Effect of Carbohydrate-Electrolyte Solution Including Bicarbonate Ion Ad Libitum Ingestion on Urine Bicarbonate Retention during Mountain Trekking: A Randomized, Controlled Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
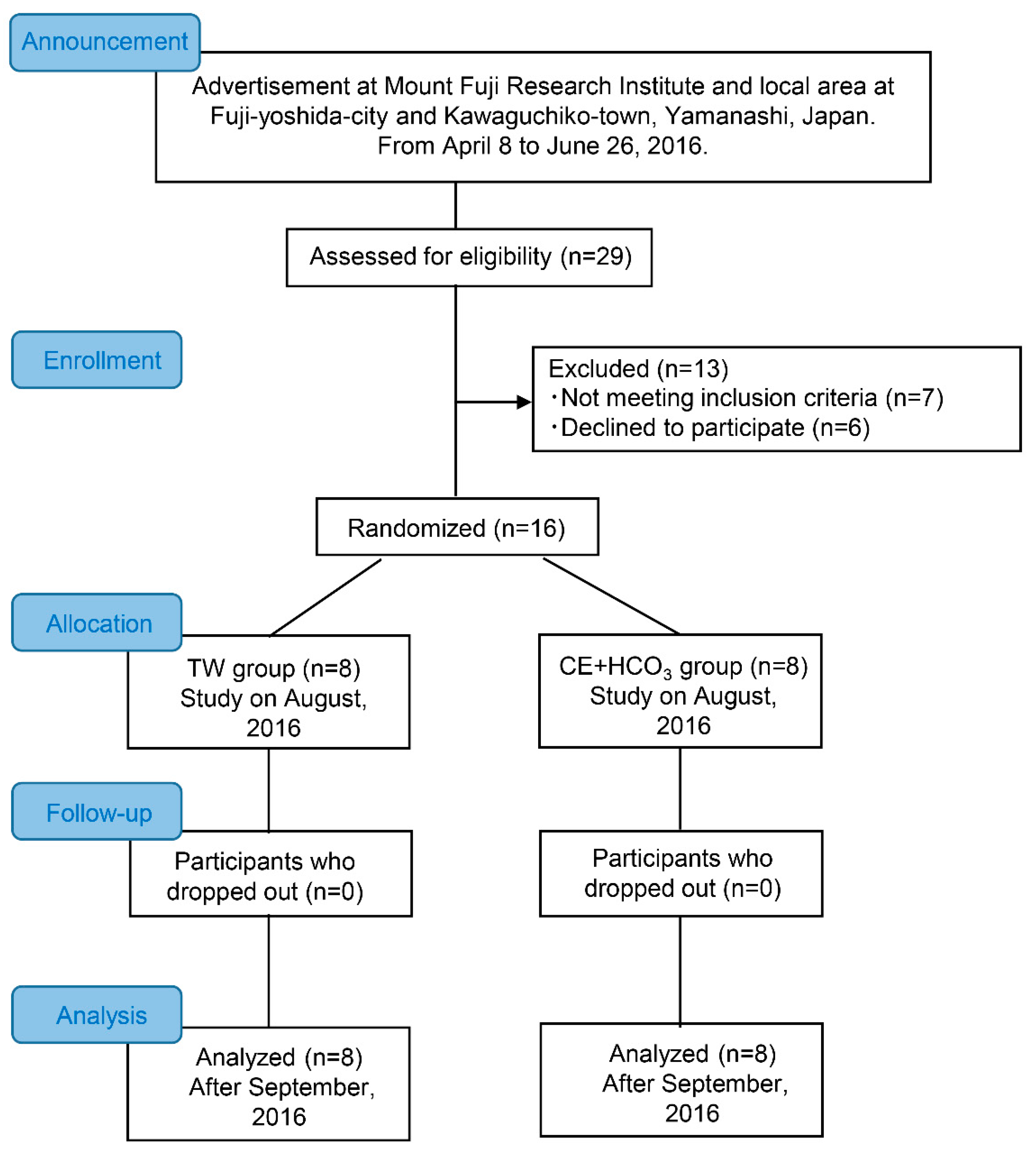
2.2. Participants
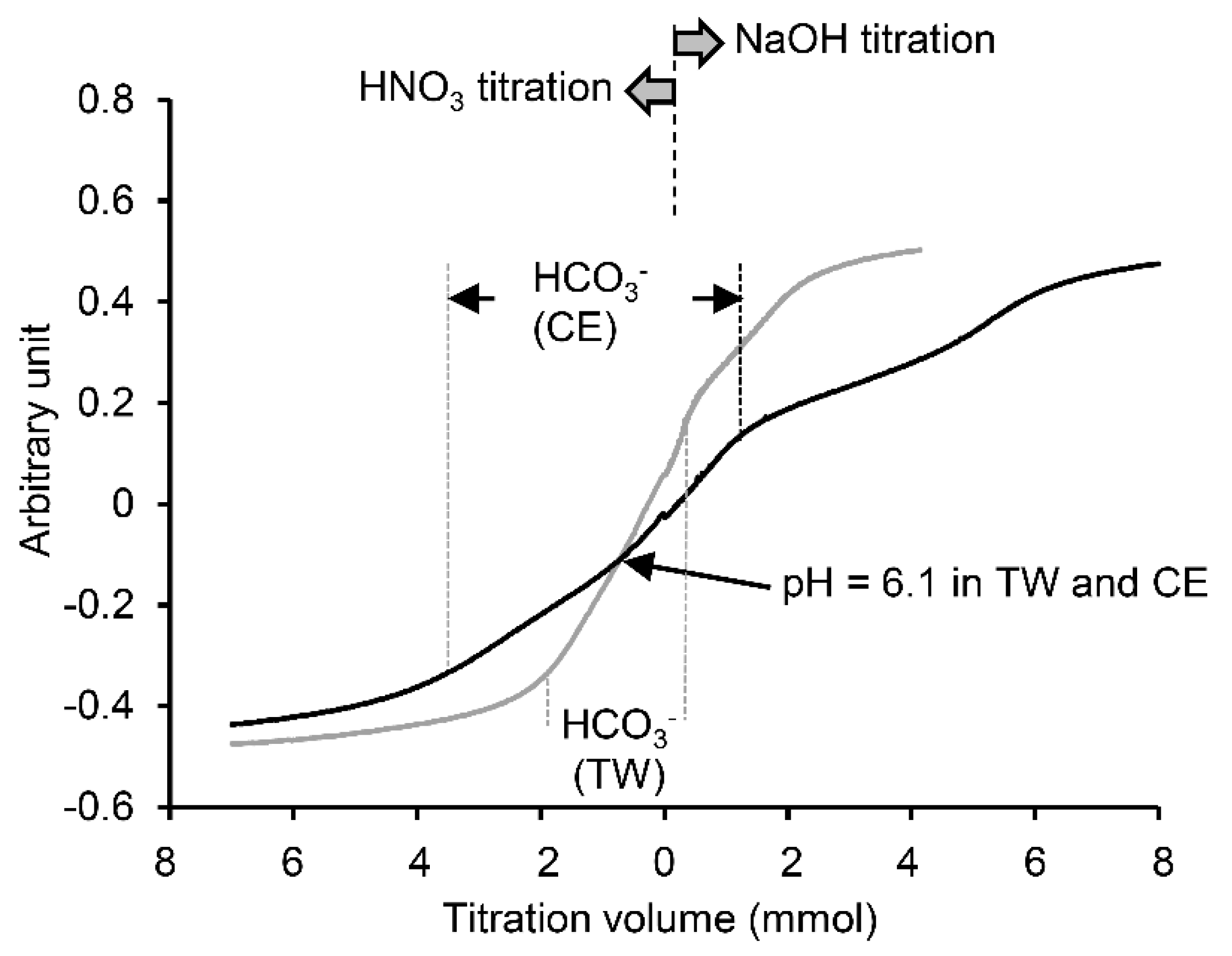
2.3. Beverage and Foods
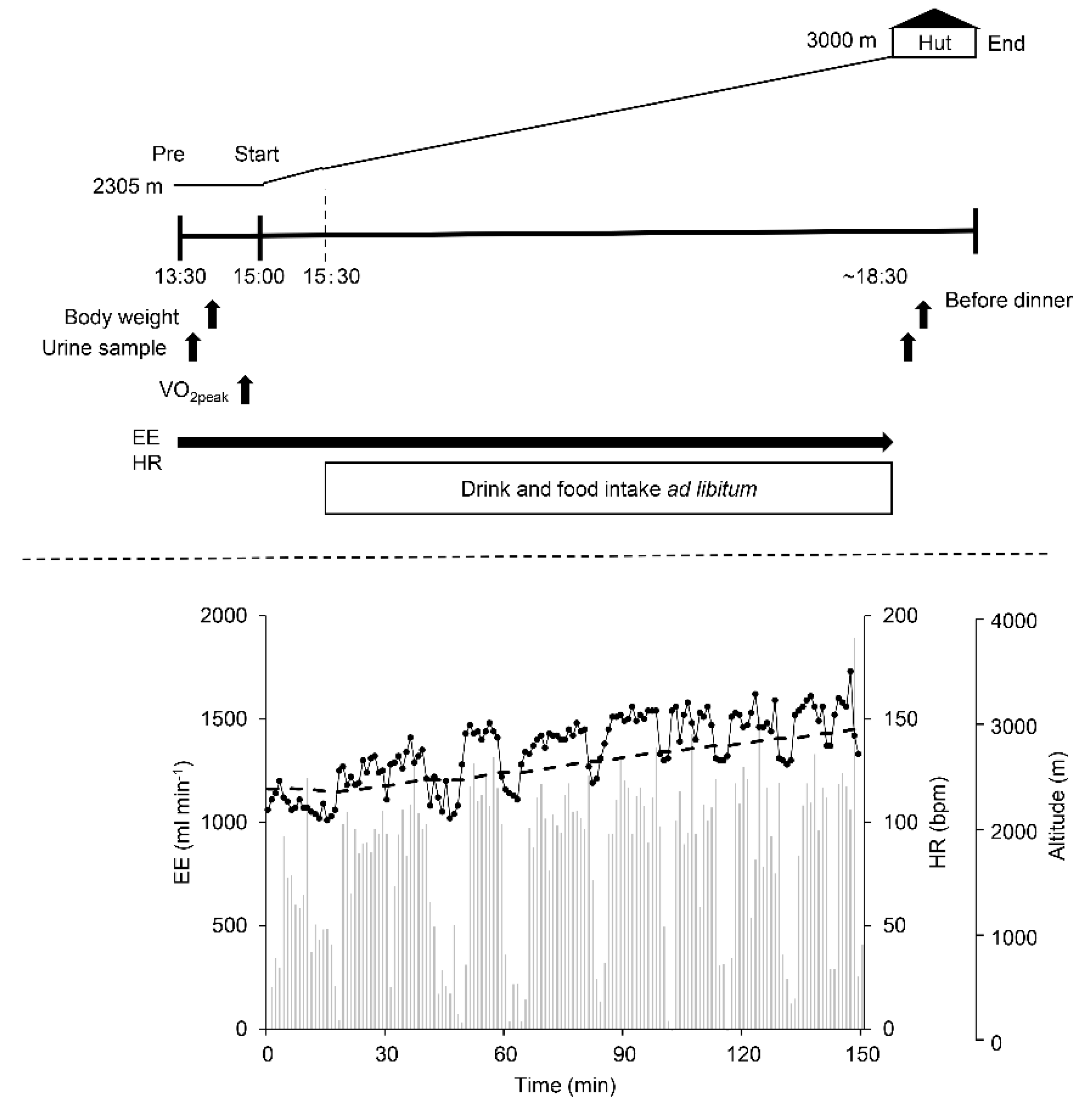
2.4. Procedure
2.5. Estimation of Peak Oxygen Uptake
2.6. Measurements during Trekking
2.7. Acute Mountain Sickness Assessment
2.8. Sample Size
2.9. Statistical Analyses
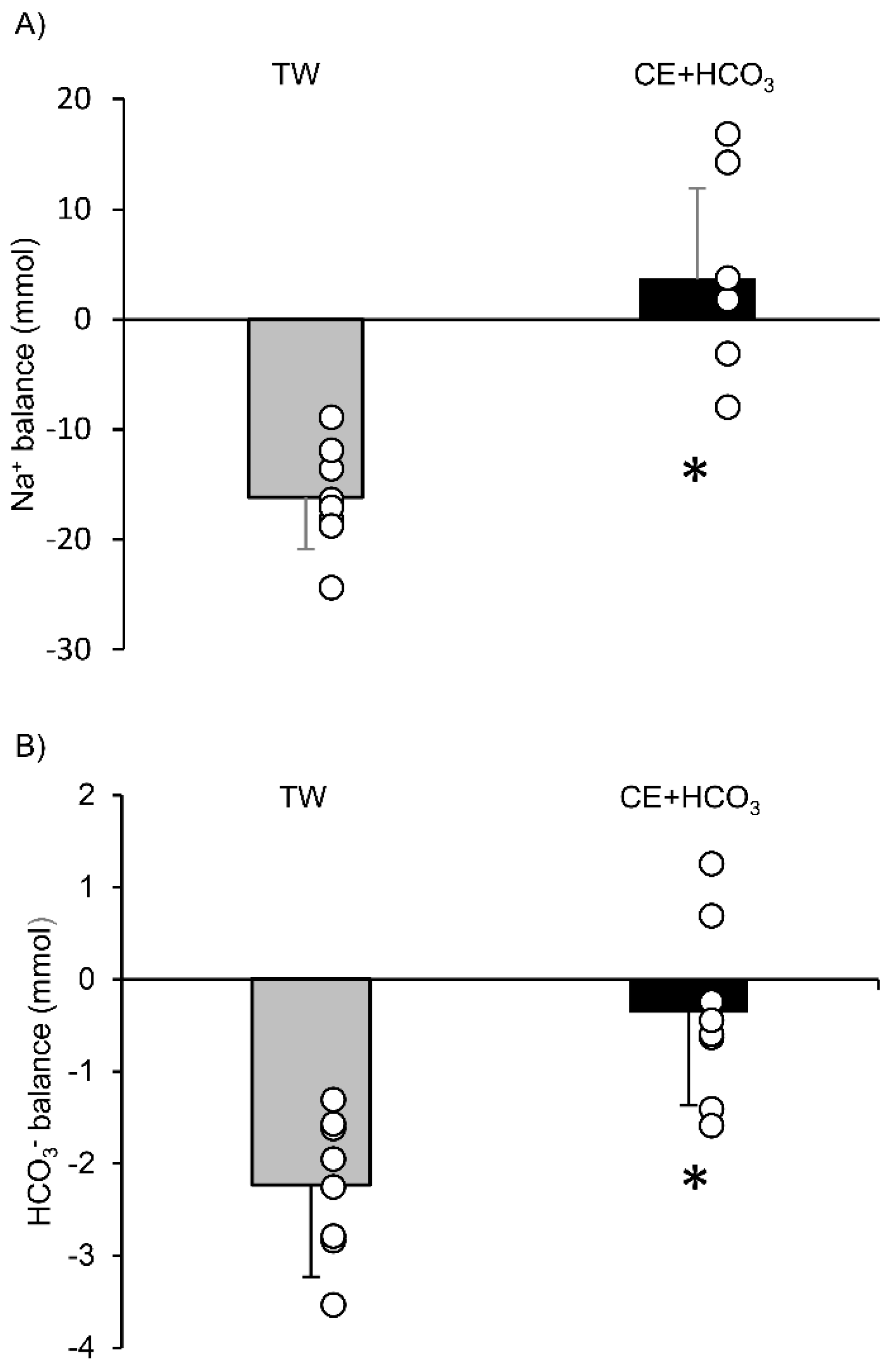
3. Results
4. Discussion
4.1. Methodological Considerations
4.2. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenefick, R.W.; Mahood, N.V.; Mattern, C.O.; Kertzer, R.; Quinn, T.J. Hypohydration adversely affects lactate threshold in endurance athletes. J. Strength Cond. Res. 2002, 16, 38–43. [Google Scholar]
- Papadopoulos, C.; Doyle, J.; Rupp, J.; Brandon, L.; Benardot, D.; Thompson, W. The effect of the hypohydration on the lactate threshold in a hot and humid environment. J. Sports Med. Phys. Fit. 2008, 48, 293–299. [Google Scholar] [CrossRef]
- Buchheit, M.; Kuitunen, S.; Voss, S.C.; Williams, B.K.; Mendez-Villanueva, A.; Bourdon, P.C. Physiological strain associated with high-intensity hypoxic intervals in highly trained young runners. J. Strength Cond. Res. 2012, 26, 94–105. [Google Scholar] [CrossRef]
- Sumi, D.; Kojima, C.; Goto, K. Impact of endurance exercise in hypoxia on muscle damage, inflammatory and performance responses. J. Strength Cond. Res. 2018, 32, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.B.; Hein, L.; Svendsen, L.B.; Secher, N.H.; Quistorff, B. Bicarbonate attenuates intracellular acidosis. Acta Anaesthesiol. Scand. 2002, 46, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Fulco, C.S.; Kambis, K.W.; Friedlander, A.L.; Rock, P.B.; Muza, S.R.; Cymerman, A. Carbohydrate supplementation improves time-trial cycle performance during energy deficit at 4300-m altitude. J. Appl. Physiol. 