; Florescu, S.; et al. The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. Atten. Deficit Hyperact. Disord. 2016, 9, 47–65. [Google Scholar] [CrossRef]
- Polanczyk, G.V.; Willcutt, E.G.; Salum, G.A.; Kieling, C.; Rohde, L.A. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014, 43, 434–442. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Paraphilic disorders. Diagn. Stat. Man. Ment. Disord. 2013. [Google Scholar] [CrossRef]
- Erskine, H.E.; Norman, R.E.; Ferrari, A.J.; Chan, G.C.K.; Copeland, W.E.; Whiteford, H.A.; Scott, J.G. Long-Term Outcomes of Attention-Deficit/Hyperactivity Disorder and Conduct Disorder: A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 841–850. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Lee, P.P.; Sharp, W.; Jeffries, N.O.; Greenstein, D.K.; Clasen, L.S.; Blumenthal, J.D.; James, R.S.; Ebens, C.L.; Walter, J.M.; et al. Developmental Trajectories of Brain Volume Abnormalities in Children and Adolescents With Attention-Deficit/Hyperactivity Disorder. JAMA 2002, 288, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Durston, S.; Pol, H.E.H.; Schnack, H.G.; Buitelaar, J.K.; Steenhuis, M.P.; Minderaa, R.B.; Kahn, R.S.; van Engeland, H. Magnetic Resonance Imaging of Boys With Attention-Deficit/Hyperactivity Disorder and Their Unaffected Siblings. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Rossi, P.D.; Watson, B.; Wharton, A.; Greenstein, D.; Raznahan, A.; Sharp, W.; Lerch, J.P.; Chakravarty, M.M. Mapping the Development of the Basal Ganglia in Children With Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 780–789.e11. [Google Scholar] [CrossRef]
- Greven, C.U.; Bralten, J.; Mennes, M.; O’Dwyer, L.; van Hulzen, K.J.E.; Rommelse, N.; Schweren, L.J.S.; Hoekstra, P.J.; Hartman, C.A.; Heslenfeld, D.; et al. Developmentally Stable Whole-Brain Volume Reductions and Developmentally Sensitive Caudate and Putamen Volume Alterations in Those with Attention-Deficit/Hyperactivity Disorder and Their Unaffected Siblings. JAMA Psychiatr. 2015, 72, 490–499. [Google Scholar] [CrossRef]
- Frodl, T.; Skokauskas, N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr. Scand. 2012, 125, 114–126. [Google Scholar] [CrossRef]
- Hoogman, M.; Rijpkema, M.; Janss, L.; Brunner, H.; Fernandez, G.; Buitelaar, J.; Franke, B.; Arias-Vásquez, A. Current Self-Reported Symptoms of Attention-Deficit/Hyperactivity Disorder Are Associated with Total Brain Volume in Healthy Adults. PLoS ONE 2012, 7, e31273. [Google Scholar] [CrossRef] [PubMed]
- Kirov, R.; Brand, S.; Banaschewski, T.; Rothenberger, A. Opposite Impact of REM Sleep on Neurobehavioral Functioning in Children with Common Psychiatric Disorders Compared to Typically Developing Children. Front. Psychol. 2016, 7, 2059. [Google Scholar] [CrossRef]
- Krauel, K.; Feldhaus, H.C.; Simon, A.; Rehe, C.; Glaser, M.; Flechtner, H.-H.H.; Heinze, H.-J.J.; Niehaus, L. Increased echogenicity of the substantia nigra in children and adolescents with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2010, 68, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Romanos, M.; Weise, D.; Schliesser, M.; Schecklmann, M.; Löffler, J.; Warnke, A.; Gerlach, M.; Classen, J.; Mehler-Wex, C. Structural abnormality of the substantia nigra in children with attention-deficit hyperactivity disorder. J. Psychiatr. Neurosci. 2010, 35, 55–58. [Google Scholar] [CrossRef]
- Kleberg, J.L.; Frick, M.A.; Brocki, K.C. Can auditory warning signals normalize eye movements in children with ADHD? Eur. Child Adolesc. Psychiatry 2020, 29, 1635–1644. [Google Scholar] [CrossRef]
