Trends in National Canadian Guideline Recommendations for the Screening and Diagnosis of Gestational Diabetes Mellitus over the Years: A Scoping Review
Abstract
1. Introduction
2. Study Design and Methods
2.1. Search Strategy
2.2. CPG Selection and Data Extraction
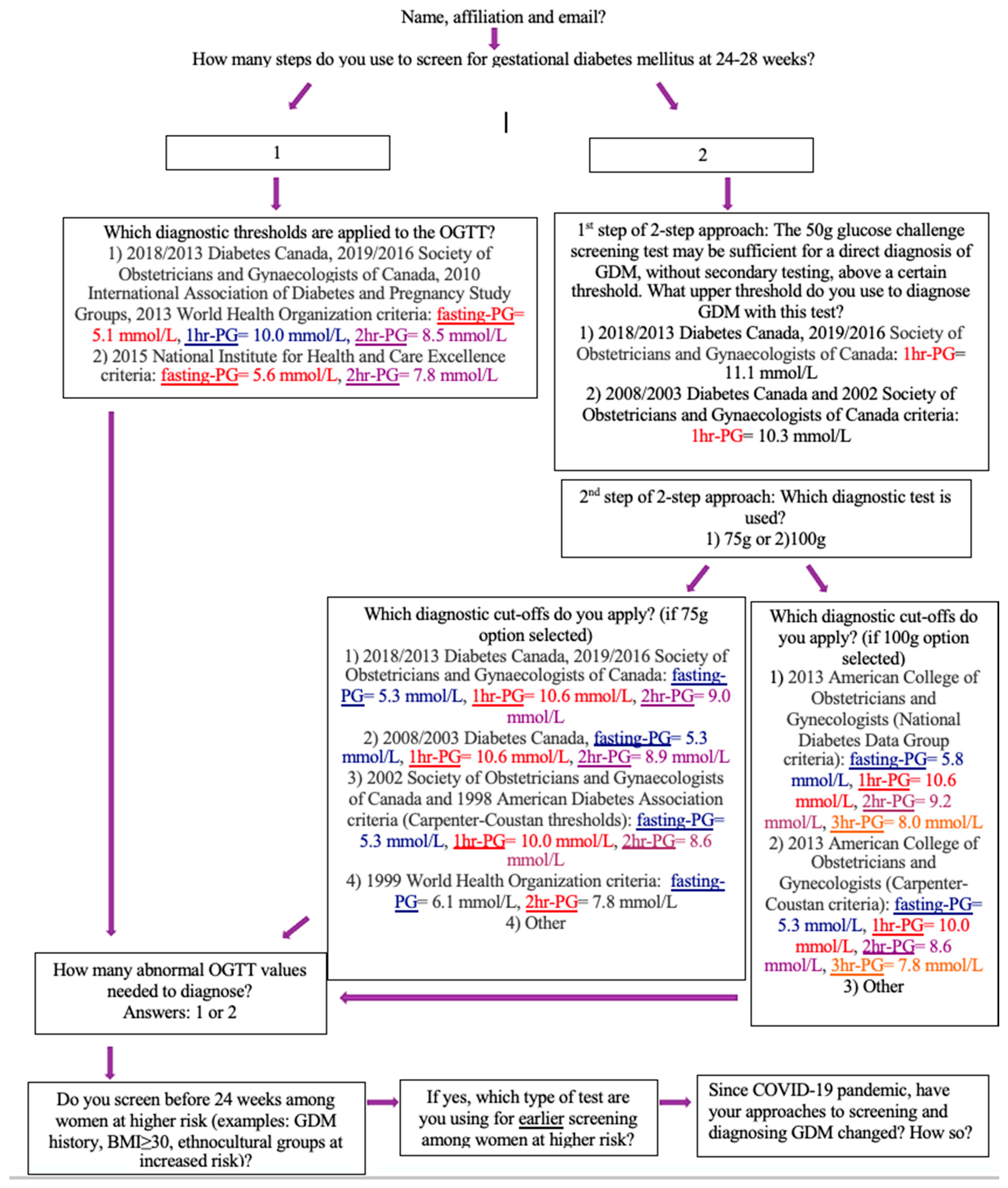
2.3. Survey Distribution
3. Results
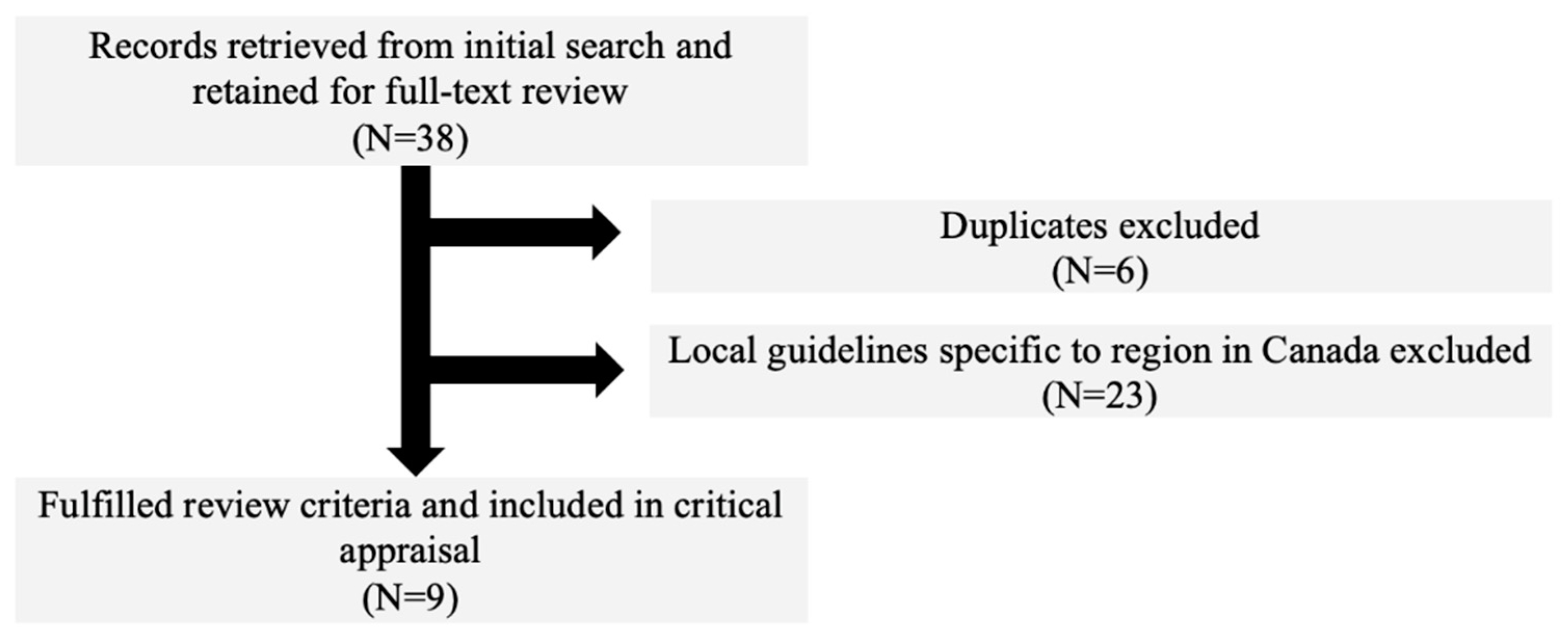
3.1. Search Results
3.2. CPG Characteristics
3.3. The Origin of Defining GDM
3.4. Evolution in Screening Approaches: Early Adoption of the 50 g GCT
3.5. Evolution of O’Sullivan’s Proposed Criteria
3.6. Universal vs. Selective Screening
3.7. Diagnostic Approaches: Variations in the Testing Times and Recommended Glucose Loads to Be Administered for OGTT
3.8. Variation in Screening and Diagnostic Approaches: Debates on Glucose Thresholds Prior to Efforts for International Consensus in 2008
3.9. The HAPO Study and Application of Its Results by the International Association of Diabetes and Pregnancy Study Groups (IADPSG)
3.10. Uniform CPG Recommendations: Recent Trends in Glucose Thresholds and Updated CPGs in Response to the 2008 HAPO Trial and the 2010 IADPSG Guidelines
- (a)
- A two-step approach (preferred by DC) which involves screening (50 g GCT) and diagnostic testing (75 g OGTT) similar to previous guidelines but basing thresholds on HAPO values signaling an OR of 2.0, rather than 1.75 as adopted by the IADPSG [9]. The higher OR corresponds to less inclusive glucose thresholds, aimed to somewhat offset increases in workload, patient burden (glucose monitoring) and associated costs [21].
