Per- and Polyfluoroalkyl Substances in Human Serum Samples of Selected Populations from Ghana
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Subjects
2.2. Examinations
2.3. Collection of Biological Samples
2.4. Analysis of Trace Elements in Biological Samples
2.5. Analysis of PFAS in Serum
2.6. Statistical Analysis
3. Results and Discussion
3.1. Background Data, Hg and Sn
3.2. Concentration and Distribution Patterns of PFASs
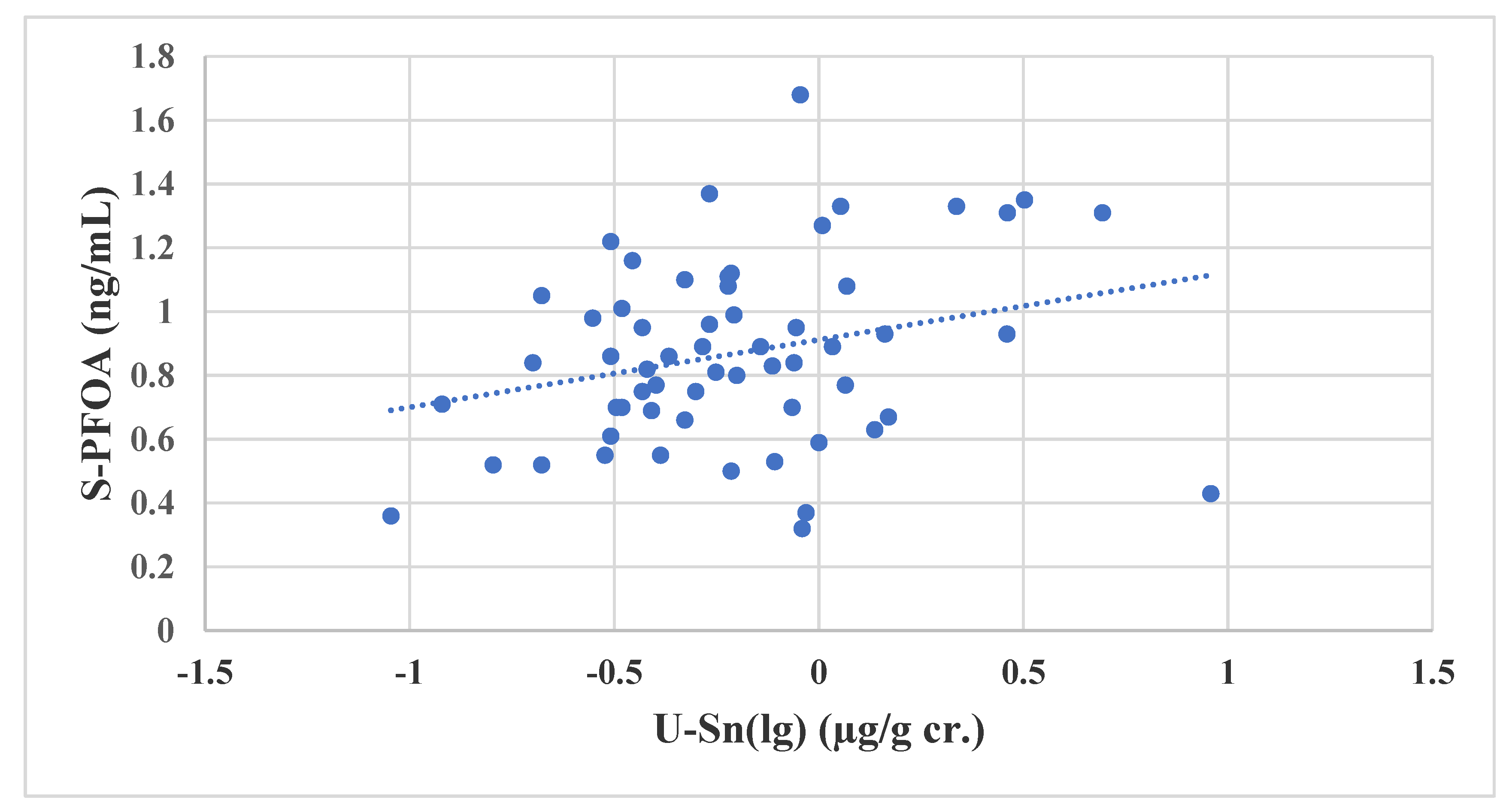
3.3. PFASs and Sn in ERWs
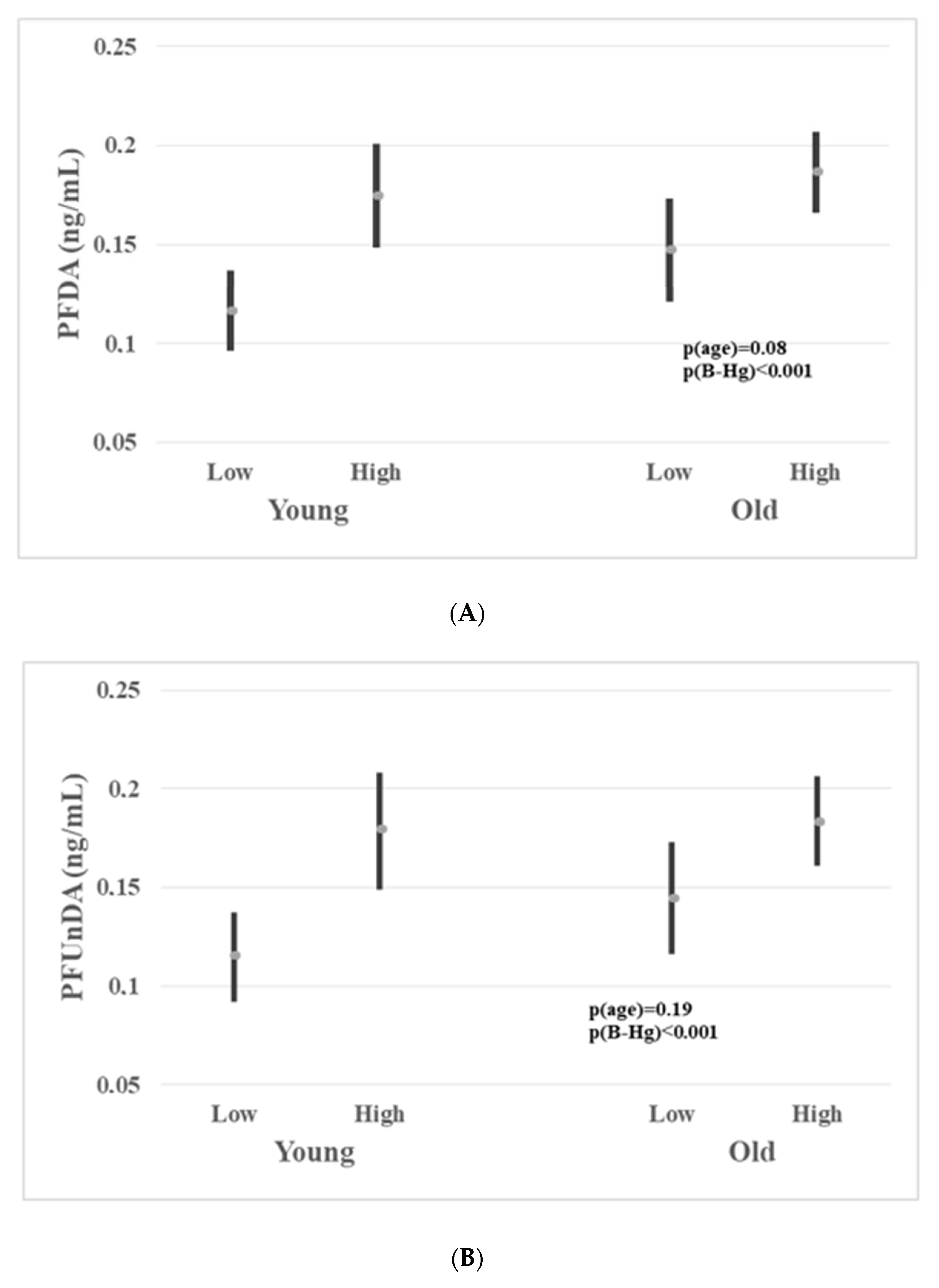
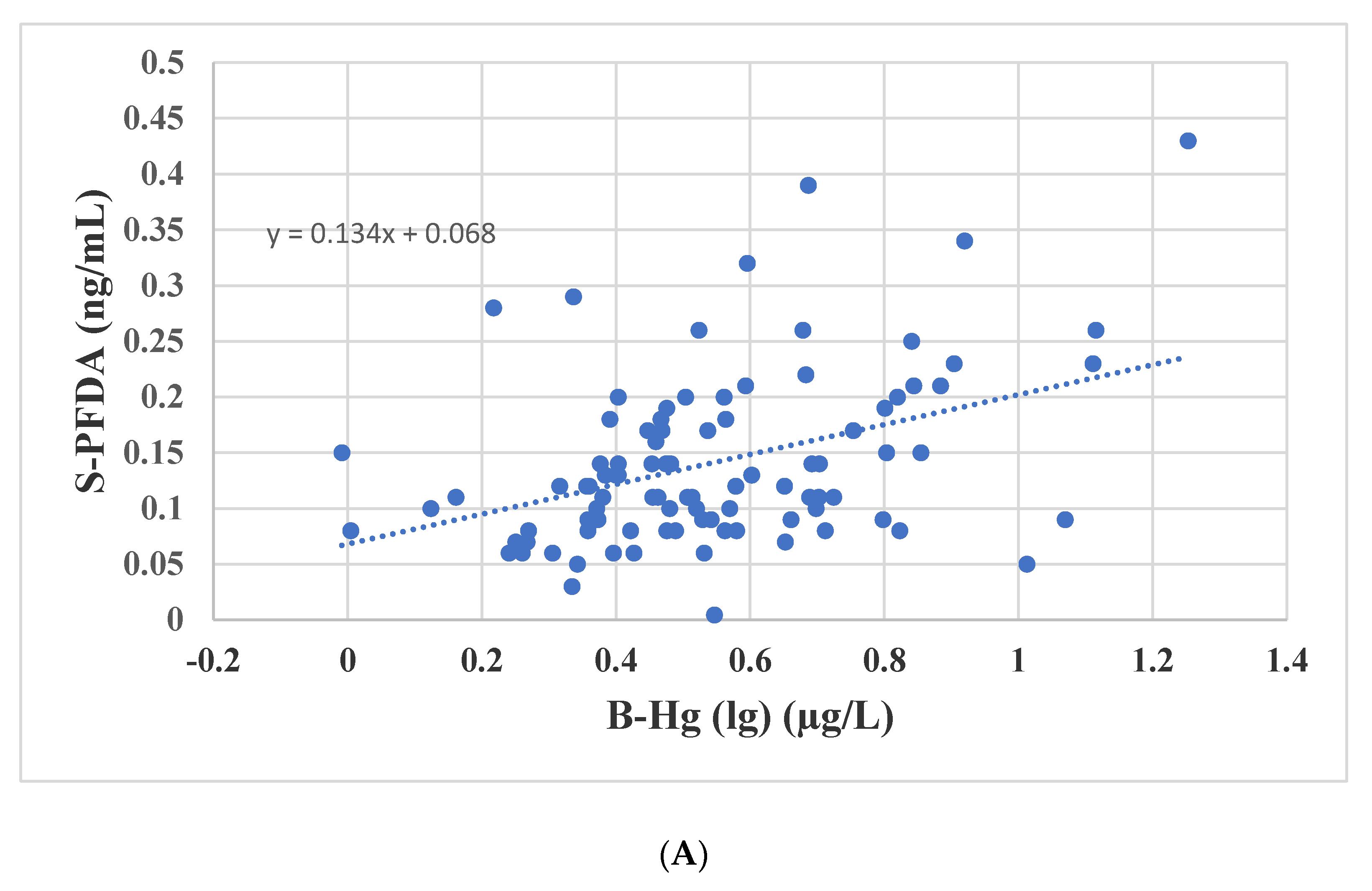
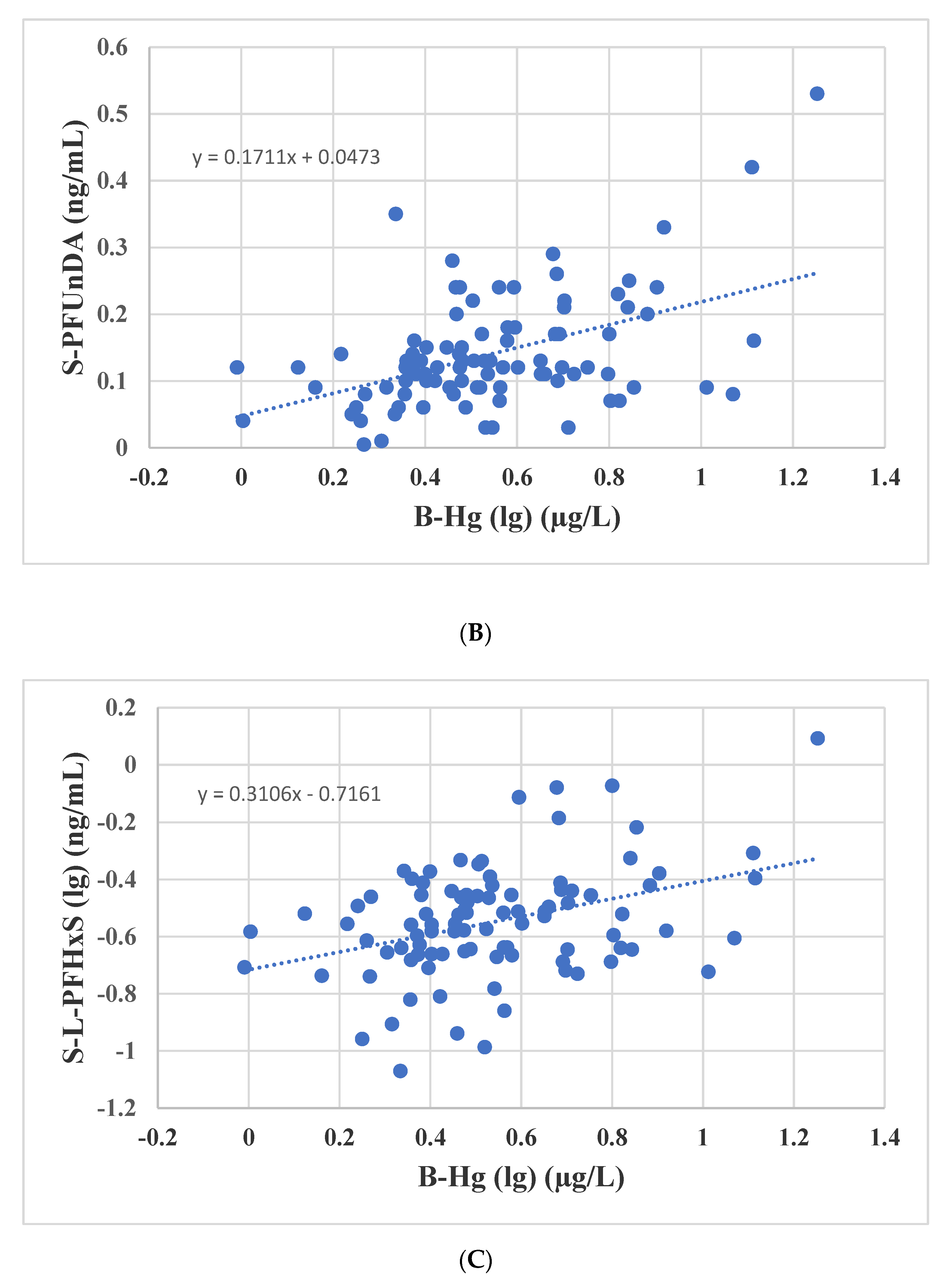
3.4. Associations between Biomarkers of Seafood Consumption and PFASs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; Van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated surfactants and repellents. In Surfactant Science Series, 2nd ed.; Marcel Dekker: New York, NY, USA, 2001; Volume 97. [Google Scholar]
- KEMI (Swedish Chemical Agency). 2015. Available online: http://www.kemi.se/en/global/rapporter/2015/ report-7-15-occurrence-and-use-of-highly-fluorinated-substances-and-alternatives.pdf (accessed on 30 January 2019).
- Kotthoff, M.; Müller, J.; Jürling, H.; Schlummer, M.; Fiedler, D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. 2015, 22, 14546–14559. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Conder, J.M.; Hoke, R.A.; De Wolf, W.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.A.; Hungerbühler, K. Bioaccumulation of perfluorinated alkyl acids: Observations and models. Environ. Sci. Technol. 2014, 48, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Perfluorooctanoic Acid (PFOA) (IARC Monographs-110). Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono110-01.pdf (accessed on 10 August 2020).