- Bast, N.; Poustka, L.; Freitag, C.M. The locus coeruleus-norepinephrine system as pacemaker of attention—A developmental mechanism of derailed attentional function in autism spectrum disorder. Eur. J. Neurosci. 2018, 47, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Mathias, C.J.; Josephs, O.; O’Doherty, J.; Zanini, S.; Dewar, B.; Cipolotti, L.; Shallice, T.; Dolan, R.J. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 2003, 126, 2139–2152. [Google Scholar] [CrossRef]
- Wass, S.V.; de Barbaro, K.; Clackson, K. Tonic and phasic co-variation of peripheral arousal indices in infants. Biol. Psychol. 2015, 111, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Wiersema, R.; Meere, J.V.D.; Roeyers, H.; Coster, R.V.; Baeyens, D. Event rate and event-related potentials in ADHD: Event rate, ERPs, ADHD. J. Child. Psychol. Psychiatry 2006, 47, 560–567. [Google Scholar] [CrossRef]
- Sergeant, J.A. Modeling Attention-Deficit/Hyperactivity Disorder: A Critical Appraisal of the Cognitive-Energetic Model. Biol. Psychiatry 2005, 57, 1248–1255. [Google Scholar] [CrossRef]
- Rodríguez, C.; González-Castro, P.; Cueli, M.; Areces, D.; González-Pienda, J.A. Attention Deficit/Hyperactivity Disorder (ADHD) Diagnosis: An Activation-Executive Model. Front. Psychol. 2016, 7, 1406. [Google Scholar] [CrossRef]
- Strauß, M.; Ulke, C.; Paucke, M.; Huang, J.; Mauche, N.; Sander, C.; Stark, T.; Hegerl, U. Brain arousal regulation in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2018, 261, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hegerl, U.; Himmerich, H.; Engmann, B.; Hensch, T. Mania and attention-deficit/hyperactivity disorder: Common symptomatology, common pathophysiology and common treatment? Curr. Opin. Psychiatry 2010, 23, 1–7. [Google Scholar] [CrossRef]
- Geissler, J.; Romanos, M.; Hegerl, U.; Hensch, T. Hyperactivity and sensation seeking as autoregulatory attempts to stabilize brain arousal in ADHD and mania? Atten. Deficit Hyperact. Disord. 2014, 6, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Wyckoff, S.N.; Strehl, U. Underarousal in Adult ADHD: How Are Peripheral and Cortical Arousal Related? Clin. EEG Neurosci. 2016, 47, 171–179. [Google Scholar] [CrossRef]
- Hegerl, U.; Hensch, T. The vigilance regulation model of affective disorders and ADHD. Neurosci. Biobehav. Rev. 2014, 44, 45–57. [Google Scholar] [CrossRef]
- Bellato, A.; Arora, I.; Hollis, C.; Groom, M.J. Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neurosci. Biobehav. Rev. 2020, 108, 182–206. [Google Scholar] [CrossRef] [PubMed]
- Negrao, B.L.; Bipath, P.; van der Westhuizen, D.; Viljoen, M. Autonomic correlates at rest and during evoked attention in children with attention-deficit/hyperactivity disorder and effects of methylphenidate. Neuropsychobiology 2011, 63, 82–91. [Google Scholar] [CrossRef]
- Imeraj, L.; Antrop, I.; Roeyers, H.; Deschepper, E.; Bal, S.; Deboutte, D. Diurnal variations in arousal: A naturalistic heart rate study in children with ADHD. Eur. Child Adolesc. Psychiatry 2011, 20, 381–392. [Google Scholar] [CrossRef]
- Imeraj, L.; Antrop, I.; Roeyers, H.; Swanson, J.; Deschepper, E.; Bal, S.; Deboutte, D. Time-of-day effects in arousal: Disrupted diurnal cortisol profiles in children with ADHD. J. Child. Psychol. Psychiatry 2012, 53, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Garfinkel, S.N. The influence of physiological signals on cognition. Curr. Opin. Behav. Sci. 2018, 19, 13–18. [Google Scholar] [CrossRef]
- Imeraj, L.; Sonuga-Barke, E.; Antrop, I.; Roeyers, H.; Wiersema, R.; Bal, S.; Deboutte, D. Altered circadian profiles in attention-deficit/hyperactivity disorder: An integrative review and theoretical framework for future studies. Neurosci. Biobehav. Rev. 2012, 36, 1897–1919. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M.; Eyre, O.; Langley, K. What have we learnt about the causes of ADHD? J. Child. Psychol Psychiatry 2013, 54, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Althaus, M.; Hartman, C.A.; Buitelaar, J.K.; Mindera, R.B.; van den Hoofdakker, B.J.; Hoekstra, P.J. Baroreflex sensitivity during rest and executive functioning in attention-deficit/hyperactivity disorder. The TRAILS study. Biol. Psychol. 2012, 90, 249–257. [Google Scholar] [CrossRef]