- (b)
- A one-step approach (alternative approach) as endorsed by the IADSPG and using the IADSPG thresholds based on the OR of 1.75 as discussed previously. The IADPSG has endorsed one-step testing as the only approach to diagnosing GDM and have concerns that many women are unable to return following a 50 g GCT. Ancillary data, along with previous retrospective studies [39], have demonstrated that most women (82%) return to complete a 75 g OGTT following a screening test and that this is not a major concern in Canada.
3.11. The Impact of Changing Diagnostic Criteria on Prevalence across Canada
3.12. The Impact of Changing Diagnostic Criteria on Health Care Economic Costs
3.13. The Impact of Changing Diagnostic Criteria on Obstetric and Neonatal Outcomes
3.14. Changes to Screening and Diagnosing GDM in the Context of the Coronavirus Disease (COVID-19)
3.15. Voluntary Online Survey Responses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Behboudi-Gandevani, S.; Amiri, M.; Bidhendi Yarandi, R.; Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 11. [Google Scholar] [CrossRef]
- Metzger, B.E.; Coustan, D.R. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998, 21 (Suppl. 2), B161–B167. [Google Scholar]
- Coustan, D.R.; Lowe, L.P.; Metzger, B.E.; Dyer, A.R. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: Paving the way for new diagnostic criteria for gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2010, 202, e651–e656. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hu, F.B.; Forman, J.P.; Chavarro, J.; Zhang, C. Increased risk of hypertension after gestational diabetes mellitus: Findings from a large prospective cohort study. Diabetes Care 2011, 34, 1582–1584. [Google Scholar] [CrossRef]
- Okoth, K.; Chandan, J.S.; Marshall, T.; Thangaratinam, S.; Thomas, G.N.; Nirantharakumar, K.; Adderley, N.J. Association between the reproductive health of young women and cardiovascular disease in later life: Umbrella review. BMJ 2020, 371, m3502. [Google Scholar] [CrossRef]
- O’Sullivan, J.B.; Mahan, C.M. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964, 13, 278–285. [Google Scholar]
- Lowe, L.P.; Metzger, B.E.; Dyer, A.R.; Lowe, J.; McCance, D.R.; Lappin, T.R.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; Hod, M.; et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 2012, 35, 574–580. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups–Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef]
- Lucas, A.; Fewtrell, M.S.; Cole, T.J. Fetal origins of adult disease-the hypothesis revisited. BMJ 1999, 319, 245–249. [Google Scholar] [CrossRef]
- Mishra, S.; Rao, C.R.; Shetty, A. Trends in the Diagnosis of Gestational Diabetes Mellitus. Scientifica 2016, 2016, 5489015. [Google Scholar] [CrossRef][Green Version]
- Blotsky, A.L.; Rahme, E.; Dahhou, M.; Nakhla, M.; Dasgupta, K. Gestational diabetes associated with incident diabetes in childhood and youth: A retrospective cohort study. CMAJ 2019, 191, E410–E417. [Google Scholar] [CrossRef]
- SOGC Expert Panel Committee. Diabetes in Pregnancy: Routine screening for gestational diabetes mellitus in pregnancy. J. Obstet. Gynaecol. Can. 1992, 1, 1–3. [Google Scholar]
- SOGC Expert Panel Committee. Screening for gestational diabetes mellitus. J. Obstet. Gynaecol. Can. 2002, 24, 894–912. [Google Scholar] [CrossRef]
- SOGC Expert Panel Committee. Diabetes in Pregnancy. J. Obstet. Gynaecol. Can. 2016, 38, 667–679. [Google Scholar] [CrossRef]
- SOGC Expert Panel Committee. Diabetes in Pregnancy. J. Obstet. Gynaecol. Can. 2019, 41, 1814–1825. [Google Scholar] [CrossRef]
- Canadian Diabetes Association Expert Panel Committee. 1998 clinical practice guidelines for the management of diabetes in Canada. Canadian Diabetes Association. CMAJ 1998, 159 (Suppl. 8), S1–S29. [Google Scholar]
