COVID-19 Community Incidence and Associated Neighborhood-Level Characteristics in Houston, Texas, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Dependent Variable
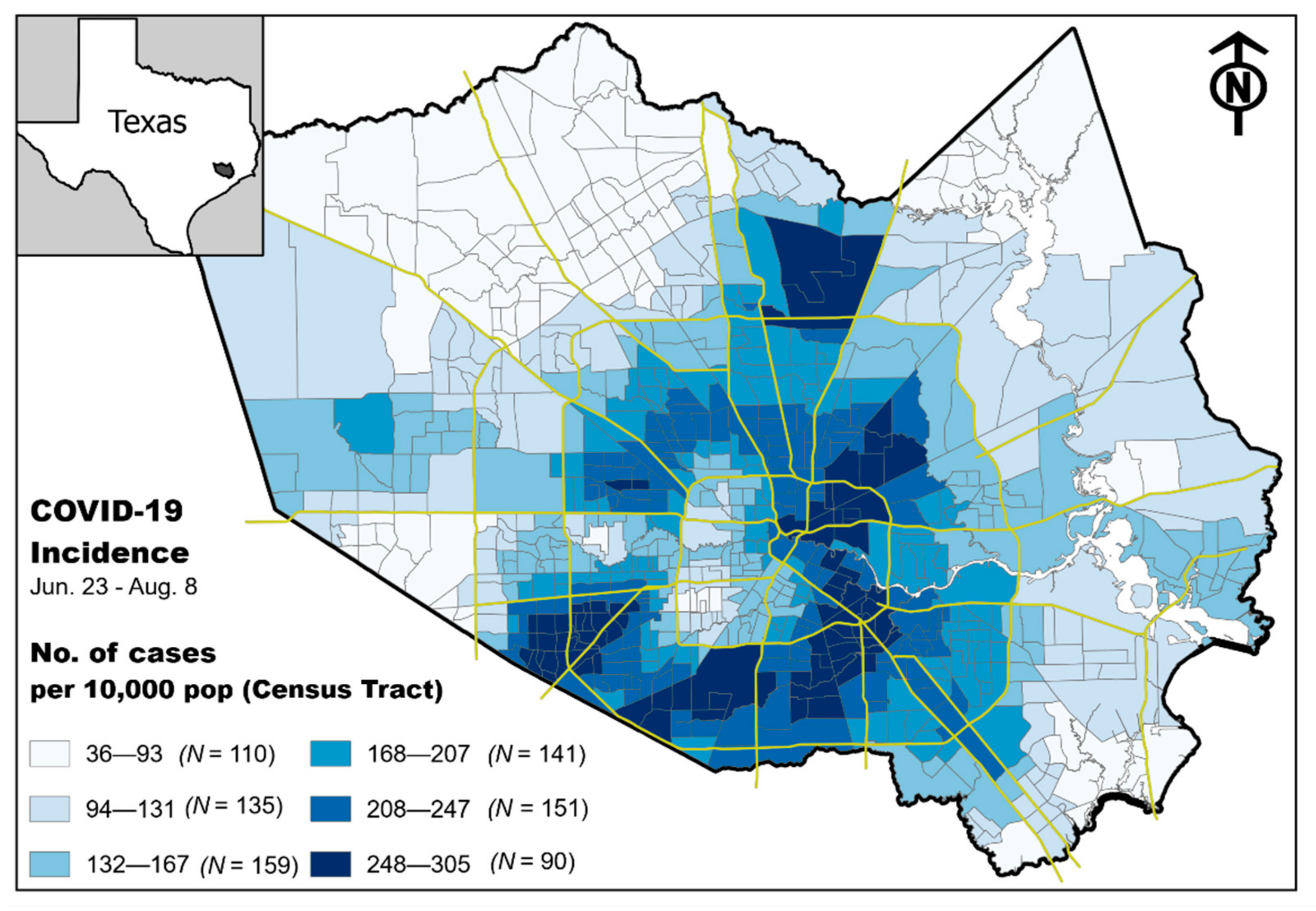
COVID-19 Neighborhood-Level Community Incidence
2.3. Independent Variables
2.4. Data Analysis
3. Results
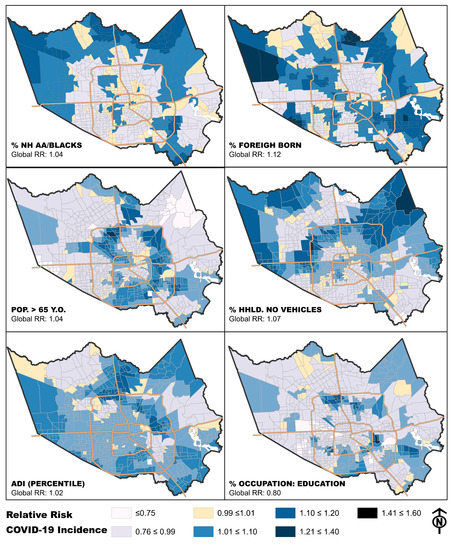
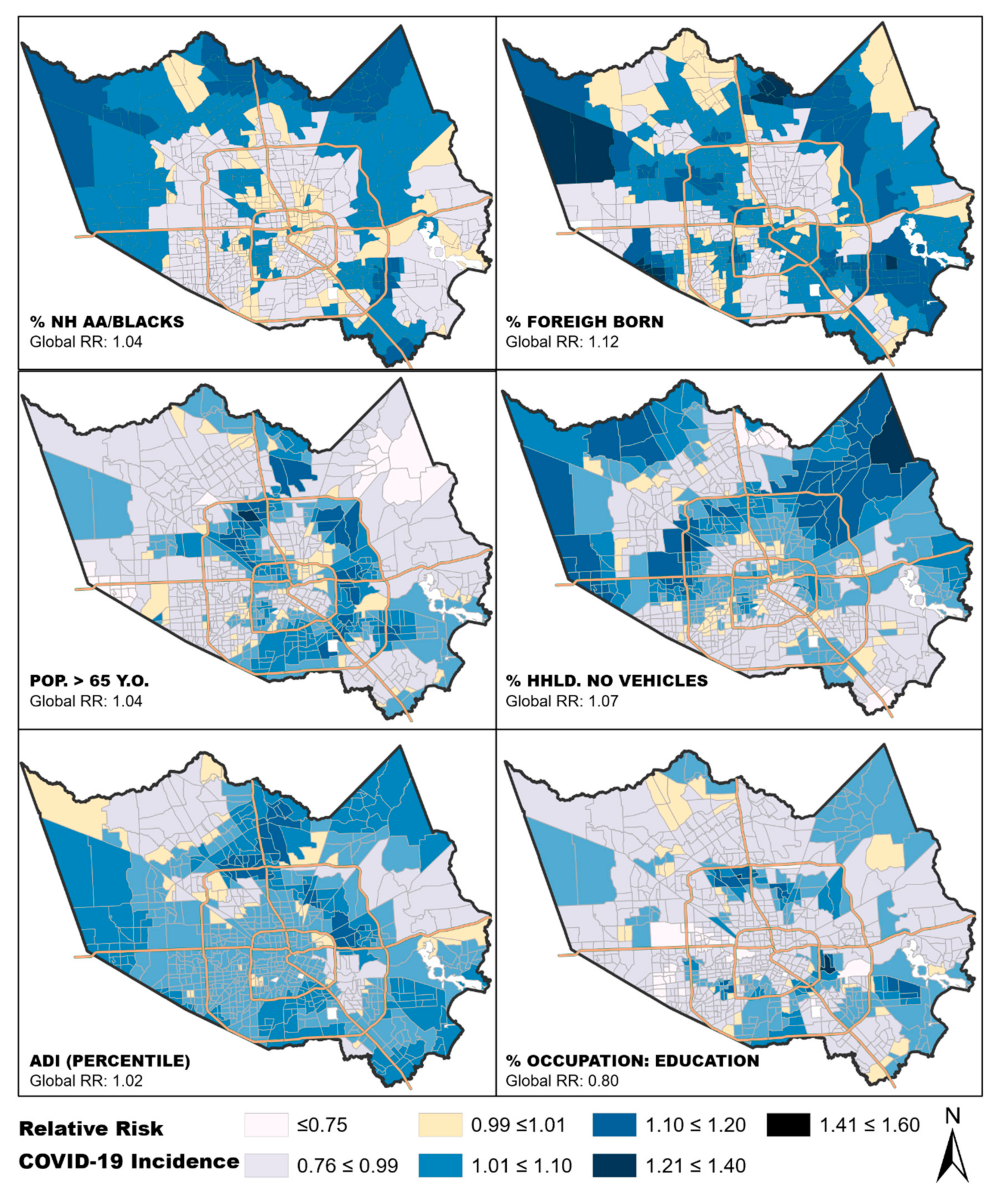
3.1. Exploring Relationships between COVID-19 Incidence and Individual Neighborhood Factors
3.2. Domain-Specific Relationships between Neighborhood Factors and COVID-19
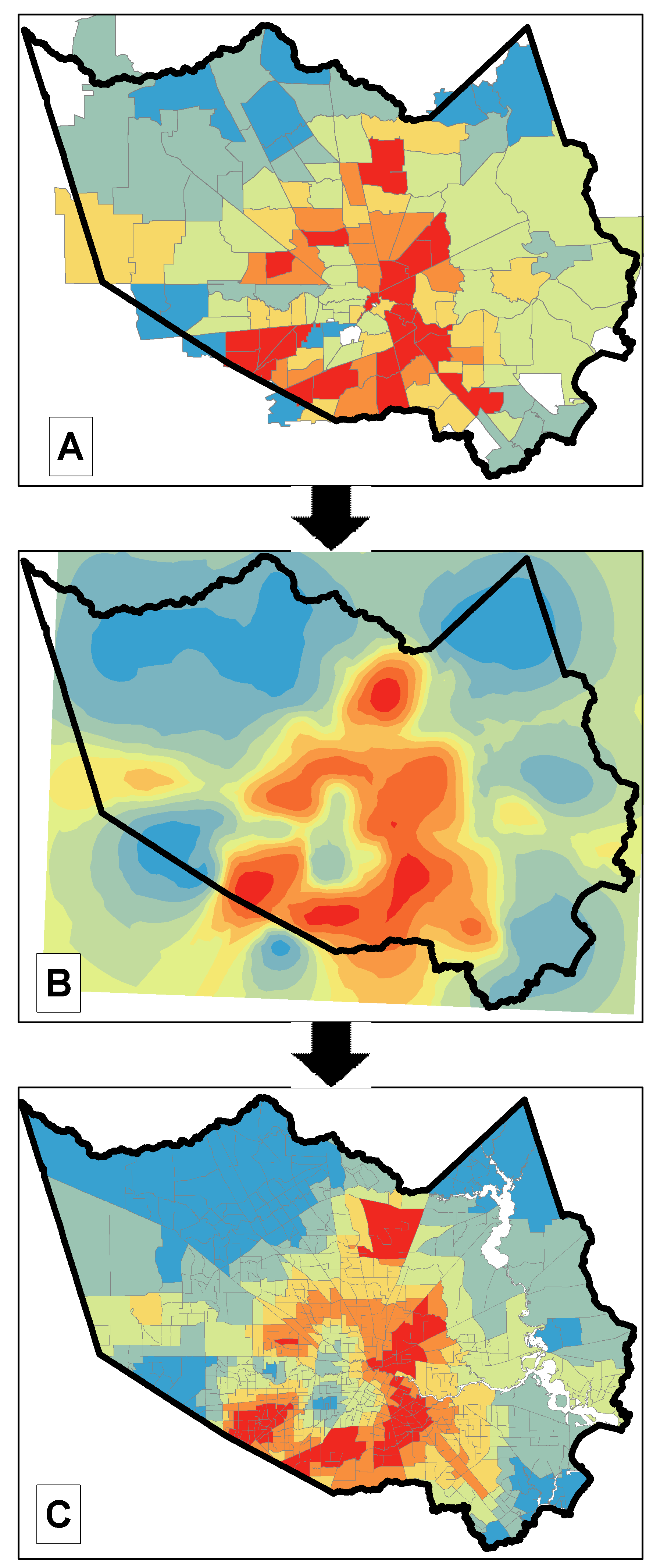
3.3. Across-Domains Relationships between Neighborhood Factor and COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramzy, A.; McNeil, D. WHO Declares Global Emergency as Wuhan Coronavirus Spreads. The New York Times. Available online: https://nyti.ms/2RER70M (accessed on 22 October 2020).