- Fromme, H.; Tittlemier, S.A.; Völkel, W.; Wilhelm, M.; Twardella, D. Perfluorinated compounds—Exposure assessment for the general population in western countries. Int. J. Hyg. Environ. Health 2009, 212, 239–270. [Google Scholar] [CrossRef] [PubMed]
- Dassuncao, C.; Hu, X.C.; Nielsen, F.; Weihe, P.; Grandjean, P.; Sunderland, E.M. Shifting global exposures to poly- and perfluoralkyl substances (PFASs) evident in longitudinal birth cohorts from a seafood consuming population. Environ. Sci. Technol. 2018, 52, 3738–3747. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Perra, G.; Corsolini, S.; Focardi, S.E. Pilot study on levels of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in selected food stuffs and human milk from Italy. Food Chem. 2013, 140, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; Glynn, A.; Darnerud, P.O.; Berger, U. Perfluoroalkyl acids and their precursosrs in Swedish food: The relative importance of direct and indirect dietary exposure. Environ. Pollut. 2015, 198, 108–115. [Google Scholar] [CrossRef]
- Okada, E.; Kashino, I.; Matsuura, H.; Sasaki, S.; Miyashita, C.; Yamamoto, J.; Ikeno, T.; Ito, Y.M.; Matsumura, T.; Tamakoshi, A.; et al. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environ. Int. 2013, 60, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Nøst, T.H.; Vestergren, R.; Berg, V.; Nieboer, E.; Odland, J.-Ø.; Sandanger, T.M. Repeated measurements of per-and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: Assessing time trends, compound correlations and relations to age/birth cohort. Environ. Int. 2014, 67, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Toms, L.-M.L.; Thompson, J.; Rotander, A.; Hobson, P.; Calafat, A.M.; Kato, K.; Ye, X.; Broomhall, S.; Harden, F.; Mueller, J.F.; et al. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environ. Int. 2014, 71, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Goldberg, D.; Wang, M.; Park, J.-S.; Petreas, M.; Bernstein, L.; Anton-Culver, H.; Nelson, D.O.; Reynolds, P. Time trends in per- and polyfluoroalkyl substances (PFASs) in Californian women: Declining serum levels, 2011–2015. Environ. Sci. Technol. 2018, 52, 277–287. [Google Scholar] [CrossRef]
- Hanssen, L.; Röllin, H.; Odland, J.Ø.; Moe, M.K.; Sandanger, T.M. Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: Results of a pilot study. J. Environ. Monit. 2010, 12, 1355–1361. [Google Scholar] [CrossRef]
- Müller, M.H.B.; Polder, A.; Brynildsrud, O.B.; Grønnestad, R.; Karimi, M.; Lie, E.; Manyilizu, W.B.; Mdegela, R.H.; Mokiti, F.; Murtadha, M.; et al. Prenatal exposure to persistent organic pollutants in Northern Tanzania and their distribution between breast milk, maternal blood, placenta and cord blood. Environ. Res. 2019, 170, 433–442. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Freberg, B.; Haug, L.S.; Olsen, R.; Daae, H.-L.; Hersson, M.; Thomsen, C.; Thorud, S.; Becher, G.; Molander, P.; Ellingsen, D.G. Occupational exposure to airborne perfluorinated compounds during professional ski waxing. Environ. Sci. Technol. 2010, 44, 7723–7728. [Google Scholar] [CrossRef]
- Fu, J.; Gao, Y.; Cui, L.; Wang, T.; Liang, Y.; Qu, G.; Yuan, B.; Wang, Y.; Zhang, A.; Jiang, G. Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China. Sci. Rep. 2016, 6, 38039. [Google Scholar] [CrossRef]
- Heydebreck, F.; Tang, J.; Xie, Z.; Ebinghaus, R. Emissions of per- and polyfluoroalkyl substances in a textile manufacturing plant in China and their relevance for workers’ exposure. Environ. Sci. Technol. 2016, 50, 10386–10396. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xu, X.; Peng, L.; Liu, J.; Guo, Y.; Huo, X. Association between maternal exposure to perfluorooctanic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ. Int. 2012, 48, 1–8. [Google Scholar] [CrossRef]