- Canadian Diabetes Association Expert Panel Committee. Canadian Diabetes Association 2003 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2003, 27, S1–S3. [Google Scholar]
- Canadian Diabetes Association Expert Panel Committee. Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2008, 32, S168–S180. [Google Scholar]
- Canadian Diabetes Association Expert Panel Committee. Diabetes Canada 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2013, 37, S168–S183. [Google Scholar] [CrossRef]
- Canadian Diabetes Association Expert Panel Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2018, 42, S255–S282. [Google Scholar] [CrossRef]
- Hoet, J.P.; Lukens, F.D.W. Carbohydrate Metabolism during Pregnancy. Diabetes 1954, 3, 1–12. [Google Scholar] [CrossRef]
- Knopp, R.H. John B. O’Sullivan: A pioneer in the study of gestational diabetes. Diabetes Care 2002, 25, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Negrato, C.A.; Gomes, M.B. Historical facts of screening and diagnosing diabetes in pregnancy. Diabetol. Metab. Syndr. 2013, 5, 22. [Google Scholar] [CrossRef]
- O’Sullivan, J.B. Diabetes Mellitus after GDM. Diabetes 1991, 40 (Suppl. 2), 131–135. [Google Scholar] [CrossRef]
- O’Sullivan, J.B. The Boston Gestational Diabetes Studies: Review and Perspectives. In Carbohydrate Metabolism in Pregnancy and the Newborn IV; Sutherland, H.W., Stowers, J.M., Pearson, D.W.M., Eds.; Springer Nature: London, UK, 1989; pp. 287–294. [Google Scholar]
- O’Sullivan, J.B.; Mahan, C.M.; Charles, D.; Dandrow, R.V. Screening criteria for high-risk gestational diabetic patients. Am. J. Obstet. Gynecol. 1973, 116, 895–900. [Google Scholar] [CrossRef]
- Coustan, D.R.; Nelson, C.; Carpenter, M.W.; Carr, S.R.; Rotondo, L.; Widness, J.A. Maternal age and screening for gestational diabetes: A population-based study. Obstet. Gynecol. 1989, 73, 557–561. [Google Scholar]
- National Diabetes Data Group. Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- Naylor, C.D.; Sermer, M.; Chen, E.; Farine, D. Selective Screening for Gestational Diabetes Mellitus. N. Engl. J. Med. 1997, 337, 1591–1596. [Google Scholar] [CrossRef]
- Association of Ontario Midwives: Gestational Diabetes. Available online: https://www.ontariomidwives.ca/sites/default/files/Gestational-diabetes-mellitus-backgrounder-PUB_0.pdf (accessed on 20 November 2020).
- Pöyhönen-Alho, M.; Teramo, K.A.; Kaaja, R.J.; Hiilesmaa, V.K. 50gram oral glucose challenge test combined with risk factor-based screening for gestational diabetes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 121, 34–37. [Google Scholar] [CrossRef]
- Moses, R.G.; Webb, A.J.; Comber, C.D.; Walton, J.G.; Coleman, K.J.; Davis, W.S.; McCosker, C.J. Gestational diabetes mellitus: Compliance with testing. ANZJOG 2003, 43, 469–470. [Google Scholar] [CrossRef]
- Cosson, E.; Benchimol, M.; Carbillon, L.; Pharisien, I.; Pariès, J.; Valensi, P.; Lormeau, B.; Bolie, S.; Uzan, M.; Attali, J.R. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab. 2006, 32, 140–146. [Google Scholar] [CrossRef]
- Sacks, D.A.; Greenspoon, J.S.; Abu-Fadil, S.; Henry, H.M.; Wolde-Tsadik, G.; Yao, J.F. Toward universal criteria for gestational diabetes: The 75-gram glucose tolerance test in pregnancy. Am. J. Obstet. Gynecol. 1995, 172, 607–614. [Google Scholar] [CrossRef]
- Lind, T.; Philips, P.R. A Prospective Multicentre Study to Determine the Influence of Pregnancy upon the 75-g Oral Glucose Tolerance Test (OGTT). In Carbohydrate Metabolism in Pregnancy and the Newborn IV; Sutherland, H.W., Stowers, J.M., Pearson, D.W.M., Eds.; Springer Nature: London, UK, 1989; pp. 209–226. [Google Scholar]