- Wolraich, M.; Brown, L.; Brown, R.T.; DuPaul, G.; Earls, M.; Feldman, H.M.; Ganiats, T.G.; Kaplanek, B.; Meyer, B.; Perrin, J.; et al. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011, 128. [Google Scholar] [CrossRef]
- Meyer, T.; Becker, A.; Sundermann, J.; Rothenberger, A.; Herrmann-Lingen, C. Attention deficit-hyperactivity disorder is associated with reduced blood pressure and serum vitamin D levels: Results from the nationwide German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Eur. Child Adolesc. Psychiatry 2017, 26, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Schlack, R.; Hölling, H.; Kurth, B.; Huss, M. The prevalence of attention-deficit/hyperactivity disorder (ADHD) among children and adolescents in Germany. Initial results from the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Hölling, H.; Kamtsiuris, P.; Lange, M.; Thierfelder, W.; Thamm, M.; Schlack, R. The German Health Interview and Examination Survey for Children and Adolescents (KiGGS): Study management and conduct of fieldwork. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hölling, H.; Kurth, B.-M.M.; Rothenberger, A.; Becker, A.; Schlack, R. Assessing psychopathological problems of children and adolescents from 3 to 17 years in a nationwide representative sample: Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Eur. Child Adolesc. Psychiatry 2008, 17 (Suppl. 1), 34–41. [Google Scholar] [CrossRef]
- Hölling, H.; Schlack, R.; Kamtsiuris, P.; Butschalowsky, H.; Schlaud, M.; Kurth, B.M. The KiGGS study. Nationwide representative longitudinal and cross-sectional study on the health of children and adolescents within the framework of health monitoring at the Robert Koch Institute. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2012, 55, 836–842. [Google Scholar] [CrossRef]
- Lange, M.; Hoffmann, R.; Mauz, E.; Houben, R.; Gößwald, A.; Rosario, A.S.; Kurth, B.M. Längsschnitterhebung von KiGGS Welle 2—Erhebungsdesign und Fallzahlentwicklung der KiGGS-Kohorte. J. Health Monit. 2018, 3. [Google Scholar] [CrossRef]
- Krause, L.; Mauz, E.; Houben, R.; Lange, M.; Gößwald, A. KiGGS Welle 2 (2014–2017)—Die zweite Folgeerhebung der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland. Available online: https://edoc.rki.de/handle/176904/3050?show=full&locale-attribute=en (accessed on 10 February 2021).
- Polanczyk, G.; de Lima, M.S.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef]
- Goodman, R. The Strengths and Difficulties Questionnaire: A research note. J. Child Psychol. Psychiatry 1997, 38, 581–586. [Google Scholar] [CrossRef]
- Becker, A.; Wang, B.; Kunze, B.; Otto, C.; Schlack, R.; Hölling, H.; Ravens-Sieberer, U.; Klasen, F.; Rogge, J.; Isensee, C.; et al. Normative Data of the Self-Report Version of the German Strengths and Difficulties Questionnaire in an Epidemiological Setting. Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie 2018, 46, 523–533. [Google Scholar] [CrossRef]
- Kunze, B.; Wang, B.; Isensee, C.; Schlack, R.; Ravens-Sieberer, U.; Klasen, F.; Rothenberger, A.; Becker, A.; BELLA-Study Group. Gender associated developmental trajectories of SDQ-dysregulation profile and its predictors in children. Psychol. Med. 2018, 48, 404–415. [Google Scholar] [CrossRef]
- Neuhauser, H.; Thamm, M. Blood pressure measurement in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Methodology and initial results. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 12 November 2020).