- Dartey, E.; Berlinger, B.; Weinbruch, S.; Thomassen, Y.; Odland, J.Ø.; Brox, J.; Nartey, V.K.; Yeboah, F.A.; Ellingsen, D.G. Essential and non-essential trace elements among working populations in Ghana. J. Trace Elem. Med. Biol. 2017, 44, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Brox, J. An automated high throughput SPE microelution method for perfluoroalkyl substances in human serum. Anal. Bioanal. Chem. 2015, 407, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.-W.; Qian, Z.M.; Geiger, S.D.; Liu, E.; Liu, Y.M.; Wang, S.Q.; Lawrence, W.R.; Yang, B.-Y.; Hu, L.-W.; Zeng, X.-W.; et al. Gender-specific associations between serum isomers of perfluoroalkyl substances and blood pressure among Chinese: Isomers of C8 Health Project in China. Sci. Total Environ. 2017, 607–608, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Brede, E.; Wilhelm, M.; Göen, T.; Mueller, J.; Rauchfuss, K.; Kraft, M.; Hölzer, J. Two-year follow-up biomonitoring pilot study of residents’ and controls’ PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany. Int. J. Hyg. Environ. Health 2010, 213, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; MacLeod, M.; Mueller, J.F.; Cousins, I.T. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: Evidence from population-based pharmacokinetic modeling. Environ. Sci. Technol. 2014, 48, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.H.; Tallving, P.; Jakobsson, K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018, 75, 46–51. [Google Scholar] [CrossRef]
- Fromme, H.; Mosch, C.; Morovitz, M.; Alba-Alejandre, I.; Boehmer, S.; Kiranoglu, M.; Faber, F.; Hannibal, I.; Genzel-Boroviczény, O.; Koletzko, B.; et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ. Sci. Technol. 2010, 44, 7123–7129. [Google Scholar] [CrossRef] [PubMed]
- Gomis, M.I.; Vestergren, R.; MacLeod, M.; Mueller, J.F.; Cousins, I.T. Historical human exposure to perfluoroalkyl acids in the United States and Australia reconstructed from biomonitoring data using population-based pharmacokinetic modelling. Environ. Int. 2017, 108, 92–102. [Google Scholar] [CrossRef]
- Gribble, M.O.; Bartell, S.M.; Kannan, K.; Wu, Q.; Fair, P.A.; Kamen, L. Longitudinal measures of perfluoroalkyl substances (PFAS) in serum of Gullah African Americans in South Carolina: 2003–2013. Environ. Res. 2015, 143, 82–88. [Google Scholar] [CrossRef]
- Eriksson, U.; Mueller, J.F.; Toms, L.M.L.; Hobson, P.; Kärrman, A. Temporal trends of PFSAs, PFCAs and selected precursors in Australian serum from 2002 to 2013. Environ. Pollut. 2017, 220, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Coakley, J.; Bridgen, P.; Mueller, J.; Douwes, J.; Mannetje, A. Polybrominated diphenylethers and perfluorinated alkyl substances in blood serum of New Zealand adults, 2011-2013. Chemosphere 2018, 208, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, T.; Wang, P.; Fu, Q.; Lu, Y. Effects of age, gender and region on serum concentrations of perfluorinated compounds in general population of Henan, China. Chemosphere 2014, 110, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ssebugere, P.; Sillanpää, M.; Matovu, H.; Wang, Z.; Schramm, K.-W.; Omwoma, S.; Wanasolo, W.; Ngeno, E.C.; Odongo, S. Environmental levels and human body burdens of per- and poly-fluoroalkyl substances in Africa: A critical review. Sci. Total Environ. 2020, 739, 139913. [Google Scholar] [CrossRef]
- Miralles-Marco, A.; Harrad, S. Perfluoroocatane sulfonate: A review of human exposure, biomonitoring and the environmental forensics utility of it chirality and isomer distribution. Environ. Int. 2015, 77, 148–159. [Google Scholar] [CrossRef]