2005, 99, 867–876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliver, S.J.; Golja, P.; Macdonald, J.H. Carbohydrate supplementation and exercise performance at high altitude: A randomized controlled trial. High Alt. Med. Biol. 2012, 13, 22–31. [Google Scholar] [CrossRef]
- Fulco, C.S.; Zupan, M.; Muza, S.R.; Rock, P.B.; Kambis, K.; Payn, T.; Hannon, M.; Glickman, E.; Cymerman, A. Carbohydrate supplementation and endurance performance of moderate altitude residents at 4300 m. Int. J. Sports Med. 2007, 28, 437–443. [Google Scholar] [CrossRef]
- Deb, S.K.; Gough, L.A.; Sparks, S.A.; McNaughton, L.R. Determinants of curvature constant (W’) of the power duration relationship under normoxia and hypoxia: The effect of pre-exercise alkalosis. Eur. J. Appl. Physiol. 2017, 117, 901–912. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deb, S.K.; Gough, L.A.; Sparks, S.A.; McNaughton, L.R. Sodium bicarbonate supplementation improves severe-intensity intermittent exercise under moderate acute hypoxic conditions. Eur. J. Appl. Physiol. 2018, 118, 607–615. [Google Scholar] [CrossRef]
- Driller, M.W.; Gregory, J.R.; Williams, A.D.; Fell, J.W. The effects of serial and acute NaHCO3 loading in well-trained cyclists. J. Strength Cond. Res. 2012, 26, 2791–2797. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Brown, D.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. The influence of alkalosis on repeated high-intensity exercise performance and acid-base balance recovery in acute moderate hypoxic conditions. Eur. J. Appl. Physiol. 