- Hailpern, S.M.; Egan, B.M.; Lewis, K.D.; Wagner, C.; Shattat, G.F.; Qaoud, D.I.A.; Shatat, I.F. Blood Pressure, Heart Rate, and CNS Stimulant Medication Use in Children with and without ADHD: Analysis of NHANES Data. Front. Pediatr. 2014, 2, 100. [Google Scholar] [CrossRef] [PubMed]
- Sayer, G.R.; McGough, J.J.; Levitt, J.; Cowen, J.; Sturm, A.; Castelo, E.; McCracken, J.T. Acute and Long-Term Cardiovascular Effects of Stimulant, Guanfacine, and Combination Therapy for Attention-Deficit/Hyperactivity Disorder. J. Child Adolesc. Psychopharmacol. 2016, 26, 882–888. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.; Neubert, A.; Man, K.K.C.K.; Banaschewski, T.; Buitelaar, J.; Carucci, S.; Coghill, D.; Danckaerts, M.; Falissard, B.; Garas, P.; et al. Effects of long-term methylphenidate use on growth and blood pressure: Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). BMC Psychiatry 2018, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- Busold-Hagenbeck, D.; Elmenhorst, J.; von Brenndorff, C.I.; Hilgers, R.; Hulpke-Wette, M. Frequency and individual severity of arterial blood pressure changes in children and adolescents with attention-deficit/hyperactivity disorder treated with methylphenidate hydrochloride: A prospective non-interventional study. Gen. Psychiatry 2020, 33, e100193. [Google Scholar] [CrossRef]
- Honeycutt, J.M. On the Correspondence Between Meta-Emotions, Cardiovascular Arousal, and Imagined Interaction Discrepancy. Evol. Psychol. Sci. 2020, 6, 82–91. [Google Scholar] [CrossRef]
- Dupuy, F.E.; Clarke, A.R.; Barry, R.J.; Selikowitz, M.; McCarthy, R. EEG and electrodermal activity in girls with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2014, 125, 491–499. [Google Scholar] [CrossRef] [PubMed]
- James, S.-N.; Cheung, C.H.; Rijsdijk, F.; Asherson, P.; Kuntsi, J. Modifiable Arousal in Attention-Deficit/Hyperactivity Disorder and Its Etiological Association with Fluctuating Reaction Times. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 539–547. [Google Scholar] [CrossRef]
- Hoogman, M.; Bralten, J.; Hibar, D.P.; Mennes, M.; Zwiers, M.P.; Schweren, L.S.J.; van Hulzen, K.J.E.; Medland, S.E.; Shumskaya, E.; Jahanshad, N.; et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross-sectional mega-analysis. Lancet Psychiatry 2017, 4, 310–319. [Google Scholar] [CrossRef]
- Salman, I.M. Major Autonomic Neuroregulatory Pathways Underlying Short- and Long-Term Control of Cardiovascular Function. Curr. Hypertens Rep. 2016, 18, 18. [Google Scholar] [CrossRef]
- Zamorano, F.; Billeke, P.; Kausel, L.; Larrain, J.; Stecher, X.; Hurtado, J.M.; López, V.; Carrasco, X.; Aboitiz, F. Lateral prefrontal activity as a compensatory strategy for deficits of cortical processing in Attention Deficit Hyperactivity Disorder. Sci. Rep. 2017, 7, 7181. [Google Scholar] [CrossRef]
