The Effect of Dry Carbon Dioxide Bathing on Peripheral Blood Circulation Measured by Thermal Imaging among Patients with Risk Factors of PAD
Abstract
1. Introduction
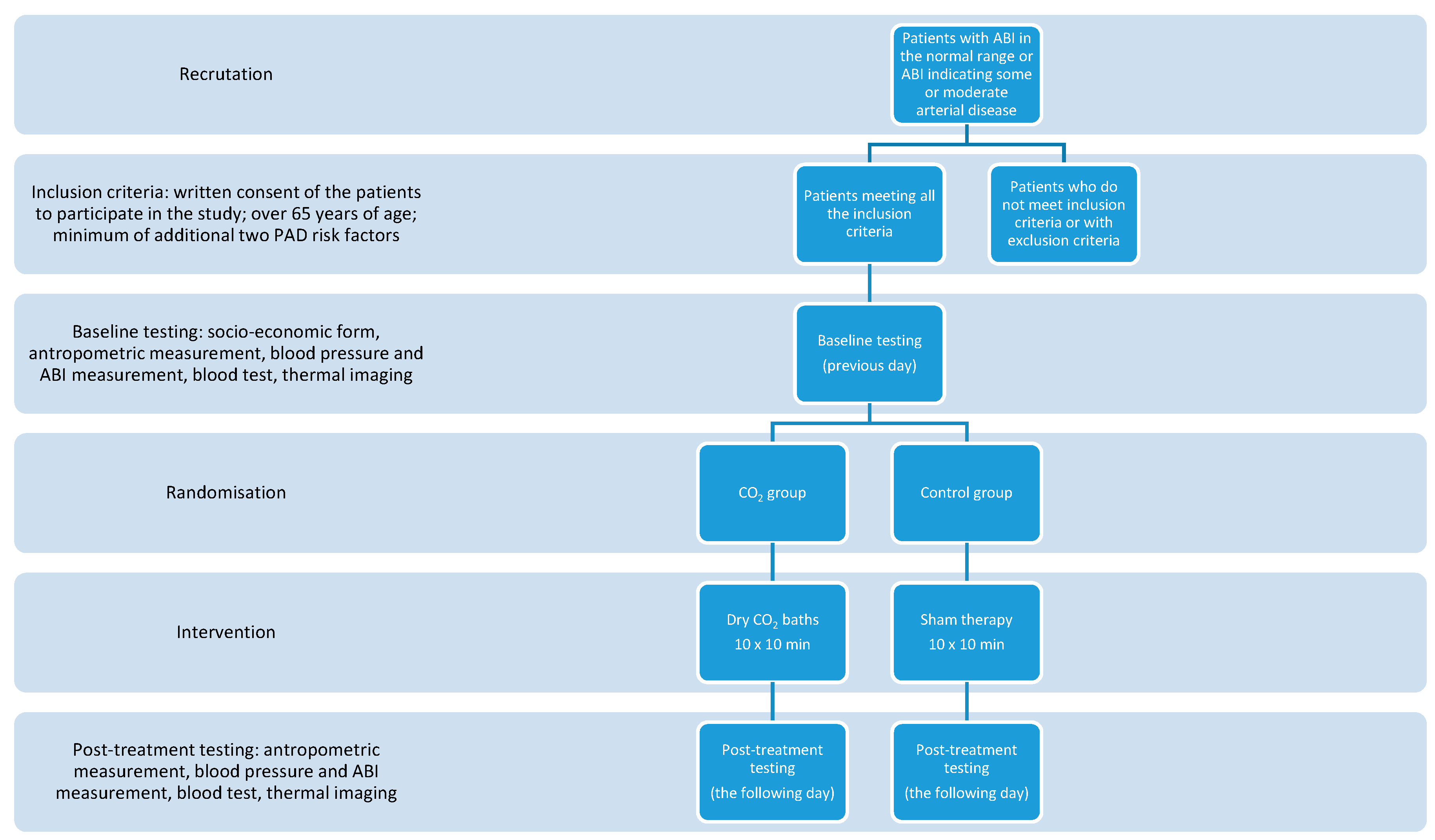
2. Materials and Methods
- Written consent of the patients to participate in the study;
- Over 65 year of age;
- Minimum of additional two PAD risk factors among smoking, diabetes mellitus, hypertension, dyslipidemia, elevated C-reactive protein (CRP), hyperviscosity or hypercoagulable state, hyperhomocysteinemia, chronic renal insufficiency.
