Abstract
Background: The fast-spreading of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli (ESBL-producing E. coli) and ESBL genes has become a big challenge to public health. The risk of spreading ESBL genes and pathogens in the environment and community has raised public health concern. The characterizing and whole-genome sequencing studies of ESBL-producing bacteria from reservoir water in Singapore is still limited. Materials and methods: The reservoir water sample was taken from two randomly selected sampling points of the Chinese Garden (Jurong river reservoir), which is a popular reservoir park in Singapore. The bacteria of the water sample were collected with 0.45 µm filter membranes and enriched before processing with ESBL-producing E. coli screening. The collected ESBL positive isolates were further characterized by both phenotypic tests including disc diffusion and microdilution Minimum Inhibitory Concentration (MIC) test, and also genotypic test as whole-genome sequencing analysis. Besides, to investigate the transferability of the resistance gene, a conjugation test was performed with the J53 E. coli strain as the gene receptor. Result: Nine ESBL-producing E. coli isolates were collected and confirmed as ESBL-producing with both phenotypic and genotypic tests. A potential pathogen as ST131 clade A isolate was identified, and all isolates were determined to harbor a blaCTX-M gene. Among them, strain J1E4 was resistant to polymyxin E and confirmed to harboring a conjugatable mcr-1 gene. Further genetic environment analysis has reflected a conversed gene cluster formed by insert sequence (IS), blaCTX-M-15, and WbuC family cupin-fold metalloprotein, which may potentially jump from the plasmids to the chromosome. Conclusion: The first time we reported the whole genome sequencing (WGS) data of ESBL-producing E. coli including potential pathogen (ST131) present in reservoir water in Singapore. The ESBL-producing E. coli from reservoir water also carrying conjugatable colistin resistance genes which may become a risk to human health.
1. Introduction
Extended-Spectrum Beta-Lactamase (ESBL)-producing Enterobacteriaceae has become a big challenge to infection control due to their resistance to most of the beta-lactams. The ESBL can hydrolyze the most commonly used penicillin, monobactam, and cephalosporin including the 3rd generation [1]. Moreover, ESBL-producing bacteria are frequently reported to carry multi-drug resistance (MDR), which limits the options of antimicrobials to treat infections caused by them [2]. The prevalence of ESBL-E. coli is increasing fast worldwide, especially some prevalent sequencing types like ST131. A study from Europe in 2018 has reported a fraction of 20.5% of ST131 among the ESBL-producing E. coli from four European hospitals [3]. As the situation gains importance, a higher requirement of sequencing information for deeper genetic alignment of the ESBL-producing E. coli isolates has been addressed. Besides the Multilocus sequence typing (MLST) with the polymerase chain reaction (PCR) and Sanger sequencing, the whole genome sequencing (WGS) has been increasingly applied in the phylogenetic analysis of ESBL-producing E. coli isolates. Further WGS analysis helps to classify ST131 isolates into different clades and makes it easier for global researchers to monitor the dissemination of them [4].
Nowadays, besides the spreading of ESBL-producing E. coli isolates, the horizontal transfer of beta-lactamase genes between different sources and species has also become a big concern. [5] Besides traditional phenotypic testing, genetic methods like PCR and Sanger sequencing targeting ESBL genes have made great contributions in monitoring the spread of ESBL genes [6]. Moreover, the sequencing technology and the Antimicrobial Resistance (AMR) gene database have been widely applied in this field to promote the study of the location and combination of the AMR gene cluster within relevant isolates. Surveillance systems from many countries have been upgraded to include the ESBL genes found in different types of isolates. Among them, the blaCTX-M genes are one of the most prevalent ESBL genes. As addressed by the Centers for Disease Control and Prevention (CDC),
(https://www.cdc.gov/drugresistance/biggest-threats.html#extend), the combination of the blaCTX-M gene and ST131 has great clinical importance.
Surface water plays an important role in providing reservoirs for microorganisms to exchange their genetic information including AMR markers. ESBL-producing bacteria have been widely reported in water bodies in many countries [7,8]. Furthermore, with the help of WGS, the genetic studies of the relationship among resistant isolates from different water sources in the same region have shown interesting new trends as per the spread of these bacteria [9].