- Coronavirus COVID-19 Global Cases by Johns Hopkins CSSE; Gisanddata. maps. arcgis. com; Johns Hopkins University (JHU): Baltimore, MD, USA, 2020.
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Jernigan, D.B. Update: Public Health Response to the Coronavirus Disease 2019 Outbreak—United STATES, 24 February 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Office of Disease Prevention and Health Promotion. Healthy People 2020. 2010. Available online: https://www.healthypeople.gov/2020 (accessed on 22 October 2020).
- Gottlieb, L.M.; Tirozzi, K.J.; Manchanda, R.; Burns, A.R.; Sandel, M.T. Moving electronic medical records upstream: Incorporating social determinants of health. Am. J. Prev. Med. 2015, 48, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Surendran, R.; Agarwal, P.; Domaratzky, M.; Li, J.; Hussain, S. It Matters! Teaching Social Determinants of Health in the Intensive Care Unit to Healthcare Providers. In C40. Critical Care: The Art of War-Innovations in Education; American Thoracic Society: New York, NY, USA, 2019; p. A4784. [Google Scholar]
- Braveman, P.; Egerter, S.; Williams, D.R. The social determinants of health: Coming of age. Annu. Rev. Public Health 2011, 32, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Shavers, V.L. Measurement of socioeconomic status in health disparities research. J. Natl. Med. Assoc. 2007, 99, 1013. [Google Scholar] [PubMed]
- Cederberg, M.; Hartsmar, N.; Lingärde, S. Thematic Report: Socioeconomic Disadvantage. Available online: https://muep.mau.se/bitstream/handle/2043/7982/ThematicSOCfinal.pdf?sequence=1 (accessed on 4 January 2021).