2018, 118, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Deb, S.K.; Brown, D.; Sparks, S.A.; McNaughton, L.R. The effects of sodium bicarbonate ingestion on cycling performance and acid base balance recovery in acute normobaric hypoxia. J. Sports Sci. 2019, 37, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Flinn, S.; Herbert, K.; Graham, K.; Siegler, J.C. Differential effect of metabolic alkalosis and hypoxia on high-intensity cycling performance. J. Strength Cond. Res. 2014, 28, 2852–2858. [Google Scholar] [CrossRef]
- Saunders, B.; Sale, C.; Harris, R.C.; Sunderland, C. Effect of sodium bicarbonate and Beta-alanine on repeated sprints during intermittent exercise performed in hypoxia. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Cripps, D. The effects of combined glucose-electrolyte and sodium bicarbonate ingestion on prolonged intermittent exercise performance. J. Sports Sci. 2012, 30, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Pyne, D.B. Bicarbonate loading to enhance training and competitive performance. Int. J. Sports Physiol. Perform. 2007, 2, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Heibel, A.B.; Perim, P.H.L.; Oliveira, L.F.; McNaughton, L.R.; Saunders, B. Time to optimize supplementation: Modifying factors influencing the individual responses to extracellular buffering agents. Front. Nutr. 2018, 5, 35. [Google Scholar] [CrossRef]
- Hackett, P.H.; Rennie, D.; Hofmeister, S.E.; Grover, R.F.; Grover, E.B.; Reeves, J.T. Fluid retention and relative hypoventilation in acute mountain sickness. Respiration 1982, 43, 321–329. [Google Scholar] [CrossRef]
- Loeppky, J.A.; Icenogle, M.V.; Maes, D.; Riboni, K.; Hinghofer-Szalkay, H.; Roach, R.C. Early fluid retention and severe acute mountain sickness. J. Appl. Physiol. 2005, 98, 591–597. [Google Scholar] [CrossRef]
- Ge, R.L.; Babb, T.G.; Sivieri, M.; Resaland, G.K.; Karlsen, T.; Stray-Gundersen, J.; Levine, B.D. Urine acid-base compensation at simulated moderate altitude. High Alt. Med. Biol. 2006, 7, 64–71. [Google Scholar] [CrossRef]
- Traynor, J.; Mactier, R.; Geddes, C.C.; Fox, J.G. How to measure renal function in clinical practice. BMJ 2006, 333, 733–737. [Google Scholar] [CrossRef]