- Onnink, A.M.H.; Zwiers, M.P.; Hoogman, M.; Mostert, J.C.; Kan, C.C.; Buitelaar, J.; Franke, B. Brain alterations in adult ADHD: Effects of gender, treatment and comorbid depression. Eur. Neuropsychopharm. 2014, 24, 397–409. [Google Scholar] [CrossRef] [PubMed]
| Variable | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Participants in the ADHD Group | Participants in the Control Group | p Value | Participants in the ADHD Group | Participants in the Control Group | p Value | |
| (n = 1219) | (n = 9741) | (n = 1190) | (n = 1190) | |||
| Age (years) | 11.25 ± 2.91 | 11.92 ± 3.13 | <0.001 | 11.26 ± 2.92 | 11.27 ± 3.07 | 0.891 |
| Sex (%) | ||||||
| Female | 26.74 | 51.48 | <0.001 | 26.81 | 26.64 | 0.963 |
| SDQ-H | 6.99 ± 1.90 | 2.54 ± 1.79 | <0.001 | 6.97 ± 1.91 | 2.80± 1.81 | <0.001 |
| SES Winkler index | 10.32 ± 4.16 | 11.58 ± 4.33 | <0.001 | 10.33 ± 4.17 | 10.35 ± 4.17 | 0.912 |
| SES Winkler Category (%) | ||||||
| Low | 37.17 | 26.13 | <0.001 | 37.23 | 36.97 | 0.907 |
| Medium | 46.61 | 47.62 | 46.47 | 46.05 | ||
| High | 16.22 | 26.25 | 16.3 | 16.97 | ||
| BMI (kg/m2) | 19.28 ± 4.20 | 19.61 ± 4.00 | 0.007 | 19.26 ± 4.19 | 19.31 ± 3.94 | 0.8 |
| BMI Category (%) | ||||||
| Underweight (BMI < 18.5) | 50.53 | 44.09 | <0.001 | 51.01 | 50 | 0.809 |
| Normal (18.5 ≤ BMI < 25.0) | 38.97 | 45.92 | 39.08 | 40.67 | ||
| Overweight (25.0 ≤ BMI < 30.0) | 8.04 | 7.31 | 8.15 | 7.9 | ||
| Obese (BMI > 30.0) | 2.46 | 2.68 | 1.76 | 1.43 | ||
| Heart rate (bpm) | 78.61 ± 11.73 | 78.47 ± 11.78 | 0.683 | 78.54 ± 23.30 | 78.26 ±11.63 | 0.565 |
| Systolic BP (mmHg) | 107.71 ± 10.69 | 110.55 ± 11.22 | <0.001 | 107.6 ± 10.66 | 109.49 ± 10.89 | <0.001 |
| Diastolic BP (mmHg) | 64.65 ± 7.52 | 66.39 ± 7.58 | <0.001 | 64.57 ± 7.48 | 65.75 ± 7.44 | <0.001 |
| Pulse pressure (mmHg) | 43.06 ± 7.81 | 44.15 ±7.92 | <0.001 | 43.03 ± 7.78 | 43.73 ± 7.89 | 0.028 |
| Variable | Before Matching (n = 10,960) | After Matching (n = 2380) | ||||||
|---|---|---|---|---|---|---|---|---|
| Exp (ß) | 95%-CI | Wald | p Value | Exp (ß) | 95%-CI | Wald | p Value | |
| Unadjusted Models | ||||||||
| Age | 0.933 | 0.915–0.952 | −6.953 | <0.001 | 0.998 | 0.972–1.025 | −0.137 | 0.891 |
| Sex | 0.344 | 0.301–0.393 | −15.74 | <0.001 | 1.009 | 0.841–1.209 | 0.093 | 0.926 |
| SES | 0.932 | 0.919–0.946 | −9.423 | <0.001 | 0.999 | 0.98–1.018 | −0.108 | 0.914 |
| BMI | 0.979 | 0.964–0.994 | −2.674 | 0.007 | 0.997 | 0.978–1.017 | −0.253 | 0.8 |
| Heart rate | 1.001 | 0.996–1.006 | 0.408 | 0.683 | 1.002 | 0.995–1.009 | 0.575 | 0.565 |
| Systolic BP | 0.976 | 0.971–0.982 | −8.3 | <0.001 | 0.984 | 0.976–0.991 | −4.233 | <0.001 |
| Diastolic BP | 0.969 | 0.962–0.977 | −7.539 | <0.001 | 0.979 | 0.968–0.990 | −3.836 | <0.001 |
| Pulse pressure | 0.982 | 0.975–0.990 | −4.526 | <0.001 | 0.989 | 0.978–0.999 | −2.188 | 0.029 |
| Adjusted Model for Systolic BP | ||||||||
| Age | 0.986 | 0.960–1.014 | −0.967 | 0.333 | 1.053 | 1.015–1.093 | 2.772 | 0.006 |