- Sharma, S.V.; Chuang, R.J.; Rushing, M.; Naylor, B.; Ranjit, N.; Pomeroy, M.; Markham, C. Social Determinants of Health-Related Needs During COVID-19 Among Low-Income Households With Children. Prev. Chronic Dis. 2020, 17, E119. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.E.; Rehkopf, D.H. US disparities in health: Descriptions, causes, and mechanisms. Annu. Rev. Public Health 2008, 29, 235–252. [Google Scholar] [CrossRef]
- Macintyre, S.; Ellaway, A. Neighborhoods and health: An overview. Neighb. Health 2003, 20, 42. [Google Scholar] [CrossRef]
- Robert, S.A. Socioeconomic position and health: The independent contribution of community socioeconomic context. Annu. Rev. Sociol. 1999, 25, 489–516. [Google Scholar] [CrossRef]
- Rosenkrantz, L.; Schuurman, N.; Bell, N.; Amram, O. The need for GIScience in mapping COVID-19. Health Place 2020, 67, 102389. [Google Scholar] [CrossRef]
- Franch-Pardo, I.; Napoletano, B.M.; Rosete-Verges, F.; Billa, L. Spatial analysis and GIS in the study of COVID-19. A review. Sci. Total Environ. 2020, 739, 140033. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, M.; Hohl, A.; Delmelle, E. Rapid surveillance of COVID-19 in the United States using a prospective space-time scan statistic: Detecting and evaluating emerging clusters. Appl. Geogr. 2020, 118, 102202. [Google Scholar] [CrossRef]
- Khose, S.; Moore, J.X.; Wang, H.E. Epidemiology of the 2020 Pandemic of COVID-19 in the State of Texas: The First Month of Community Spread. J. Community Health 2020, 45, 696–701. [Google Scholar] [CrossRef]
- Zhang, C.H.; Schwartz, G.G. Spatial disparities in coronavirus incidence and mortality in the United States: An ecological analysis as of May 2020. J. Rural Health 2020, 36, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Bilal, U.; Barber, S.; Diez-Roux, A.V. Spatial inequities in COVID-19 outcomes in 3 US cities. Version 2. medRxiv 2020. [Google Scholar] [CrossRef]
- Millett, G.A.; Jones, A.T.; Benkeser, D.; Baral, S.; Mercer, L.; Beyrer, C.; Honermann, B.; Lankiewicz, E.; Mena, L.; Crowley, J.S.; et al. Assessing differential impacts of COVID-19 on black communities. Ann. Epidemiol. 2020, 47, 37–44. [Google Scholar] [CrossRef]
- Kopel, J.; Perisetti, A.; Roghani, A.; Aziz, M.; Gajendran, M.; Goyal, H. Racial and Gender-Based Differences in COVID-19. Front. Public Health 2020, 8, 418. [Google Scholar] [CrossRef]
- Cuomo, R.E. Shift in racial communities impacted by COVID-19 in California. J. Epidemiol. Community Health 2020. [Google Scholar] [CrossRef]
- Feinhandler, I.; Cilento, B.; Beauvais, B.; Harrop, J.; Fulton, L. Predictors of Death Rate during the COVID-19 Pandemic. Healthcare 2020, 8, 339. [Google Scholar] [CrossRef]
- Mollalo, A.; Rivera, K.M.; Vahedi, B. Artificial Neural Network Modeling of Novel Coronavirus (COVID-19) Incidence Rates across the Continental United States. Int. J. Environ. Res. Public Health 2020, 17, 4204. [Google Scholar] [CrossRef] [PubMed]
- Sugg, M.M.; Spaulding, T.J.; Lane, S.J.; Runkle, J.D.; Harden, S.R.; Hege, A.; Iyer, L.S. Mapping community-level determinants of COVID-19 transmission in nursing homes: A multi-scale approach. Sci. Total Environ. 2020, 752, 141946. [Google Scholar] [CrossRef] [PubMed]
- Mollalo, A.; Vahedi, B.; Rivera, K.M. GIS-based spatial modeling of COVID-19 incidence rate in the continental United States. Sci. Total Environ. 2020, 728, 138884. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, I.J.; Lee, J. COVID-19 Emergence and Social and Health Determinants in Colorado: A Rapid Spatial Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3856. [Google Scholar] [CrossRef]
- Maroko, A.R.; Nash, D.; Pavilonis, B.T. COVID-19 and Inequity: A Comparative Spatial Analysis of New York City and Chicago Hot Spots. J. Urban Health 2020, 97, 461–470. [Google Scholar] [CrossRef]
- Werner, A.K.; Strosnider, H. Developing a surveillance system of sub-county data: Finding suitable population thresholds for geographic aggregations. Spat. Spatio Temporal Epidemiol. 2020, 33, 100339. [Google Scholar] [CrossRef]
- Werner, A.K.; Strosnider, H.; Kassinger, C.; Shin, M.; Workgroup, S.-C.D.P. Lessons Learned From the Environmental Public Health Tracking Sub-County Data Pilot Project. J. Public Health Manag. Pract. 2018, 24, E20. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. QuickFacts: Harris County, Texas. 2017. Available online: https://www.census.gov/quickfacts/table/PST045215/48201,00 (accessed on 10 May 2017).
- United States Census Bureau. QuickFacts: Houston city, Texas; Harris County, Texas; United States. 2020. Available online: https://www.census.gov/quickfacts/fact/table/houstoncitytexas,harriscountytexas,US/RHI425219#RHI425219 (accessed on 29 July 2020).