- Horiuchi, M.; Endo, J.; Dobashi, S.; Kiuchi, M.; Koyama, K.; Subudhi, A.W. Effect of progressive normobaric hypoxia on dynamic cerebral autoregulation. Exp. Physiol. 2016, 101, 1276–1284. [Google Scholar] [CrossRef]
- Horiuchi, M.; Endo, J.; Kondo, K.; Uno, T.; Morikawa, M.; Nose, H. Impact of carbohydrate-electrolyte beverage ingestion on heart rate response while climbing mountain fuji at ~3000 m. BioMed Res. Int. 2017, 2017, 3919826. [Google Scholar] [CrossRef]
- Yamazaki, T.; Gen-No, H.; Kamijo, Y.; Okazaki, K.; Masuki, S.; Nose, H. A new device to estimate VO2 during incline walking by accelerometry and barometry. Med. Sci. Sports Exerc. 2009, 41, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-Predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Shimizu, M.; Miyagawa, K.; Iwashita, S.; Noda, T.; Hamada, K.; Genno, H.; Nose, H. Energy expenditure during 2-day trail walking in the mountains (2857 m) and the effects of amino acid supplementation in older men and women. Eur. J. Appl. Physiol. 2012, 112, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Roach, R.C.; Hackett, P.H.; Oelz, O.; Bartsch, P.; Luks, A.M.; MacInnis, M.J.; Baillie, J.K.; The Lake Louise AMS Score Consensus Committee. The 2018 lake louise acute mountain sickness score. High Alt. Med. Biol. 2018, 19, 4–6. [Google Scholar] [CrossRef]
- Zouboules, S.M.; Lafave, H.C.; O’Halloran, K.D.; Brutsaert, T.D.; Nysten, H.E.; Nysten, C.E.; Steinback, C.D.; Sherpa, M.T.; Day, T.A. Renal reactivity: Acid-Base compensation during incremental ascent to high altitude. J. Physiol. 2018, 596, 6191–6203. [Google Scholar] [CrossRef]
- Carr, A.J.; Gore, C.J.; Dawson, B. Induced alkalosis and caffeine supplementation: Effects on 2000-m rowing performance. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 357–364. [Google Scholar] [CrossRef]