- Smoking–habit present or not (according to the patient-filled questionnaire);
- Diabetes mellitus–previously diagnosed diabetes mellitus, use of diabetic drug or fasting plasma glucose ≥ 126 mg/dL;
- Hypertension—previously diagnosed hypertension, use of blood pressure-lowering drug or systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg;
- Dyslipidemia—previously diagnosed hypertension, use of a lipid-lowering drug, or any one of the following: total cholesterol level ≥ 240 mg/dL, triglyceride level ≥ 150 mg/dL, low-density-lipoprotein (LDL) cholesterol level ≥ 140 mg/dL, high-density-lipoprotein (HDL) cholesterol level < 40 mg/dL;
- Elevated c-reactive protein—CRP > 3.0 mg/L;
- Hyperviscosity or hypercoagulable state—previously diagnosed hyperviscosity or hypercoagulable state;
- Hyperhomocysteinemia—blood homocysteine concentration > 15 µmol/l; chronic renal insufficiency—previously diagnosed chronic renal insufficiency.
- Severe arterial disease defined by ankle–brachial index < 0.5;
- Diagnosis of Buerger’s disease;
- Current chemotherapy or radiotherapy;
- Current therapy with any immunosuppressive agent;
- Planned amputation within 6 months;
- Concurrent participation in another clinical study.
2.1. Thermal Imaging Measurements
- Frontal plane front upper body;
- Frontal plane front lower body;
- Frontal plane back upper body;
- Frontal plane back lower body.
- Upper back (UB);
- Lower back (LB);
- Chest (CH);
- Abdomen (AB);
- Upper limb (UL);
- Lower limb (LL).
- arm anterior right (AAR);
- Arm anterior left (AAL);
- Forearm anterior right (FRAR);
- Forearm anterior left (FRAL);
- Hand palmar right (HPR);
- Hand palmar left (HPL);
- Arm posterior right (APR);
- Arm posterior left (APL);
- Forearm posterior right (FRPR);
- Forearm posterior left (FRPL);
- Hand dorsal right (HDR);
- Hand dorsal left (HDL).
- Thigh anterior right (TAR);
- Thigh anterior left (TAL);
- Leg anterior right (LAR);
- Leg anterior left (LAL);
- Thigh posterior right (TPR);
- Thigh posterior left (TPL);
- Leg posterior right (LPR);
- Leg posterior left (LPL).