The reservoirs and the rainwater catchment systems in Singapore are very unique and play an important role in freshwater supply. To avoid pollution, the wastewater collection system is separated from the rainwater collection. However, the reservoir water may still have the potential to obtain AMR markers like ESBL genes originating in other environmental and anthropogenic sources, such as soil, air, plants, animals, and human beings [10]. The Jurong Lake reservoir is located in a 90-hectare new national garden in western Singapore and is planning to build the largest commercial and regional center outside Singapore’s city center. It is available for water sports, popular for community activities. The water samples were taken from two points of the reservoir to collect ESBL-producing E. coli strain potential contaminated from the community. Based on our knowledge, our study is the first WGS analysis study of ESBL-producing E. coli from the reservoir water in Singapore. This study aims towards a genetic characterization of ESBL-producing E. coli from reservoir water, further, investigate their phylogenetic relationship with isolates from humans, report the acquired ESBL genes as well as other resistance genes harbored by the isolates, predict the transferability of the AMR genes with sequencing data. With the reported data, we have provided some new insights for discussing the potential role of this water environment in the spread of clinically relevant AMR in Singapore.
2. Materials and Methods
2.1. Sample Collection and Processing
Eight hundred milliliters of surface water (1 m depth to the surface) was collected from two randomly selected sampling points in Chines Garden and transported to the lab at 4 °C within 4 h. The full water sample was then filtered through 0.45 µm filter membranes (Sigma, Germany). The filter membranes were incubated overnight in Mueller Hinton (MH) Broth under 37 °C.
2.2. ESBL-Producing Bacteria Isolation
The overnight cultures were streaked on chromogenic screening plates as Brilliance ESBL agar plates (Thermo scientific, USA). Blue or pink colonies were picked as presumptive positive for E. coli and re-streaked on an MH agar for further purification. The pure colonies were picked for overnight liquid culture in MH broth at 37 °C. The overnight culture was then modified to long-term stock containing 20% (v/v) of glycerol and stored at −80 °C for further processing.
2.3. Antimicrobial Susceptibility Test
The ESBL screening was performed with a double-disc synergy test. Discs of amoxicillin/clavulanic acid (AMC, 30 µg) were placed adjacent to four cephalosporin discs including ceftriaxone (CRO, 30 µg), ceftazidime (CAZ, 30 µg), cefotaxime (CTX, 30 µg), and cefepime (FEP, 30 µg) with an 8-channel dispenser (Thermo Scientific, USA). The result was considered positive when elliptical clearing was apparent between the AMC disc and any of the cephalosporin discs.
The ESBL confirmation was processed using Sensititre™ ESBL Plate (Thermo Scientific, USA) with microdilution methods. The details of the antimicrobials are listed in Table S1. The Minimum Inhibitory Concentration (MIC) was defined as the lowest concentration to inhibit visible growth. The isolates were considered as ESBL-producing when an 8 times decrease of MICs appeared when cephalosporin combined with clavulanate acid compared to cephalosporin alone.
2.4. DNA extraction and Next-Generation Sequencing
The bacterial genomic DNA was extracted using the Qiagen DNA miniprep kit (Germany) with the modified protocol [11]. The genomic DNA was then processed with Hiseq sequencing (Illumina, USA) based on the methods described before [12].
2.5. Whole-Genome Sequencing Analysis
The raw reads from Hiseq were assembled with Assembler1.2 [13]. The sequencing analysis tools used in this research is provided by the Center for Genomic Epidemiology (CGE) server [14]. The default setting was used for all tools. The genome annotation was performed with RAST [15]. The location of the beta-lactamase genes, fluoroquinolone resistance genes, and mcr genes were determined by the alignment of contigs carrying AMR genes with BLASTn. For isolates J1E1, J2E2, and J2E4, as the contigs carrying AMR genes were over the size limit of BLASTn, 20,000 bp fragments containing the AMR genes were extracted for Blast. The insert sequence was detected with ISFinder [16].
2.6. Phylogenetic Analysis
Multilocus sequence typing was processed with MLST 2.0 CGE server. As strain J2E1 has been defined to be ST131, a pack of ST131 isolate from different countries from the clinic was chosen to do the phylogenetic analysis with J2E1. The WGS raw reads of clinical E. coli isolates were selected from Bioproject accession No.PRJNA398288, as clinical isolates of ST131 from the year 2015 to 2017; the isolates details can be found in supplementary Table S2. The phylogenetic analysis was processed with the CGE server based on the core gene single-nucleotide polymorphism (SNP). The phylogenetic tree was annotated with iToL tools [17]. The alignment of contigs carrying beta-lactamase genes was processed with BLASTn and plotted with SnapGene software (from GSL Biotech; available at snapgene.com).