- Pitts, R.F.; Ayer, J.L.; Schiess, W.A.; Miner, P. The renal regulation of acid-base balance in man. III. The reabsorption and excretion of bicarbonate. J. Clin. Investig. 1949, 28, 35–44. [Google Scholar] [CrossRef]
- Moriguchi, T.; Tomoda, A.; Ichimura, S.; Odagiri, Y.; Inoue, S.; Nagasawa, T.; Tanaka, H.; Nakagawa, N.; Shimomitsu, T. Significance of post-exercise increment of urinary bicarbonate and pH in subjects loaded with submaximal cycling exercise. Tohoku J. Exp. Med. 2004, 202, 203–211. [Google Scholar] [CrossRef]
- Cheung, S.S.; Mutanen, N.E.; Karinen, H.M.; Koponen, A.S.; Kyrolainen, H.; Tikkanen, H.O.; Peltonen, J.E. Ventilatory chemosensitivity, cerebral and muscle oxygenation, and total hemoglobin mass before and after a 72-day mt. Everest expedition. High Alt. Med. Biol. 2014, 15, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Stelter, G.P.; Vogel, J.A. Arterial pyruvate, lactate, pH, and PCO2 during work at sea level and high altitude. J. Appl. Physiol. 1967, 23, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: What, why and how it influences muscle function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Jones, R.L.; Stellingwerff, T.; Artioli, G.G.; Saunders, B.; Cooper, S.; Sale, C. Dose-Response of sodium bicarbonate ingestion highlights individuality in time course of blood analyte responses. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 445–453. [Google Scholar] [CrossRef]
- Bernardi, M.; Guerra, E.; Rodio, A.; Dante, D.; Castellano, V.; Peluso, I.; Schena, F.; Bhambhani, Y. Assessment of exercise stroke volume and its prediction from oxygen pulse in paralympic athletes with locomotor impairments: Cardiac long-term adaptations are possible. Front. Physiol. 2019, 10, 1451. [Google Scholar] [CrossRef]
- Honigman, B.; Theis, M.K.; Koziol-McLain, J.; Roach, R.; Yip, R.; Houston, C.; Moore, L.G.; Pearce, P. Acute mountain sickness in a general tourist population at moderate altitudes. Ann. Intern. Med. 1993, 118, 587–592. [Google Scholar] [CrossRef]