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Crawford, F.; Welch, K.; Andras, A.; Chappell, F.M. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Morley, R.L.; Sharma, A.; Horsch, A.D.; Hinchliffe, R.J. Peripheral Artery Disease. BMJ 2018, 374, 861–871. [Google Scholar] [CrossRef]
- Finzgar, M.; Melik, Z.; Cankar, K. Effect of transcutaneous application of gaseous carbon dioxide on cutaneous microcirculation. Clin. Hemorheol. Microcirc. 2015, 60, 423–435. [Google Scholar] [CrossRef]
- Brandi, C.; Grimaldi, L.; Nisi, G.; Brafa, A.; Campa, A.; Calabrò, M.; Campana, M.; D’Aniello, C. The role of carbon dioxide therapy in the treatment of chronic wounds. In Vivo 2010, 24, 223–226. [Google Scholar] [PubMed]
- Dogliotti, G.; Galliera, E.; Iorio, E.; Valserra, M.D.B.D.; Solimene, U.; Corsi, M.M. Effect of immersion in CO2-enriched water on free radical release and total antioxidant status in peripheral arterial occlusive disease. Int. Angiol. 2011, 30, 12–17. [Google Scholar]
- Hartmann, B.R.; Bassenge, E.; Hartmann, M. Effects of Serial Percutaneous Application of Carbon Dioxide in Intermittent Claudication: Results of a Controlled Trial. Angiology 1997, 48, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Toriyama, T.; Kumada, Y.; Matsubara, T.; Murata, A.; Ogino, A.; Hayashi, H.; Nakashima, H.; Takahashi, H.; Matsuo, H.; Kawahara, H. Effect of artificial carbon dioxide foot bathing on critical limb ischemia (Fontaine IV) in peripheral arterial disease patients. Int. Angiol. 2002, 21, 367. [Google Scholar] [PubMed]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
- Carpes, F.P.; Mello-Carpes, P.B.; Priego Quesada, J.I.; Pérez-Soriano, P.; Salvador Palmer, R.; Ortiz de Anda, R.M.C. Insights on the use of thermography in human physiology practical classes. Adv. Physiol. Educ. 2018, 42, 521–525. [Google Scholar] [CrossRef]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.; Costa, C.M.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Marconi, E.; Moura-Fernandes, M.C.; Lopes-Souza, P.; Teixeira-Silva, Y.; Reis-Silva, A.; Marchon, R.M.; Guedes-Aguiar, E.D.O.; Paineiras-Domingos, L.L.; Sá-Caputo, D.D.C.D.; Morel, D.S.; et al. Evaluation of the temperature of posterior lower limbs skin during the whole body vibration measured by infrared thermography: Cross-sectional study analysis using linear mixed effect model. PLoS ONE 2019, 14, e0212512. [Google Scholar] [CrossRef]
- Marins, J.C.B.; Moreira, D.G.; Cano, S.P.; Quintana, M.S.; Soares, D.D.; Fernandes, A.D.A.; Da Silva, F.S.; Costa, C.M.A.; Amorim, P.R.D.S. Time required to stabilize thermographic images at rest. Infrared Phys. Technol. 2014, 65, 30–35. [Google Scholar] [CrossRef]
- Fernández-Cuevas, I.; Sillero-Quintana, M.; Garcia-Concepcion, M.A.; Serrano, J.R.; Gomez-Carmona, P.; Marins, J.B. Monitoring skin thermal response to training with Infrared Thermography. New Stud. Athl. 2014, 29, 57–71. [Google Scholar]
- Lahiri, B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical applications of infrared thermography: A review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Ring, E.F.; Ammer, K. Infrared thermal imaging in medicine. Physiol Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef]
- Fiodorenko-Dumas, Ż.; Rabczyński, M.; Paprocka-Borowicz, M.; Małecki, R.; Adamiec, R. Changes in the haemodynamics of blood flow to the lower limbs on example of thermography measurements. Acta Angiol. 2014, 2, 141–146. [Google Scholar]
- McDivitt, J.D.; Braun, M.; Kassop, D. Cardiovascular Disease: Lower Extremity Peripheral Artery Disease. FP Essent. 2019, 479, 11–15. [Google Scholar]
- Firnhaber, J.M.; Powell, C.S. Lower Extremity Peripheral Artery Disease: Diagnosis and Treatment. Am. Fam. Physician 2019, 99, 362–369. [Google Scholar]
- Fiodorenko-Dumas, Ż.; Paprocka-Borowicz, M.; Dumas, I. Analiza temperatury powierzchni skóry u pacjentów z niedokrwieniem kończyn dolnych. Acta Bio-Opt. Inform. Medica 2012, 3, 177–181. [Google Scholar]
- Conte, M.S.; Pomposelli, F.B.; Clair, D.G.; Geraghty, P.J.; McKinsey, J.F.; Mills, J.L.; Moneta, G.L.; Murad, M.H.; Powell, R.J.; Reed, A.B.; et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: Management of asymptomatic disease and claudication [published correction appears. J. Vasc. Surg. 2015, 61 (Suppl. 3), S2–S41. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.-H.; Han, S.-H.; Chung, N.-S.; Hyun, H.-S. The validity of ankle-brachial index for the differential diagnosis of peripheral arterial disease and lumbar spinal stenosis in patients with atypical claudication. Eur. Spine J. 2011, 21, 1165–1170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Sillero-Quintana, M. A checklist for measuring skin temperature with infrared thermography in sport and exercise medicine. Thermol. Int. 2017, 27, 141–143. [Google Scholar]
- WHO. Cardiovascular Diseases (CVDs). 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 24 November 2019).