| Sex | 0.318 | 0.277–0.365 | −16.329 | <0.001 | 0.955 | 0.794–1.148 | −0.491 | 0.623 |
| SES | 0.93 | 0.916–0.944 | −9.465 | <0.001 | 0.998 | 0.979–1.018 | −0.174 | 0.862 |
| BMI | 1.03 | 1.011–1.050 | 3.073 | 0.002 | 1.023 | 0.998–1.049 | 1.826 | 0.068 |
| Heart rate | 1.004 | 0.998–1.010 | 1.252 | 0.211 | 1.007 | 0.999–1.014 | 1.75 | 0.08 |
| Systolic BP | 0.968 | 0.961–0.975 | −8.259 | <0.001 | 0.971 | 0.961–0.981 | −5.624 | <0.001 |
| Adjusted Model for Diastolic BP | ||||||||
| Age | 0.961 | 0.936–0.986 | −3.021 | 0.003 | 1.027 | 0.993–1.063 | 1.534 | 0.125 |
| Sex | 0.333 | 0.291–0.382 | −15.777 | <0.001 | 0.987 | 0.821–1.186 | −0.14 | 0.888 |
| SES | 0.929 | 0.915–0.943 | −9.588 | <0.001 | 0.999 | 0.979–1.018 | −0.136 | 0.892 |
| BMI | 1.011 | 0.993–1.029 | 1.168 | 0.243 | 1.005 | 0.982–1.029 | 0.451 | 0.652 |
| Heart rate | 1.003 | 0.997–1.009 | 1.057 | 0.291 | 1.006 | 0.999–1.014 | 1.646 | 0.1 |
| Diastolic BP | 0.969 | 0.960–0.978 | −6.539 | <0.001 | 0.973 | 0.961–0.985 | −4.368 | <0.001 |
| Adjusted Model for Pulse Pressure | ||||||||
| Age | 0.948 | 0.924–0.972 | −4.121 | <0.001 | 1.015 | 0.981–1.049 | 0.841 | 0.401 |
| Sex | 0.333 | 0.291–0.382 | −15.768 | <0.001 | 0.988 | 0.823–1.187 | −0.128 | 0.898 |
| SES | 0.929 | 0.916–0.944 | −9.481 | <0.001 | 0.999 | 0.979–1.019 | −0.119 | 0.905 |
| BMI | 1.01 | 0.991–1.029 | 1.031 | 0.303 | 1.005 | 0.982–1.03 | 0.448 | 0.654 |
| Heart rate | 0.998 | 0.993–1.004 | −0.656 | 0.512 | 1.002 | 0.995–1.009 | 0.568 | 0.57 |
| Pulse pressure | 0.983 | 0.974–0.992 | −3.747 | <0.001 | 0.985 | 0.973–0.997 | −2.422 | 0.016 |
| Variable | Participants in the ADHD Group | Participants in the Control Group | p Value |
|---|---|---|---|
| (n = 273) | (n = 323) | ||
| Measurement at Baseline | |||
| Age (years) | 10.60 ± 2.93 | 10.56 ± 2.99 | 0.879 |
| Sex (%) | |||
| Female | 28.57 | 25.39 | 0.435 |
| SDQ-H | 6.97 ± 1.93 | 2.74 ± 1.82 | <0.001 |
| SES Winkler index | 10.97 ± 3.98 | 10.65 ± 4.13 | 0.342 |
| SES Winkler Category (%) | |||
| Low | 29.3 | 35.6 | 0.252 |
| Medium | 50.92 | 45.51 | |
| High | 19.78 | 18.89 | |
| BMI (kg/m2) | 18.59 ± 4.11 | 18.46 ± 3.56 | 0.684 |
| BMI Category (%) | |||
| Underweight (BMI < 18.5) | 61.9 | 60.06 | 0.425 |
| Normal (18.5 ≤ BMI < 25.0) | 30.77 | 33.44 | |
| Overweight (25.0 ≤ BMI < 30.0) | 5.86 | 6.19 | |
| Obese (BMI > 30.0) | 1.47 | 0.31 | |
| Heart rate (bpm) | 80.00 ± 11.34 | 78.96 ± 10.93 | 0.252 |
| Systolic BP (mmHg) | 106.14 ± 10.74 | 107.73 ± 10.69 | 0.071 |
| Diastolic BP (mmHg) | 63.8 ± 7.59 | 64.99 ± 7.44 | 0.055 |
| Pulse pressure (mmHg) | 42.34 ± 7.32 | 42.74 ± 7.91 | 0.519 |
| Measurement at Follow-Up | |||
| Age (years) | 21.53 ± 2.92 | 21.48 ± 3.04 | 0.856 |
| BMI at (kg/m2) | 24.81 ± 5.40 | 23.86 ± 4.02 | 0.014 |
| BMI Category (%) | |||
| Underweight (BMI < 18.5) | 4.76 | 5.26 | 0.017 |