- Wu, T.Y.; Ding, S.Q.; Zhang, S.L.; Duan, J.Q.; Li, B.Y.; Zhan, Z.Y.; Wu, Q.L.; Baomu, S.; Liang, B.Z.; Han, S.R.; et al. Altitude illness in Qinghai-Tibet railroad passengers. High Alt. Med. Biol. 2010, 11, 189–198. [Google Scholar] [CrossRef]
- Richalet, J.P.; Larmignat, P.; Poitrine, E.; Letournel, M.; Canoui-Poitrine, F. Physiological risk factors for severe high-altitude illness: A prospective cohort study. Am. J. Respir. Crit. Care Med. 2012, 185, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.E.; Turk, A.J.; Maggiorini, M.; Hess, T.; Merz, T.; Bosch, M.M.; Barthelmes, D.; Hefti, U.; Pichler, J.; Senn, O.; et al. Effect of ascent protocol on acute mountain sickness and success at Muztagh Ata, 7546 m. High Alt. Med. Biol. 2009, 10, 25–32. [Google Scholar] [CrossRef]
- Erba, P.; Anastasi, S.; Senn, O.; Maggiorirni, M.; Bloch, K.E. Acute mountain sickness is related to nocturnal hypoxemia but not to hypoventilation. Eur. Respir. J. 2004, 24, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.H.; Gao, X.B.; Wu, X.J.; Yu, J.; Chen, J.F.; Bian, S.Z.; Ding, X.H.; Huang, L. Correlation between blood pressure changes and AMS, sleeping quality and exercise upon high-altitude exposure in young Chinese men. Mil. Med. Res. 2014, 1, 19. [Google Scholar] [CrossRef][Green Version]
- Oliver, S.J.; Sanders, S.J.; Williams, C.J.; Smith, Z.A.; Lloyd-Davies, E.; Roberts, R.; Arthur, C.; Hardy, L.; Macdonald, J.H. Physiological and psychological illness symptoms at high altitude and their relationship with acute mountain sickness: A prospective cohort study. J. Travel Med. 2012, 19, 210–219. [Google Scholar] [CrossRef]
- Mairer, K.; Wille, M.; Bucher, T.; Burtscher, M. Prevalence of acute mountain sickness in the Eastern Alps. High Alt. Med. Biol. 2009, 10, 239–245. [Google Scholar] [CrossRef]