- Vilcahuaman, L.; Harba, R.; Canals, R.; Zequera, M.; Wilches, C.; Arista, M.T.; Torres, L.; Arbañil, H. Detection of diabetic foot hyperthermia by infrared imaging. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4831–4834. [Google Scholar] [CrossRef]
- Van Netten, J.J.; van Baal, J.G.; Liu, C.; van der Heijden, F.; Bus, S.A. Infrared thermal imaging for automated detection of diabetic foot complications. J. Diabetes Sci. Technol. 2013, 7, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Holtz-Neiderer, K.; Wendel, C.; Mohler, M.J.; Kimbriel, H.R.; Lavery, L.A. Skin Temperature Monitoring Reduces the Risk for Diabetic Foot Ulceration in High-risk Patients. Am. J. Med. 2007, 120, 1042–1046. [Google Scholar] [CrossRef]
- Lavery, L.A.; Higgins, K.R.; Lanctot, D.R.; Constantinides, G.P.; Zamorano, R.G.; Armstrong, D.G.; Athanasiou, K.A.; Agrawal, C.M. Home Monitoring of Foot Skin Temperatures to Prevent Ulceration. Diabetes Care 2004, 27, 2642–2647. [Google Scholar] [CrossRef]
- Lavery, L.A.; Higgins, K.R.; Lanctot, D.R.; Constantinides, G.P.; Zamorano, R.G.; Athanasiou, K.A.; Armstrong, D.G.; Agrawal, C.M. Preventing Diabetic Foot Ulcer Recurrence in High-Risk Patients: Use of temperature monitoring as a self-assessment tool. Diabetes Care 2006, 30, 14–20. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Spagna, P.E.; Taylor, M.C. Sacral Skin Temperature Assessed by Thermal Imaging: Role of Patient Vascular Attributes. J. Wound Ostomy Cont. Nurs. 2018, 45, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Tang, Q.; Jiang, M.; Zhong, N.; Liu, G. Infrared thermal imaging and Doppler vessel pressurization ultrasonography to detect lower extremity deep vein thrombosis: Diagnostic accuracy study. Clin. Respir. J. 2017, 12, 1118–1124. [Google Scholar] [CrossRef]
| Population Characteristics | |
|---|---|
| n = 46 | |
| Age, years | 71.7 (±4.95) |
| Male: female sex ratio | 30:70 |
| White ethnicity, n (%) | 46 (100) |
| Smoking status, n (%) Current smoker Ex-smoker Non-smoker | 5 (10.9) 18 (39.1) 23 (50.0) |
| body mass index, kg/m2 | 30.1 (±5.9) |
| Hypertension, n (%) | 46 (100.0) |
| Diabetes mellitus, n (%) | 34 (73.9) |
| Dyslipidemia, n (%) | 43 (93.5) |
| Hyperviscosity or hypercoagulable state, n (%) | 5 (10.9) |
| Elevated inflammation markers, n (%) | 5 (10.9) |
| Hyperhomocysteinemia, n (%) | 6 (13.0) |
| Chronic renal insufficiency, n (%) | 0 (0.0) |