| Normal (18.5 ≤ BMI < 25.0) | 56.41 | 60.37 | |
| Overweight (25.0 ≤ BMI < 30.0) | 22.34 | 26.32 | |
| Obese (BMI > 30.0) | 16.48 | 8.05 | |
| Heart rate (bpm) | 75.46 ± 12.93 | 73.55 ± 11.80 | 0.06 |
| Systolic BP (mmHg) | 123.4 ± 10.65 | 123.78 ± 11.1 | 0.675 |
| Diastolic BP (mmHg) | 71.86 ± 6.84 | 71.85 ± 7.06 | 0.992 |
| Pulse pressure (mmHg) | 51.55 ± 7.86 | 51.93 ± 8.05 | 0.561 |
| Baseline vs. Follow-Up Change | |||
| BMI at (kg/m2) | 6.22 ± 3.9.0 | 5.41 ± 3.22 | 0.006 |
| Heart rate (bpm) | −4.54 ± 13.04 | −5.41 ± 13.37 | 0.427 |
| Systolic BP (mmHg) | 17.27 ± 11.44 | 16.05 ± 12.73 | 0.223 |
| Diastolic BP (mmHg) | 8.05 ± 8.00 | 6.86 ± 8.65 | 0.083 |
| Pulse pressure (mmHg) | 9.21 ± 9.81 | 9.19 ± 10.45 | 0.976 |
| BP at Baseline (n =596) | BP at Follow-Up (n =596) | Baseline vs. Follow-Up Change (n = 596) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp (ß) | 95%-CI | Wald | p Value | Exp (ß) | 95%-CI | Wald | p Value | Exp (ß) | 95%-CI | Wald | p Value | ||
| Adjusted Model for Systolic BP at Baseline or Follow-Up | |||||||||||||
| Age | 1.065 | 0.987–1.149 | 1.624 | 0.104 | Age | 1.004 | 0.949–1.063 | 0.145 | 0.885 | 1.024 | 0.962–1.091 | 0.749 | 0.454 |
| Sex | 1.118 | 0.771–1.621 | 0.586 | 0.558 | Sex | 1.065 | 0.709–1.599 | 0.302 | 0.763 | 1.271 | 0.853–1.894 | 1.179 | 0.239 |
| SES | 1.017 | 0.977–1.060 | 0.837 | 0.403 | SES | 1.027 | 0.986–1.069 | 1.261 | 0.207 | 1.024 | 0.983–1.067 | 1.129 | 0.259 |
| BMI at baseline | 1.039 | 0.985–1.096 | 1.4 | 0.161 | BMI at follow-up | 1.053 | 1.015–1.093 | 2.751 | 0.006 | 1.05 | 1.013–1.090 | 2.65 | 0.008 |
| Heart rate at baseline | 1.014 | 0.998–1.030 | 1.687 | 0.092 | Heart rate at follow-up | 1.014 | 1.000–1.028 | 1.991 | 0.046 | 1.012 | 0.998–1.026 | 1.727 | 0.084 |
| Systolic BP at baseline | 0.969 | 0.949–0.991 | −2.83 | 0.005 | Systolic BP at follow-up | 0.992 | 0.975–1.009 | −0.959 | 0.337 | 1.013 | 0.997–1.029 | 1.602 | 0.109 |
| Adjusted Model for Diastolic BP at Baseline or Follow-Up | |||||||||||||
| Age | 1.046 | 0.973–1.123 | 1.221 | 0.222 | Age | 1.005 | 0.949–1.065 | 0.169 | 0.865 | 1.012 | 0.956–1.072 | 0.419 | 0.675 |
| Sex | 1.129 | 0.779–1.635 | 0.639 | 0.523 | Sex | 1.128 | 0.770–1.652 | 0.619 | 0.536 | 1.17 | 0.801–1.709 | 0.813 | 0.416 |
| SES | 1.022 | 0.981–1.064 | 1.053 | 0.292 | SES | 1.026 | 0.985–1.069 | 1.256 | 0.209 | 1.026 | 0.985–1.069 | 1.221 | 0.222 |
| BMI at baseline | 1.021 | 0.971–1.074 | 0.805 | 0.421 | BMI at follow-up | 1.05 | 1.012–1.089 | 2.629 | 0.009 | 1.053 | 1.016–1.093 | 2.774 | 0.006 |
| Heart rate at baseline | 1.015 | 0.999–1.031 | 1.814 | 0.07 | Heart rate at follow-up | 1.014 | 1.000–1.028 | 1.956 | 0.05 | 1.012 | 0.999–1.026 | 1.762 | 0.078 |
| Diastolic BP at baseline | 0.967 | 0.942–0.992 | −2.604 | 0.009 | Diastolic BP at follow-up | 0.993 | 0.968–1.018 | −0.572 | 0.567 | 1.02 | 0.999–1.041 | 1.878 | 0.06 |