- Arias, E.; Escobedo, L.A.; Kennedy, J.; Fu, C.; Cisewski, J.A. US Small-Area Life Expectancy Estimates Project: Methodology and Results Summary; CDC: Atlanta, GA, USA, 2018. [Google Scholar]
- Census Bureau. Geographic Areas Reference Manual, Chapter 10: Census Tracts and Block Numbering Areas; Census Bureau: Suitland, MD, USA, 1994. [Google Scholar]
- Wortham, J.M. Characteristics of Persons Who Died with COVID-19—United States, February 12–May 18, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 923–929. [Google Scholar] [CrossRef]
- ESRI. Using Areal Interpolation to Perform Polygon-to-Polygon Predictions. Available online: https://pro.arcgis.com/en/pro-app/help/analysis/geostatistical-analyst/using-areal-interpolation-to-predict-to-new-polygons.htm (accessed on 3 July 2020).
- Hallisey, E.; Tai, E.; Berens, A.; Wilt, G.; Peipins, L.; Lewis, B.; Graham, S.; Flanagan, B.; Lunsford, N.B. Transforming geographic scale: A comparison of combined population and areal weighting to other interpolation methods. Int. J. Health Geogr. 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Krivoruchko, K.; Gribov, A.; Krause, E. Multivariate areal interpolation for continuous and count data. Procedia Environ. Sci. 2011, 3, 14–19. [Google Scholar] [CrossRef]
- Comber, A.; Zeng, W. Spatial interpolation using areal features: A review of methods and opportunities using new forms of data with coded illustrations. Geogr. Compass 2019, 13, e12465. [Google Scholar] [CrossRef]
- Census. American Community Survey Information Guide. 2017. Available online: https://www.census.gov/content/dam/Census/programs-surveys/acs/about/ACS_Information_Guide.pdf (accessed on 20 April 2019).
- Knighton, A.J.; Savitz, L.; Belnap, T.; Stephenson, B.; VanDerslice, J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: Implications for population health. eGEMs 2016, 4, 1238. [Google Scholar] [CrossRef]
- Singh, G.K. Area deprivation and widening inequalities in US mortality, 1969–1998. Am. J. Public Health 2003, 93, 1137–1143. [Google Scholar] [CrossRef]
- Cameron, A.C.; Trivedi, P.K. Regression Analysis of Count Data; Cambridge University Press: Cambridge, UK, 2013; Volume 53. [Google Scholar]
- Lord, D. Modeling motor vehicle crashes using Poisson-gamma models: Examining the effects of low sample mean values and small sample size on the estimation of the fixed dispersion parameter. Accid. Anal. Prev. 2006, 38, 751–766. [Google Scholar] [CrossRef]
- Brunsdon, C.; Fotheringham, A.S.; Charlton, M.E. Geographically weighted regression: A method for exploring spatial nonstationarity. Geogr. Anal. 1996, 28, 281–298. [Google Scholar] [CrossRef]
- Fotheringham, A.S.; Brunsdon, C.; Charlton, M. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Nakaya, T.; Fotheringham, A.S.; Brunsdon, C.; Charlton, M. Geographically weighted Poisson regression for disease association mapping. Stat. Med. 2005, 24, 2695–2717. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Szefler, S.J. COVID-19 and the impact of social determinants of health. Lancet Respir. Med. 2020, 8, 659–661. [Google Scholar] [CrossRef]
- Cummins, S.; Curtis, S.; Diez-Roux, A.V.; Macintyre, S. Understanding and representing ‘place’in health research: A relational approach. Soc. Sci. Med. 2007, 65, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Boyce, W.F.; Simpson, K.; Pickett, W. Influence of individual-and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. Am. J. Clin. Nutr. 2006, 83, 139–145. [Google Scholar] [CrossRef]
- Diez Roux, A.V.; Mair, C. Neighborhoods and health. Ann. N. Y. Acad. Sci. 2010, 1186, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Haan, M.; Kaplan, G.A.; Camacho, T. Poverty and health. Prospective evidence from the Alameda County Study. Am. J. Epidemiol. 1987, 125, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Sydenstricker, E. The incidence of influenza among persons of different economic status during the epidemic of 1918. Public Health Rep. 1931, 121, 191–204, discussion 190. [Google Scholar] [CrossRef]