- Aeberli, I.; Erb, A.; Spliethoff, K.; Meier, D.; Gotze, O.; Fruhauf, H.; Fox, M.; Finlayson, G.S.; Gassmann, M.; Berneis, K.; et al. Disturbed eating at high altitude: Influence of food preferences, acute mountain sickness and satiation hormones. Eur. J. Nutr. 2013, 52, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Caravita, S.; Faini, A.; Lombardi, C.; Valentini, M.; Gregorini, F.; Rossi, J.; Meriggi, P.; Di Rienzo, M.; Bilo, G.; Agostoni, P.; et al. Sex and acetazolamide effects on chemoreflex and periodic breathing during sleep at altitude. Chest 2015, 147, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Takase, K.; Nishiyasu, T.; Asano, K. Modulating effects of the menstrual cycle on cardiorespiratory responses to exercise under acute hypobaric hypoxia. Jpn. J. Physiol. 2002, 52, 553–560. [Google Scholar] [CrossRef] [PubMed]
| TW | CE+HCO3 | p Values | |
|---|---|---|---|
| (6 M and 2 W) | (6 M and 2 W) | ||
| Age, years | 34 ± 9 | 33 ± 10 | 0.794 |
| Height, cm | 169 ± 7 | 170 ± 7 | 0.730 |
| Body weight, kg | 61.7 ± 8.1 | 62.8 ± 9.9 | 0.799 |
| BMI, kg·(m2)−1 | 21.6 ± 2.2 | 21.7 ± 3.0 | 0.930 |
| HR at standing rest, bpm | 80 ± 2 | 78 ± 4 | 0.391 |
| HRpeak during 9 min walking, bpm | 159 ± 15 | 155 ± 9 | 0.509 |
| Estimated VO2peak, mL·kg−1·min−1 | 39.3 ± 9.0 | 41.7 ± 13.1 | 0.676 |
| SpO2 at rest, % | 94 ± 2 | 95 ± 1 | 0.862 |
| SpO2nadir, % | 83 ± 3 | 87 ± 3 | 0.022 |
| TW | CE+HCO3 | p Values | |
|---|---|---|---|
| (6 M and 2 W) | (6 M and 2 W) | ||
| Walking time, min | 121 ± 3 | 118 ± 3 | 0.104 |
| Resting time, min | 32 ± 5 | 31 ± 2 | 0.618 |
| During walking | |||
| Average EE, mL·min−1 | 880 ± 130 | 951 ± 179 | 0.379 |
| Average HR, mL·min−1 | 138 ± 10 | 128 ± 12 | 0.071 |
| Average EE/HR, mL·min−1·bpm−1 | 6.38 ± 0.87 | 7.48 ± 1.32 | 0.068 |
| TW | CE+HCO3 | Two-Way ANOVA p Values | ||||
|---|---|---|---|---|---|---|
| (6 M and 2 W) | (6 M and 2 W) | Condition | Time | Interaction | ||