- Jackson, S.L.; Safo, S.E.; Staimez, L.R.; Olson, D.E.; Narayan, K.M.V.; Long, Q.; Lipscomb, J.; Rhee, M.K.; Wilson, P.W.F.; Tomolo, A.M.; et al. Glucose challenge test screening for prediabetes and early diabetes. Diabet. Med. 2016, 34, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Esakoff, T.F.; Block-Kurbisch, I.; Ustinov, A.; Shafer, S.; Caughey, A.B. Screening or diagnostic: Markedly elevated glucose loading test and perinatal outcomes. J. Matern. Neonatal Med. 2006, 19, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.M. Gestational diabetes mellitus: An update on the current international diagnostic criteria. World J. Diabetes 2015, 6, 782–791. [Google Scholar] [CrossRef]
- Feig, D.S.; Hwee, J.; Shah, B.R.; Booth, G.L.; Bierman, A.S.; Lipscombe, L.L. Trends in Incidence of Diabetes in Pregnancy and Serious Perinatal Outcomes: A Large, Population-Based Study in Ontario, Canada, 1996–2010. Diabetes Care 2014, 37, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.M.; Dhatt, G.; Othman, Y. Gestational diabetes: Differences between the current international diagnostic criteria and implications of switching to IADPSG. J. Diabetes Complicat. 2015, 29, 544–549. [Google Scholar] [CrossRef]
- Pouliot, A.; Elmahboubi, R.; Adam, C. Incidence and Outcomes of Gestational Diabetes Mellitus Using the New International Association of Diabetes in Pregnancy Study Group Criteria in Hôpital Maisonneuve-Rosemont. Can. J. Diabetes 2019, 43, 594–599. [Google Scholar] [CrossRef]
- Agarwal, M.M.; Dhatt, G.S.; Othman, Y. Gestational diabetes in a tertiary care hospital: Implications of applying the IADPSG criteria. Arch. Gynecol. Obstet. 2012, 286, 373–378. [Google Scholar] [CrossRef]
- Meltzer, S.J.; Snyder, J.; Penrod, J.R.; Nudi, M.; Morin, L. Gestational diabetes mellitus screening and diagnosis: A prospective randomised controlled trial comparing costs of one-step and two-step methods. Int. J. Obstet. Gynaecol. 2010, 117, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Sharifi, F. Perinatal outcomes for untreated women with gestational diabetes by IADPSG criteria: A population-based study. Int. J. Obstet. Gynaecol. 2020, 127, 116–122. [Google Scholar] [CrossRef]
- Sacks, D.A.; Black, M.H.; Li, X.; Montoro, M.N.; Lawrence, J.M. Adverse Pregnancy Outcomes Using the International Association of the Diabetes and Pregnancy Study Groups Criteria. Obstet. Gynecol. 2015, 126, 67–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meltzer, S.; Snyder, J.; Morin, L.; Nudi, M. Maternal and Neonatal Outcomes from a Large Randomized Trial of Three Methods of Gestational Diabetes Diagnosis. In Proceedings of the Diabetologia International Diabetes Federation Meeting, Cape Town, South Africa, 3–7 December 2006. Abstract #1413. [Google Scholar]
- Benhalima, K.; Hanssens, M.; Devlieger, R.; Verhaeghe, J.; Mathieu, C. Analysis of Pregnancy Outcomes Using the New IADPSG Recommendation Compared with the Carpenter and Coustan Criteria in an Area with a Low Prevalence of Gestational Diabetes. Int. J. Endocrinol. 2013, 2013, 248121. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Moses, R.G. Response to Comment on McIntyre and Moses the Diagnosis and Management of Gestational Diabetes Mellitus in the Context of the COVID-19 Pandemic. Diabetes Care 2020, 43, e193. [Google Scholar] [CrossRef]
- Yamamoto, J.M.; Donovan, L.E.; Feig, D.S.; Berger, H. Urgent Update–Temporary Alternative Screening Strategy for Gestational Diabetes Screening during the COVID-19 Pandemic. Available online: https://sogc.org/common/Uploaded%20files/GDM-COVID-19%20temporary%20screening%20guidelines%20-%2020200402%20Agreed%20Final.pdf (accessed on 25 November 2020).