- Suganuma, K. Advances in lead free electronic soldering. Curr. Opin. Solid State Mater. Sci. 2001, 5, 55–64. [Google Scholar] [CrossRef]
- Sinclair, E.; Kim, S.E.; Akinleye, H.B.; Kannan, K. Quantitation of Gas.-Phase Perfluoroalkyl Surfactants and Fluorotelomer Alcohols Released from Nonstick Cookware and Microwave Popcorn Bags. Environ. Sci. Technol. 2007, 41, 1180–1185. [Google Scholar] [CrossRef]
- Kerger, B.D.; Loccisano, A.E.; Gerads, R.; Glassman, M.J. Small chamber study of lead exposures from manual soldering of microelectronics. Hum. Ecol. Risk Assess. Int. J. 2020. [Google Scholar] [CrossRef]
- Nordberg, G.; Fowler, B.A.; Nordberg, M. (Eds.) Handbook on the Toxicology of Metals, 4th ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 1013–1075, 1241–1285. [Google Scholar]
- MOFAD. Ministry of Fisheries and Aquaculture Development; Fishery and Aquaculture Country Profiles: Accra, Ghana, 2015. [Google Scholar]
- FAO. Fishery and Aquaculture Country Profiles—The Republic of Ghana; FAO: Roma, Italy, 2016. [Google Scholar]
- Agorku, E.S.; Voegborlo, R.B.; Adimado, A.A. Total mercury levels in nine species of freshwater fish from two hydroelectric reservoirs and a crater lake in Ghana. Environ. Monit. Assess. 2009, 153, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Oppong, S.O.B.; Voegborlo, R.B.; Agorku, S.E.; Adimado, A.A. Total Mercury in Fish, Sediments and Soil from the River Pra Basin, Southwestern Ghana. Bull. Environ. Contam. Toxicol. 2010, 85, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, L.; Cui, Y.; Chen, M.; Zhu, L. Probing mechanisms for bioaccumulation of perfluoroalkyl acids in carp (Cyprinus carpio): Impacts of protein binding affinities and elimination pathways. Sci. Total Environ. 2019, 647, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.Y.; Raymond, M.; Blackowicz, M.; Liu, Y.; Thompson, B.A.; Anderson, H.A.; Turyk, M. Perfluoroalkyl substances and fish consumption. Environ. Res. 2017, 154, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.; Kannan, K.; Isobe, T.; Takahasi, S.; Yamada, T.K.; Miyazaki, N.; Tanabe, S. Time Trends and Transplacental Transfer of Perfluorinated Compounds in Melon-Headed Whales Stranded Along the Japanese Coast in 1982, 2002/2002, and 2006. Environ. Sci. Technol. 2008, 42, 7132–7137. [Google Scholar] [CrossRef]
- Fair, P.A.; Wolf, B.; White, N.D.; Arnott, S.A.; Kannan, K.; Karthikraj, R.; Vena, J.E. Perfluoroalkyl substances (PFASs) in edible fish species from Charleston Harbor and tributaries, South Carolina, United States: Exposure and risk assessment. Environ. Res. 2019, 171, 266–277. [Google Scholar] [CrossRef] [PubMed]


| Referents (N = 65) | ERWs (N = 64) | LBRWs (N = 64) | FPTs (N = 25) | ||
|---|---|---|---|---|---|
| AM * (Min–Max) | AM (Min–Max) | AM (Min–Max) | AM (Min–Max) | ||
| pANOVA | |||||
| Age (years.) c | 30.2 (18–50) | 32.6 (18–50) | 31.8 (20–49) | 34.2 (20–49) | 0.17 |
| BMI (kg/m2) c | 23.0 (16.5–31.5) | 22.8 (17.9–29.4) | 23.3 (17.3–31.1) | 28.2 (16.2–40.0) | <0.001 |
| Work-years b | 8.9 (1–30) | 10.8 (2–30) | 11.4 (1–30) | 6.2 (1–20) | 0.007 |
| Smokers (in %) | 1.6 | 0 | 3.1 | 0 | Na § |
| Alcohol users (in %) | 20.3 | 23.4 | 15.6 | 4.0 | na |
| U-Sn (µg/g. cr.) †a | 0.32 (<LOD ‡–8.1) | 0.62 (0.09–9.1) | 0.24 (0.06–8.1) | 0.36 (0.11–1.9) | <0.01 |
| U-Hg (µg/g cr.) †c | 0.26 (<LOD–1.2) | 0.27 (<LOD–2.6) | 0.22 (<LOD–4.2) | 0.42 (<LOD–6.9) | 0.05 |
| B-Hg (µg/L) †b | 4.3 (1.5–18) | 3.6 (1.3–13) | 3.6 (1.0–9.3) | 3.6 (2.1–15) | 0.14 |
| Referents 1 | ERWs | LBRWs | FPTs | ||
|---|---|---|---|---|---|
| (N = 65) | (N = 64) | (N = 64) | (N = 25) | ||
| AM (Min–Max) | AM (Min–Max) | AM (Min–Max) | AM (Min–Max) | pANOVA | |