- Bambra, C.; Riordan, R.; Ford, J.; Matthews, F. The COVID-19 pandemic and health inequalities. J. Epidemiol. Community Health 2020, 74, 964–968. [Google Scholar] [CrossRef]
- Murray, C.J.; Lopez, A.D.; Chin, B.; Feehan, D.; Hill, K.H. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918-20 pandemic: A quantitative analysis. Lancet 2006, 368, 2211–2218. [Google Scholar] [CrossRef]
- Bengtsson, T.; Dribe, M.; Eriksson, B. Social Class and Excess Mortality in Sweden During the 1918 Influenza Pandemic. Am. J. Epidemiol. 2018, 187, 2568–2576. [Google Scholar] [CrossRef]
- Mamelund, S.E. A socially neutral disease? Individual social class, household wealth and mortality from Spanish influenza in two socially contrasting parishes in Kristiania 1918-19. Soc. Sci. Med. 2006, 62, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Jarvis, M.J.; Steggles, N.; Sutton, S.; Williamson, S.; Farrimond, H.; Cartwright, M.; Simon, A.E. Socioeconomic disparities in cancer-risk behaviors in adolescence: Baseline results from the Health and Behaviour in Teenagers Study (HABITS). Prev. Med. 2003, 36, 721–730. [Google Scholar] [CrossRef]
- Butler, D.C.; Petterson, S.; Phillips, R.L.; Bazemore, A.W. Measures of Social Deprivation That Predict Health Care Access and Need within a Rational Area of Primary Care Service Delivery. Health Serv. Res. 2013, 48, 539–559. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Azuine, R.E.; Siahpush, M. Widening Socioeconomic, Racial, and Geographic Disparities in HIV/AIDS Mortality in the United States, 1987–2011. Adv. Prev. Med. 2013, 2013, 657961. [Google Scholar] [CrossRef]
- Hatef, E.; Chang, H.Y.; Kitchen, C.; Weiner, J.P.; Kharrazi, H. Assessing the Impact of Neighborhood Socioeconomic Characteristics on COVID-19 Prevalence Across Seven States in the United States. Front. Public Health 2020, 8, 571808. [Google Scholar] [CrossRef]
- Malik, A.A.; McFadden, S.M.; Elharake, J.; Omer, S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 2020, 26, 100495. [Google Scholar] [CrossRef] [PubMed]
- Kearns, R.; Moon, G. From medical to health geography: Novelty, place and theory after a decade of change. Prog. Hum. Geogr. 2002, 26, 605–625. [Google Scholar] [CrossRef]
| Race/Ethnicity and Nativity |
| % Non-Hispanic white pop. |
| % Black or African American pop. |
| % Asian pop. |
| % Other race + two or more races pop. |
| % Hispanic or Latino pop. |
| % Foreign-born pop. who is not a United States citizen |
| Socioeconomic Disadvantage |
| Area Deprivation Index (ADI) * |
| Disaster Vulnerabilities |
| % of households with no vehicle available |
| % of adults 18 y and over who have limited English ability |
| % of pop. with a disability |
| % of pop. with no health insurance coverage |
| Over 65 Years Old |
| % of pop. that is 65 y and over |
| % of pop. 65 y and over who live alone |
| % of pop. 65 y and over with a disability |
| % of pop. 65 y and over living in quarters |
| Occupation |
| % Health and healthcare support |
| % Human Services |
| % Management, science, technology |
| % Mobile workers, construction, maintenance |
| % Food preparation |
| % Personal care |
| % Education/Training/Library |
| Senior Care Facilities |
| No. of assisted living inside census tract |
| Capacity of assisted living inside census tract |
| No. of nursing homes inside census tract |
| Capacity of nursing homes inside census tract |
| Access to Technology |
| % of Households that have no computer, smartphone, or tablet |
| % Households with cellular data plan with no other type of internet subscription |
| % of Households with no internet access |
| Independent Variables | Coeff. | Coeff. 95% CI | IRR | IRR 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| Race, ethnicity, nativity | |||||||
| % Non-Hispanic White pop | −0.099 | −0.108 | −0.091 | 0.906 | 0.989 | 0.991 | <0.001 |
| % Black or African American pop | 0.043 | 0.029 | 0.056 | 1.044 | 1.003 | 1.006 | <0.001 |
| % Asian pop. | −0.105 | −0.139 | −0.071 | 0.900 | 0.986 | 0.993 | <0.001 |
| % Other race + two or more races pop | −0.755 | −0.906 | −0.605 | 0.470 | 0.913 | 0.941 | <0.001 |
| % Hispanic or Latino pop | 0.074 | 0.064 | 0.083 | 1.077 | 1.006 | 1.008 | <0.001 |