| Adjusted Model for Pulse Pressure at Baseline or Follow-Up | |||||||||||||
| Age | 1.016 | 0.949–1.088 | 0.463 | 0.644 | Age | 1 | 0.945–1.058 | −0.004 | 0.997 | 1.003 | 0.944–1.065 | 0.101 | 0.919 |
| Sex | 1.174 | 0.812–1.696 | 0.854 | 0.393 | Sex | 1.076 | 0.715–1.619 | 0.351 | 0.726 | 1.16 | 0.779–1.726 | 0.731 | 0.465 |
| SES | 1.022 | 0.981–1.064 | 1.04 | 0.299 | SES | 1.027 | 0.986–1.069 | 1.263 | 0.207 | 1.026 | 0.985–1.069 | 1.241 | 0.214 |
| BMI at baseline | 1.016 | 0.965–1.070 | 0.613 | 0.54 | BMI at follow-up | 1.054 | 1.015–1.094 | 2.732 | 0.006 | 1.049 | 1.013–1.089 | 2.605 | 0.009 |
| Heart rate at baseline | 1.009 | 0.994–1.024 | 1.134 | 0.257 | Heart rate at follow-up | 1.013 | 0.999–1.026 | 1.818 | 0.069 | 1.013 | 0.999–1.027 | 1.862 | 0.063 |
| Pulse pressure at baseline | 0.99 | 0.966–1.015 | −0.781 | 0.435 | Pulse pressure at follow-up | 0.991 | 0.968–1.014 | −0.801 | 0.423 | 1.002 | 0.984–1.020 | 0.167 | 0.868 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, J.; Huber, F.; Schlack, R.; Hölling, H.; Ravens-Sieberer, U.; Meyer, T.; Poustka, L.; Rothenberger, A.; Wang, B.; Becker, A. The Association between Low Blood Pressure and Attention-Deficit Hyperactivity Disorder (ADHD) Observed in Children/Adolescents Does Not Persist into Young Adulthood. A Population-Based Ten-Year Follow-Up Study. Int. J. Environ. Res. Public Health 2021, 18, 1864. https://doi.org/10.3390/ijerph18041864
Schulz J, Huber F, Schlack R, Hölling H, Ravens-Sieberer U, Meyer T, Poustka L, Rothenberger A, Wang B, Becker A. The Association between Low Blood Pressure and Attention-Deficit Hyperactivity Disorder (ADHD) Observed in Children/Adolescents Does Not Persist into Young Adulthood. A Population-Based Ten-Year Follow-Up Study. International Journal of Environmental Research and Public Health. 2021; 18(4):1864. https://doi.org/10.3390/ijerph18041864
Chicago/Turabian StyleSchulz, Jan, Franziska Huber, Robert Schlack, Heike Hölling, Ulrike Ravens-Sieberer, Thomas Meyer, Luise Poustka, Aribert Rothenberger, Biyao Wang, and Andreas Becker. 2021. "The Association between Low Blood Pressure and Attention-Deficit Hyperactivity Disorder (ADHD) Observed in Children/Adolescents Does Not Persist into Young Adulthood. A Population-Based Ten-Year Follow-Up Study" International Journal of Environmental Research and Public Health 18, no. 4: 1864. https://doi.org/10.3390/ijerph18041864
APA StyleSchulz, J., Huber, F., Schlack, R., Hölling, H., Ravens-Sieberer, U., Meyer, T., Poustka, L., Rothenberger, A., Wang, B., & Becker, A. (2021). The Association between Low Blood Pressure and Attention-Deficit Hyperactivity Disorder (ADHD) Observed in Children/Adolescents Does Not Persist into Young Adulthood. A Population-Based Ten-Year Follow-Up Study. International Journal of Environmental Research and Public Health, 18(4), 1864. https://doi.org/10.3390/ijerph18041864