| PFOA abc | 0.66 (0.24–1.4) | 0.87 (0.32–1.7) | 0.52 (0.16–0.91) | 0.40 (0.17–0.61) | <0.001 |
| PFNA bc | 0.29 (0.11–0.84) | 0.28 (0.07–0.56) | 0.25 (0.08–0.55) | 0.24 (0.10–0.49) | 0.07 |
| PFDA | 0.17 (0.01–0.43) | 0.15 (0.05–0.39) | 0.15 (0.03–0.40) | 0.16 (0.05–0.33) | 0.58 |
| PFUnDA | 0.17 (<MLD ‡–0.53) | 0.15 (0.03–0.42) | 0.14 (<MLD–0.39) | 0.14 (0.06–0.33) | 0.20 |
| Σ-PFCA abc | 1.3 (0.39–3.3) | 1.5 (0.60–2.3) | 1.1 (0.50–1.9) | 0.95 (0.40–1.7) | <0.001 |
| L-PFHxS †c | 0.35 (0.09–1.2) | 0.37 (0.12–1.1) | 0.31 (0.10–0.83) | 0.21 (0.07–3.4) | <0.001 |
| Br-PFHxS †a | 0.49 (0.01–4.1) | 0.78 (0.07–5.3) | 0.65 (0.09–5.5) | 0.44 (0.09–6.9) | 0.02 |
| Σ-PFHxS †a | 0.99 (0.35–4.2) | 1.3 (0.25–6.1) | 1.1 (0.25–5.8) | 0.75 (0.23–7.1) | 0.008 |
| L-PFHpS c | 0.08 (<MLD–0.28) | 0.08 (<MLD–0.16) | 0.08 (0.04–0.19) | 0.02 (<MLD–0.12) | <0.001 |
| Br-PFHpS c | 0.02 (<MLD–0.06) | 0.03 (<MLD–0.06) | 0.02 (<MLD–0.04) | 0.01 (<MLD–0.04) | <0.001 |
| Σ-PFHpS c | 0.10 (<MLD–0.32) | 0.11 (<MLD–0.21) | 0.10 (0.05–0.22) | 0.03 (<MLD–0.16) | <0.001 |
| L-PFOS b | 1.8 (0.33–5.3) | 1.7 (0.49–4.4) | 1.4 (0.20–3.6) | 1.4 (0.53–3.3) | 0.04 |
| Br-PFOS c | 1.4 (0.43–3.6) | 1.4 (0.37–3.3) | 1.2 (0.46–2.9) | 0.80 (0.29–1.7) | <0.001 |
| Σ-PFOS bc | 3.2 (0.88–7.4) | 3.2 (1.1–6.6) | 2.7 (0.77–5.6) | 2.2 (0.82–4.5) | 0.002 |
| Σ-PFSA c | 4.5 (1.4–10.1) | 4.9 (1.7–11.5) | 4.1 (1.7–8.0) | 3.4 (1.2–9.3) | 0.003 |
| ERW | B-Hg (lg) | Age | BMI | Sex | Multipler | |
|---|---|---|---|---|---|---|
| PFOA | 0.29 *** | 0.32 *** | - | - | 0.18 ** | 0.59 *** |
| PFNA | - | 0.19 *** | 0.002 * | −0.004 * | - | 0.43 *** |
| PFDA | - | 0.13 *** | 0.001 * | - | - | 0.40 *** |
| PFUnDA | - | 0.14 *** | - | - | - | 0.35 *** |
| L-PFHxS (lg) | - | 0.26 *** | 0.008 *** | - | 0.22 *** | 0.53 *** |
| Br-PFHxS (lg) | 0.16 * | - | - | - | - | 0.17 * |
| Σ-PFHxS (lg) | 0.09 * | - | - | - | 0.14 * | 0.23 ** |
| L-PFHpS | - | 0.05 *** | 0.001 *** | - | 0.06 *** | 0.59 *** |
| Br-PFHpS | 0.005 * | 0.01 ** | 0.001 *** | - | 0.02 *** | 0.55 *** |
| Σ-PFHpS | - | 0.06 *** | 0.002 *** | - | 0.08 *** | 0.60 *** |
| L-PFOS | - | 1.2 *** | 0.02 * | −0.04 ** | - | 0.37 *** |
| Br-PFOS | - | 0.79 *** | 0.02 *** | - | 0.57 *** | 0.54 *** |
| Σ-PFOS | - | 1.9 *** | 0.03 ** | - | 0.83 ** | 0.47 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dartey, E.; Ellingsen, D.G.; Berlinger, B.; Thomassen, Y.; Odland, J.Ø.; Brox, J.; Nartey, V.K.; Yeboah, F.A.; Huber, S. Per- and Polyfluoroalkyl Substances in Human Serum Samples of Selected Populations from Ghana. Int. J. Environ. Res. Public Health 2021, 18, 1581. https://doi.org/10.3390/ijerph18041581
Dartey E, Ellingsen DG, Berlinger B, Thomassen Y, Odland JØ, Brox J, Nartey VK, Yeboah FA, Huber S. Per- and Polyfluoroalkyl Substances in Human Serum Samples of Selected Populations from Ghana. International Journal of Environmental Research and Public Health. 2021; 18(4):1581. https://doi.org/10.3390/ijerph18041581
Chicago/Turabian StyleDartey, Emmanuel, Dag G. Ellingsen, Balazs Berlinger, Yngvar Thomassen, Jon Ø. Odland, Jan Brox, Vincent K. Nartey, Francis A. Yeboah, and Sandra Huber. 2021. "Per- and Polyfluoroalkyl Substances in Human Serum Samples of Selected Populations from Ghana" International Journal of Environmental Research and Public Health 18, no. 4: 1581. https://doi.org/10.3390/ijerph18041581
APA StyleDartey, E., Ellingsen, D. G., Berlinger, B., Thomassen, Y., Odland, J. Ø., Brox, J., Nartey, V. K., Yeboah, F. A., & Huber, S. (2021). Per- and Polyfluoroalkyl Substances in Human Serum Samples of Selected Populations from Ghana. International Journal of Environmental Research and Public Health, 18(4), 1581. https://doi.org/10.3390/ijerph18041581