| % Foreign-born pop. who is not a United States citizen | 0.109 | 0.090 | 0.128 | 1.115 | 1.009 | 1.013 | <0.001 |
| Socioeconomic disadvantage | |||||||
| Area Deprivation Index (ADI) | 0.710 | 0.627 | 0.792 | 2.034 | 1.873 | 2.208 | <0.001 |
| Disaster vulnerabilities | |||||||
| % of households with no vehicle available | 0.266 | 0.225 | 0.307 | 1.305 | 1.023 | 1.031 | <0.001 |
| % of adults 18 y and over who have limited English ability | 0.104 | 0.090 | 0.118 | 1.109 | 1.009 | 1.012 | <0.001 |
| % of pop. with a disability | 0.153 | 0.091 | 0.215 | 1.166 | 1.009 | 1.022 | <0.001 |
| % of pop. with no health insurance coverage | 0.186 | 0.166 | 0.206 | 1.204 | 1.017 | 1.021 | <0.001 |
| Over 65 years old | |||||||
| % of pop. that is 65 y and over | −0.097 | −0.150 | −0.043 | 0.908 | 0.985 | 0.996 | <0.001 |
| % of pop. 65 y and over who lives alone | 0.033 | 0.013 | 0.052 | 1.033 | 1.001 | 1.005 | 0.001 |
| % of pop. 65 y and over with a disability | 0.063 | 0.044 | 0.083 | 1.065 | 1.004 | 1.008 | <0.001 |
| % of pop. 65 y and over living in quarters | 0.035 | −0.005 | 0.075 | 1.035 | 0.999 | 1.007 | 0.090 |
| Occupation | |||||||
| % Health and healthcare support | −0.025 | −0.148 | 0.098 | 0.976 | 0.985 | 1.010 | 0.693 |
| % Human services | −0.109 | −0.173 | −0.045 | 0.897 | 0.983 | 0.996 | 0.001 |
| % Management, science, technology | −0.075 | −0.084 | −0.066 | 0.928 | 0.992 | 0.993 | <0.001 |
| % Mobile workers, construction, maintenance | 0.034 | 0.016 | 0.052 | 1.034 | 1.002 | 1.005 | <0.001 |
| % Food preparation | 0.260 | 0.194 | 0.326 | 1.296 | 1.020 | 1.033 | <0.001 |
| % Personal care | 0.215 | 0.107 | 0.323 | 1.240 | 1.011 | 1.033 | <0.001 |
| % Education/Training/Library | −0.427 | −0.477 | −0.377 | 0.652 | 0.953 | 0.963 | <0.001 |
| Access to technology | |||||||
| % of households that have no computer, smartphone, or tablet | 0.196 | 0.172 | 0.220 | 1.217 | 1.017 | 1.022 | <0.001 |
| % Households with cellular data plan; no other type of internet | 0.182 | 0.159 | 0.205 | 1.200 | 1.016 | 1.021 | <0.001 |
| % of households with no internet access | 0.161 | 0.144 | 0.179 | 1.175 | 1.014 | 1.018 | <0.001 |
| Senior care facilities | |||||||
| No. of assisted living inside census tract | −0.314 | −0.619 | −0.009 | 0.731 | 0.940 | 0.999 | 0.044 |
| Capacity of assisted living inside census tract | −0.016 | −0.023 | −0.010 | 0.984 | 0.998 | 0.999 | <0.001 |
| No. of nursing homes inside census tract | −0.402 | −1.084 | 0.280 | 0.669 | 0.897 | 1.028 | 0.248 |
| Capacity of nursing homes inside census tract | −0.002 | −0.007 | 0.004 | 0.998 | 0.999 | 1.000 | 0.575 |
| Independent Variable | Coeff. | Coeff. 95% CI | IRR | IRR 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| Race, ethnicity, nativity | |||||||
| % Non-Hispanic white pop. | n.s. | ||||||
| % Black or African American pop. | 0.0831 | 0.072 | 0.094 | 1.087 | 1.075 | 1.098 | <0.001 |
| % Other race + two or more races pop. | n.s. | ||||||
| % Hispanic or Latino pop. | 0.0739 | 0.064 | 0.084 | 1.077 | 1.066 | 1.088 | <0.001 |
| % Foreign-born pop. not a United States citizen | 0.0635 | 0.044 | 0.083 | 1.066 | 1.045 | 1.087 | <0.001 |
| Socioeconomic disadvantage | |||||||
| Area Deprivation Index (ADI) | 0.7100 | 0.630 | 0.790 | 2.034 | 1.878 | 2.203 | <0.001 |
| Disaster vulnerabilities | |||||||
| % of households with no vehicle available | 0.1268 | 0.086 | 0.168 | 1.135 | 1.090 | 1.182 | <0.001 |
| % of adults 18 y and over who have limited English ability | 0.0324 | 0.008 | 0.057 | 1.033 | 1.008 | 1.058 | 0.009 |
| % of pop. with a Disability | 0.1125 | 0.054 | 0.171 | 1.119 | 1.056 | 1.186 | <0.001 |
| % of pop. with No health insurance coverage | 0.1166 | 0.079 | 0.154 | 1.124 | 1.082 | 1.167 | <0.001 |
| Over 65 years old | |||||||
| % of pop. that is 65 y and over | −0.0949 | −0.111 | −0.079 | 0.910 | 0.895 | 0.924 | <0.001 |
| % of pop. 65 y and over who lives alone | 0.0306 | 0.025 | 0.036 | 1.031 | 1.026 | 1.036 | 0.017 |
| % of pop. 65 y and over with a disability | 0.0585 | 0.053 | 0.064 | 1.060 | 1.054 | 1.066 | <0.001 |
| % of pop. 65 y and over living in quarters | n.s. | ||||||