| Unless otherwise stated, data are presented as mean (standard deviation). | |
| Health-Related Characteristics | Control Group | CO2 Group | ||||
|---|---|---|---|---|---|---|
| T0 | T1 | p-Value | T0 | T1 | p-Value | |
| Waist circumference, cm | ||||||
| Men | 106.8 (±11.1) | 104.8 (±11.1) | 0.95 | 102.9 (±9.5) | 103.1 (±7.8) | 0.23 |
| Women | 93.1 (±14.0) | 92.2 (±14.0) | 0.92 | 97.9 (±10.8) | 96.9 (±10.9) | 0.89 |
| Waist-to-hip ratio | ||||||
| Men | 0.99 (±0.03) | 0.97 (±0.02) | 0.98 | 0.96 (±0.05) | 0.97 (±0.03) | 0.18 |
| Women | 0.88 (±0.05) | 0.88 (±0.05) | 0.40 | 0.93 (±0.03) | 0.93 (±0.03) | 0.53 |
| Ankle–brachial index | 1.1 (±0.2) | 1.1 (±0.2) | 0.91 | 1.0 (±0.2) | 1.0 (±0.2) | 0.86 |
| Fasting glucose, (mg/dL) | 112.1 (±26.8) | 110.8 (±22.0) | 0.81 | 114.4 (±29.3) | 118.5 (±34.2) | 0.59 |
| Glycated hemoglobin, (%) | 5.9 (±0.5) | 6.0 (±0.6) | 0.99 | 6.1 (±0.7) | 6.1 (±0.7) | 0.99 |
| Total cholesterol, (mg/dL) | 184.7 (±43.3) | 184.0 (±37.1) | 0.98 | 180.5 (±41.0) | 174.6 (±39.8) | 0.98 |
| LDL-cholesterol, (mg/dL) | 112.0 (±40.2) | 113.4 (±34.7) | 0.67 | 116.1 (±40.4) | 110.9 (±40.8) | 0.95 |
| HDL-cholesterol, (mg/dL) | 62.0 (±16.1) | 60.5 (±16.1) | 1.00 | 52.3 (±13.3) | 53.5 (±12.4) | 1.00 |
| Triglycerides, (mg/dL) | 135.2 (±67.9) | 133.0 (±68.4) | 0.98 | 166.7 (±102.4) | 135.5 (±66.6) | 0.99 |
| C-reactive protein, (mg/L) | 2.5 (±2.1) | 3.0 (±3.6) | 0.79 | 2.6 (±1.8) | 4.1 (±4.4) | 0.99 |
| Homocysteine, (mol/L) | 13.3 (±2.5) | 13.4 (±3.2) | 0.86 | 16.3 (±6.1) | 15.7 (±5.5) | 0.27 |
| Systolic blood pressure, (mmHg) | 140.0 (±22.0) | 139.7 (±22.7) | 0.28 | 157.8 (±23.3) | 153.5 (±19.4) | 0.99 |
| Diastolic blood pressure, (mmHg) | 72.2 (±10.6) | 72.2 (±10.8) | 0.84 | 79.4 (±11.6) | 80.0 (±10.5) | 0.79 |
| Control Group | CO2 Group | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | ||||||||||
| Region of interest | ΔT | p-value T0 vs. T1 | ΔT | p-value T0 vs. T1 | p-value T1 placebo vs. T1 CO2 | ||||||||
| temp. (°C) | ±SD | temp. (°C) | ±SD | temp. (°C) | ±SD | temp. (°C) | ±SD | ||||||
| Arm anterior right | 31.73 | 1.15 | 32.40 | 1.10 | −0.67 | 0.682 | 32.13 | 1.14 | 32.01 | 0.12 | 0.84 | 0.075 | 0.205 |
| Forearm anterior right | 31.65 | 1.20 | 32.70 | 1.07 | −1.04 | 0.982 | 31.76 | 0.87 | 31.65 | 0.11 | 0.78 | 0.007 | 0.001 |
| Hand palmar right | 28.71 | 2.91 | 31.79 | 1.51 | −3.08 | 0.288 | 29.47 | 3.14 | 28.54 | 0.93 | 2.70 | 0.001 | 0.001 |