| Professional Society, Year | Screening Population | Test | # of Abnormal Diagnostic Values | Fasting Glucose (mmol/L) | 1 h Post Glucose Loading (mmol/L) | 2 h Post Glucose Loading (mmol/L) | 3 h Post Glucose Loading (mmol/L) | Estimated Prevalence of GDM in Canada § |
|---|---|---|---|---|---|---|---|---|
| Society of Obstetricians and Gynaecologists of Canada (SOGC) | ||||||||
| SOGC, 1992 | Universal | 2 step 3 h 100g * | 2 | 5.3 or 5.8 | 10.0 or 10.6 | 8.6 or 9.2 | 7.8 or 8.0 | 3.8–6.5% |
| SOGC, 2002 | Selective | 2 step 2 h 75 g | 2 | 5.3 | 10.0 | 8.6 | -- | 3.8–6.5% |
| 2 step 3 h 100 g * | 2 | 5.3 or 5.8 | 10.0 or 10.6 | 8.6 or 9.2 | 7.8 or 8.0 | 3.8–6.5% | ||
| SOGC, 2016 | Universal | 2 step 2 h 75 g † | 1 | 5.3 | 10.6 | 9.0 | -- | 7.0% |
| 1 step 2 h 75 g | 1 | 5.1 | 10.0 | 8.5 | -- | 16.1% | ||
| SOGC, 2019 | Universal | 2 step 2 h 75 g † | 1 | 5.3 | 10.6 | 9.0 | -- | 7.0% |
| 1 step 2 h 75 g | 1 | 5.1 | 10.0 | 8.5 | -- | 16.1% | ||
| Diabetes Canada (DC) ‡ | ||||||||
| DC, 1998 | Selective | 2 step 2 h 75 g † | 2 | 5.3 | 10.6 | 8.9 | -- | 2.0–4.0% |
| 2 step 3 h 100 g | 2 | 5.3 | 10.0 | 8.6 | 7.8 | 2.0–4.0% | ||
| DC, 2003 | Universal | 2 step 2 h 75 g | 2 | 5.3 | 10.6 | 8.9 | -- | 3.7% |
| DC, 2008 | Universal | 2 step 2 h 75 g | 2 | 5.3 | 10.6 | 8.9 | -- | 3.7% |
| DC, 2013 | Universal | 2 step 2 h 75 g † | 1 | 5.3 | 10.6 | 9.0 | -- | 7.0% |
| 1 step 2 h 75 g | 1 | 5.1 | 10.0 | 8.5 | -- | 16.1% | ||
| DC, 2018 | Universal | 2 step 2 h 75 g † | 1 | 5.3 | 10.6 | 9.0 | -- | 7.0% |
| 1 step 2 h 75 g | 1 | 5.1 | 10.0 | 8.5 | -- | 16.1% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussa, J.; Meltzer, S.; Bond, R.; Garfield, N.; Dasgupta, K. Trends in National Canadian Guideline Recommendations for the Screening and Diagnosis of Gestational Diabetes Mellitus over the Years: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 1454. https://doi.org/10.3390/ijerph18041454
Mussa J, Meltzer S, Bond R, Garfield N, Dasgupta K. Trends in National Canadian Guideline Recommendations for the Screening and Diagnosis of Gestational Diabetes Mellitus over the Years: A Scoping Review. International Journal of Environmental Research and Public Health. 2021; 18(4):1454. https://doi.org/10.3390/ijerph18041454
Chicago/Turabian StyleMussa, Joseph, Sara Meltzer, Rachel Bond, Natasha Garfield, and Kaberi Dasgupta. 2021. "Trends in National Canadian Guideline Recommendations for the Screening and Diagnosis of Gestational Diabetes Mellitus over the Years: A Scoping Review" International Journal of Environmental Research and Public Health 18, no. 4: 1454. https://doi.org/10.3390/ijerph18041454
APA StyleMussa, J., Meltzer, S., Bond, R., Garfield, N., & Dasgupta, K. (2021). Trends in National Canadian Guideline Recommendations for the Screening and Diagnosis of Gestational Diabetes Mellitus over the Years: A Scoping Review. International Journal of Environmental Research and Public Health, 18(4), 1454. https://doi.org/10.3390/ijerph18041454