| Occupation | |||||||
| % Healthcare | n.s. | ||||||
| % Human services | n.s. | ||||||
| % Management, science, technology | −0.0442 | −0.060 | −0.029 | 0.957 | 0.942 | 0.972 | <0.001 |
| % Mobile workers, construction, maintenance | 0.0580 | 0.043 | 0.073 | 1.060 | 1.044 | 1.075 | <0.001 |
| % Food preparation | n.s. | ||||||
| % Personal care | 0.0996 | 0.008 | 0.191 | 1.105 | 1.008 | 1.210 | <0.001 |
| % Education/Training/Library | −0.2612 | −0.345 | −0.177 | 0.770 | 0.708 | 0.838 | <0.001 |
| Access to technology | |||||||
| % of households that have no computer, smartphone, or tablet | n.s. | ||||||
| % Households with cellular data plan; no other type of internet | 0.0943 | 0.068 | 0.121 | 1.099 | 1.070 | 1.128 | <0.001 |
| % of Households with no internet access | 0.1144 | 0.093 | 0.136 | 1.121 | 1.098 | 1.145 | <0.001 |
| Senior care facilities | |||||||
| No. of assisted living inside census tract | n.s. | ||||||
| Capacity of assisted living inside census tract | −0.016 | −0.023 | −0.010 | 0.984 | 0.978 | 0.990 | <0.001 |
| No. of nursing homes inside census tract | n.s. | ||||||
| Capacity of nursing homes inside census tract | n.s. | ||||||
| Independent Variable | Coeff. | Coeff. 95% CI | IRR | IRR 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| % Black or African American pop. | 0.0267 | 0.0131 | 0.040 | 1.027 | 1.013 | 1.041 | <0.001 |
| % Foreign-born pop. not a United States citizen | 0.1066 | 0.0806 | 0.133 | 1.112 | 1.084 | 1.142 | <0.001 |
| Area Deprivation Index (ADI) | 0.2709 | 0.1700 | 0.372 | 1.311 | 1.185 | 1.450 | <0.001 |
| % of households with no vehicle available | 0.0741 | 0.0327 | 0.116 | 1.077 | 1.033 | 1.122 | <0.001 |
| % of pop. that is 65 y and over | 0.0588 | 0.0085 | 0.109 | 1.061 | 1.009 | 1.115 | 0.022 |
| % Education/Training/Library occupation | −0.1941 | −0.2497 | −0.139 | 0.824 | 0.779 | 0.871 | <0.001 |
| Capacity of assisted living inside census tract | −0.0077 | −0.0130 | −0.002 | 0.992 | 0.987 | 0.998 | 0.004 |
| Local Terms | Mean | STD | Min. | Lower Quartile | Median | Upper Quartile | Max. |
|---|---|---|---|---|---|---|---|
| Intercept | −47.19 | 8.07 | −81.00 | −51.51 | −45.18 | −41.65 | −22.34 |
| % NH Black or African American pop. | 0.00 | 0.06 | −0.24 | −0.03 | −0.01 | 0.03 | 0.18 |
| % Foreign-born pop. not a United States citizen | 0.02 | 0.08 | −0.29 | −0.02 | 0.02 | 0.07 | 0.29 |
| Area Deprivation Index (ADI) | 0.06 | 0.07 | −0.18 | 0.02 | 0.05 | 0.10 | 0.34 |
| % of households with no vehicle available | 0.01 | 0.12 | −0.48 | −0.05 | 0.01 | 0.07 | 0.38 |
| % of pop. that is 65 y and over | −0.01 | 0.13 | −0.43 | −0.09 | −0.01 | 0.07 | 0.34 |
| % Education/Training/Library occupation | −0.06 | 0.14 | −0.65 | −0.12 | −0.05 | 0.02 | 0.47 |
| Indicators | GWPR | Global Model |
|---|---|---|
| AIC | 1422.19 | 6242.63 |
| AICc | 1254.09 | 6242.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluyomi, A.O.; Gunter, S.M.; Leining, L.M.; Murray, K.O.; Amos, C. COVID-19 Community Incidence and Associated Neighborhood-Level Characteristics in Houston, Texas, USA. Int. J. Environ. Res. Public Health 2021, 18, 1495. https://doi.org/10.3390/ijerph18041495
Oluyomi AO, Gunter SM, Leining LM, Murray KO, Amos C. COVID-19 Community Incidence and Associated Neighborhood-Level Characteristics in Houston, Texas, USA. International Journal of Environmental Research and Public Health. 2021; 18(4):1495. https://doi.org/10.3390/ijerph18041495
Chicago/Turabian StyleOluyomi, Abiodun O., Sarah M. Gunter, Lauren M. Leining, Kristy O. Murray, and Chris Amos. 2021. "COVID-19 Community Incidence and Associated Neighborhood-Level Characteristics in Houston, Texas, USA" International Journal of Environmental Research and Public Health 18, no. 4: 1495. https://doi.org/10.3390/ijerph18041495
APA StyleOluyomi, A. O., Gunter, S. M., Leining, L. M., Murray, K. O., & Amos, C. (2021). COVID-19 Community Incidence and Associated Neighborhood-Level Characteristics in Houston, Texas, USA. International Journal of Environmental Research and Public Health, 18(4), 1495. https://doi.org/10.3390/ijerph18041495