2.7. Conjugation Experiment to Test the Transferability of the Mcr-1 Gene
As harboring the mcr-1 gene, strain J1E4 was chosen as the donor strain for the conjugation test. E. coli strain J53 with sodium azide resistance was chosen as recipients. Briefly, single colonies of both J1E4 and J53 was picked from overnight culture MH agar plate, respectively, and cultured in MH broth at 37 °C with 150 rpm for 4 h to reach the early log phase. The donor and recipient culture were mixed in a 3:1 (V: V) ratio and cultured at 37 °C overnight statically. The MH agars contain 200 µg/mL sodium azide was used to calculate the total concentration of the J53 strain. The MH agars contain 200 µg/mL sodium azide and 4 µg/mL colistin were used to select the J53 harboring mcr-1 transconjugants. The antimicrobial susceptibility of successful transconjugants was determined with the microdilution MIC test and disc diffusion test. The co-transferability of blaCTX-M-15, blaTEM-1B genes with the mcr-1 gene was determined with the PCR test, the primers are shown as Table S3, and the result was checked with agarose (2%, w/v) gel electrophoresis.
3. Results
3.1. Genome Profile of ESBL-Producing E. coli Isolates
Nine ESBL-producing E. coli were isolated from the water sample and sent for WGS. The basic genomic and typing information is listed in Table 1. The genome size of the nine isolates is between 4,866,444 bp to 5,408,697 bp and content GC percentage between 50.3% to 50.7%. The MLST types of isolates were all different. Two isolates had up to 5 plasmid replicons, three isolates had 2 origins of replications (Ori), the last four isolates carried 3–4 Oris. The most common Ori was IncFII, which was found in six isolates. The MLST types and plasmids are listed in Table 1. Among them, ST131 has been reported to cause infections in Singapore before and clinic isolates information is shown in Table S2 and phylogenetic tree.

Table 1.
Basic information of the isolates.
3.2. AMR Genes of the Isolates
The AMR genes detected from each isolate are listed in Table 2 and shown in Figure 1. All the nine isolates shown positive ESBL in double-disc diffusion were confirmed to carrying ESBL genes. Besides, 8/9 except J1E2 harboring resistance genes subject to at least three classes, which from the genetic angle predict as MDR. As carrying the most AMR genes, strain J2E3 had been detected to carry 17 resistance genes subject to nine types of antimicrobials. All isolates were carrying blaCTX-M genes, and two of the isolates were carrying a blaTEM-1B gene besides the blaCTX-M-15. blaCTX-M-15 was the most prevalent beta-lactamase as it was detected in 5 isolates. Besides the beta-lactamase genes, the macrolide, lincosamide, and streptogramin B (MLS) classes of resistance genes were the most common and were detected in all isolates. Moreover, the variant type is mainly mph(A) and mdf(A).

Table 2.
Acquired resistance genes and resistance-related mutations detected in isolates by ResFinder.
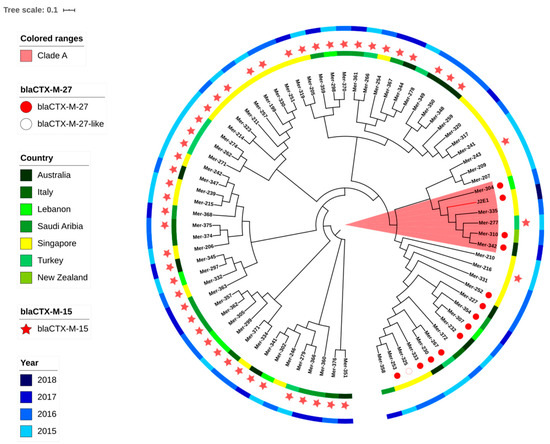
Figure 1.
The phylogenetic tree of ST131 isolates from both the clinic and reservoir based on core genome single-nucleotide polymorphisms (SNPs). The tree was built with E. coli MG1655 as the reference genome and branch length was ignored. J2E1 was isolated in this research and or the other isolates were from NCBI. The metadata is shown in the supplementary.