| Arm posterior right | 30.33 | 1.18 | 31.57 | 1.20 | −1.24 | 0.973 | 30.34 | 1.41 | 30.22 | 0.12 | 1.27 | 0.001 | 0.001 |
| Forearm posterior right | 31.73 | 0.88 | 32.60 | 1.06 | −0.86 | 0.296 | 31.69 | 1.04 | 31.28 | 0.41 | 1.11 | 0.004 | 0.001 |
| Hand dorsal right | 28.11 | 3.11 | 31.66 | 1.49 | −3.56 | 0.108 | 29.40 | 2.65 | 28.01 | 1.39 | 3.10 | 0.001 | 0.001 |
| Arm anterior left | 31.85 | 1.27 | 32.57 | 1.17 | −0.73 | 0.766 | 31.88 | 0.83 | 31.87 | 0.01 | 0.68 | 0.076 | 0.044 |
| Forearm anterior left | 31.75 | 1.21 | 32.73 | 1.16 | −0.97 | 0.394 | 31.79 | 0.88 | 31.58 | 0.21 | 0.76 | 0.015 | 0.001 |
| Hand palmar left | 28.69 | 2.64 | 31.80 | 1.29 | −3.11 | 0.233 | 29.85 | 2.97 | 28.70 | 1.14 | 3.42 | 0.001 | 0.001 |
| Arm posterior left | 30.17 | 1.21 | 31.43 | 1.06 | −1.25 | 0.860 | 30.24 | 1.34 | 30.17 | 0.07 | 1.27 | 0.001 | 0.002 |
| Forearm posterior left | 31.65 | 1.19 | 32.56 | 1.30 | −0.91 | 0.242 | 31.43 | 0.90 | 31.10 | 0.33 | 0.98 | 0.030 | 0.001 |
| Hand dorsal left | 28.31 | 2.63 | 31.31 | 1.50 | −3.00 | 0.371 | 28.97 | 3.31 | 28.16 | 0.80 | 2.70 | 0.001 | 0.001 |
| Thigh anterior right | 30.04 | 1.29 | 32.08 | 1.27 | −2.04 | 0.511 | 30.43 | 1.23 | 30.20 | 0.23 | 1.12 | 0.001 | 0.001 |
| Leg anterior right | 31.83 | 0.65 | 32.81 | 0.89 | −0.98 | 0.025 | 32.00 | 1.15 | 31.24 | 0.76 | 1.05 | 0.001 | 0.001 |
| Thigh posterior right | 30.62 | 1.15 | 32.53 | 1.15 | −1.91 | 0.079 | 30.90 | 1.17 | 30.30 | 0.60 | 1.09 | 0.001 | 0.001 |
| Leg posterior right | 31.01 | 0.78 | 32.28 | 0.75 | −1.27 | 0.046 | 31.10 | 1.08 | 30.53 | 0.57 | 0.76 | 0.001 | 0.001 |
| Thigh anterior left | 30.24 | 1.65 | 32.13 | 1.30 | −1.88 | 0.408 | 30.28 | 1.20 | 30.00 | 0.28 | 1.09 | 0.001 | 0.001 |
| Leg anterior left | 32.09 | 1.01 | 32.81 | 0.98 | −0.72 | 0.092 | 31.62 | 1.38 | 31.01 | 0.62 | 1.02 | 0.031 | 0.001 |
| Thigh posterior left | 30.62 | 1.31 | 32.40 | 1.15 | −1.78 | 0.175 | 31.04 | 1.19 | 30.59 | 0.45 | 1.01 | 0.001 | 0.001 |
| Leg posterior left | 31.11 | 0.67 | 32.25 | 0.77 | −1.14 | 0.197 | 30.98 | 1.13 | 30.60 | 0.38 | 0.82 | 0.001 | 0.001 |
| Chest | 32.37 | 1.09 | 33.32 | 0.92 | −0.95 | 0.767 | 32.47 | 0.83 | 32.54 | −0.08 | 0.95 | 0.006 | 0.011 |
| Abdomen | 31.72 | 1.40 | 32.79 | 1.19 | −1.07 | 0.272 | 31.60 | 1.10 | 31.24 | 0.36 | 1.08 | 0.015 | 0.001 |
| Upper back | 32.39 | 1.27 | 33.03 | 1.05 | −0.63 | 0.775 | 32.40 | 1.00 | 32.32 | 0.08 | 0.79 | 0.103 | 0.034 |
| Lower back | 31.93 | 1.38 | 32.59 | 1.40 | −0.66 | 0.507 | 31.93 | 1.18 | 31.71 | 0.21 | 0.97 | 0.214 | 0.022 |