| Body weight, kg | Pre | 61.66 ± 8.13 | 63.07 ± 10.24 | 0.780 | <0.001 | 0.135 |
| Hut | 61.19 ± 7.97 | 62.40 ± 10.21 | ||||
| [Na+]u, meq·L−1 | Pre | 26.0 ± 14.0 | 29.0 ± 20.0 | 0.701 | <0.001 | 0.262 |
| Hut | 55.7 ± 21.4 | 46.4 ± 21.3 | ||||
| [HCO3−]u, meq·L−1 | Pre | 2.04 ± 0.28 | 2.23 ± 0.77 | 0.075 | 0.020 | 0.068 |
| Hut | 1.99 ± 0.15 | 3.36 ± 1.42 | ||||
| pH, u | Pre | 7.00 ± 0.76 | 7.19 ± 1.07 | 0.956 | 0.158 | 0.272 |
| Hut | 6.94 ± 0.62 | 6.72 ± 0.58 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiuchi, M.; Hasegawa, T.; Nose, H. Effect of Carbohydrate-Electrolyte Solution Including Bicarbonate Ion Ad Libitum Ingestion on Urine Bicarbonate Retention during Mountain Trekking: A Randomized, Controlled Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 1441. https://doi.org/10.3390/ijerph18041441
Horiuchi M, Hasegawa T, Nose H. Effect of Carbohydrate-Electrolyte Solution Including Bicarbonate Ion Ad Libitum Ingestion on Urine Bicarbonate Retention during Mountain Trekking: A Randomized, Controlled Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(4):1441. https://doi.org/10.3390/ijerph18041441
Chicago/Turabian StyleHoriuchi, Masahiro, Tatsuya Hasegawa, and Hiroshi Nose. 2021. "Effect of Carbohydrate-Electrolyte Solution Including Bicarbonate Ion Ad Libitum Ingestion on Urine Bicarbonate Retention during Mountain Trekking: A Randomized, Controlled Pilot Study" International Journal of Environmental Research and Public Health 18, no. 4: 1441. https://doi.org/10.3390/ijerph18041441
APA StyleHoriuchi, M., Hasegawa, T., & Nose, H. (2021). Effect of Carbohydrate-Electrolyte Solution Including Bicarbonate Ion Ad Libitum Ingestion on Urine Bicarbonate Retention during Mountain Trekking: A Randomized, Controlled Pilot Study. International Journal of Environmental Research and Public Health, 18(4), 1441. https://doi.org/10.3390/ijerph18041441