| Variable | ROI | p-Value | Cor/Rho/tau | Correlation Type |
|---|---|---|---|---|
| Total cholesterol | TPL | 0.037 | 0.481 | Pearson’s |
| LPL | 0.046 | 0.462 | Pearson’s | |
| Body fat | LAR | 0.022 | −0.523 | Pearson’s |
| Homocysteine | APR | 0.049 | −0.456 | Pearson’s |
| Waist-to-hip ratio | TPR | 0.049 | −0.456 | Pearson’s |
| Hypertension | HPR | 0.039 | −0.408 | Kendall |
| Diabetes | APR | 0.023 | −0.450 | Kendall |
| LAR | 0.021 | −0.457 | Kendall | |
| LPL | 0.037 | −0.414 | Kendall |
| Variable | ROI | p-Value | Cor/Rho/tau | Correlation Type |
|---|---|---|---|---|
| Systolic blood pressure | AAR | 0.031 | 0.450 | Pearson’s |
| AB | 0.022 | 0.475 | Pearson’s | |
| CRP | TPL | 0.036 | −0.438 | Spearman |
| LDL-cholesterol | LAR | 0.015 | 0.500 | Pearson’s |
| Fasting glucose | TPL | 0.034 | −0.446 | Spearman |
| TAL | 0.048 | −0.416 | Spearman | |
| TPR | 0.039 | −0.433 | Spearman | |
| HDR | 0.034 | −0.442 | Spearman | |
| Hypercholesterolemia | APR | 0.017 | −0.428 | Kendall |
| APL | 0.021 | −0.413 | Kendall | |
| LAR | 0.025 | −0.402 | Kendall | |
| LB | 0.017 | −0.425 | Kendall |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbroja, H.; Kowalski, M.; Lubkowska, A. The Effect of Dry Carbon Dioxide Bathing on Peripheral Blood Circulation Measured by Thermal Imaging among Patients with Risk Factors of PAD. Int. J. Environ. Res. Public Health 2021, 18, 1490. https://doi.org/10.3390/ijerph18041490
Zbroja H, Kowalski M, Lubkowska A. The Effect of Dry Carbon Dioxide Bathing on Peripheral Blood Circulation Measured by Thermal Imaging among Patients with Risk Factors of PAD. International Journal of Environmental Research and Public Health. 2021; 18(4):1490. https://doi.org/10.3390/ijerph18041490
Chicago/Turabian StyleZbroja, Hanna, Mateusz Kowalski, and Anna Lubkowska. 2021. "The Effect of Dry Carbon Dioxide Bathing on Peripheral Blood Circulation Measured by Thermal Imaging among Patients with Risk Factors of PAD" International Journal of Environmental Research and Public Health 18, no. 4: 1490. https://doi.org/10.3390/ijerph18041490
APA StyleZbroja, H., Kowalski, M., & Lubkowska, A. (2021). The Effect of Dry Carbon Dioxide Bathing on Peripheral Blood Circulation Measured by Thermal Imaging among Patients with Risk Factors of PAD. International Journal of Environmental Research and Public Health, 18(4), 1490. https://doi.org/10.3390/ijerph18041490