Fluoroquinolone resistance gene qnrS1 was also detected in four isolates including a variant strain of qnrS1 with 99.85% identity in J2E3. J1E4 was detected to carry both the qnS1 gene and mutations (gyrA p.S83L). The other three strains were only detected with mutations. Two mobile-colistin resistance genes mcr-1.1 and mcr-3.1 have been detected in strain J1E4 and J2E3, respectively.
3.3. Phenotype and Genotype Comparison
A brief comparison was done between the resistance characterization with phenotypic and sequencing methods. The phenotypic susceptibility was determined with the microdilution MIC test and disc diffusion according to the Clinical and Laboratory Standards Institute (CLSI) standards (Tables S1 and S5). According to the ESBL definition of the CLSI standards, all the isolates were determined with ESBL-producing phenotype based on the difference of MICs between subjects to cephalosporin and cephalosporin combined with clavulanic acid. The confirmation of ESBL-producing with MICs is agreed with the double-disc synergy test and genetic prediction (Table 3). Four out of nine isolates were confirmed as MDR with the phenotypic test. Five of the isolates were harboring resistance genes without showing the phenotype, which may due to the genes were regulated or silenced.

Table 3.
The comparison of phenotypic and genotypic resistance.
All the isolates have shown resistance to 6/8 cephems tested, except that, no isolates were resistant to cefoxitin, but the MICs of J1E1, J1G1, and J2E3 have reached the intermediate range, as MIC = 16 µg/mL. The isolates have shown different susceptibility to ceftazidime and ceftazidime/clavulanic acid. Among the five isolates that harbored blaCTX-M-15, four are resistant to ceftazidime, except J2E2 only reached the intermedium range. Besides, strain J2E3 is also resistant to ceftazidime, the blaCTX-M-55 it harbored is a variant of blaCTX-M-15. As a beta-lactamase inhibitor, clavulanic acid significantly influenced the susceptibility to ceftazidime and cefotaxime. The MIC of J1E4 to ceftazidime decreased 1024-fold when it was combined with the clavulanic acid. Except that, the resistance of J1E1 to cefotaxime was not inhibited by clavulanic acid, which is different from the response of the other four blaCTX-M-15 harbored isolates.
Among the 4 isolates which were carrying qnrS1 genes, strain J1E4 and J2E4 have shown resistance to ciprofloxacin, but J2E2 and J2E3 were sensitive to ciprofloxacin. For the three isolates that were only detected with ciprofloxacin resistance mutations, J1E3 was sensitive to ciprofloxacin, the other two were resistant to ciprofloxacin.
Only the J1E4 strain has been determined to be resistant to colistin as MIC = 4 µg/mL, which may because of the mcr-1 gene, besides, the mcr-1 gene is confirmed to be conjugatable and successfully provide resistance to the new receipts strain J53. Strain J2E3 is still sensitive to colistin even though harboring an mcr-3 gene.
3.4. Phylogenetic Analysis
Considering the clinic importance of E. coli ST131, a phylogenetic analysis was applied including the J2E1 ST131 and other ST131 clinical isolates from seven countries including Singapore as shown in Figure 1. If shown with the branch length, the branch containing J2E1 has a further evolution distance compared to other branches. All isolates on this branch including J2E1 were carrying fimH41, which may reflect that this branch belonged to ST131 clade A. The nearest strain to J2E1 on the tree is strain Mer-335 which has been isolated from the blood of a patient in Singapore in 2016. Besides this strain, 3 clinical isolates from other countries: Australia, New Zealand, and Indonesia were also on the same branch as J2E1. However, as the SNP difference was over 350, this may suggest a more distant common ancestor, instead of direct relationships between the clinical isolates and J2E1.
Even though no direct link was found between the J2E1 and clinic isolates, the genetic analysis has shown some similarity of AMR markers including the similar types of beta-lactamase genes and other mutations among isolates on clade A branch (Table S3). Four out of six isolates on this branch including J2E1 were carrying blaCTX-M-27, except Mer-277 and Mer-335, harboring blaCTX-M-15 and blaCTX-M-14, respectively. The blaCTX-M-27 of J2E1 and Mer-304 were both located on short contigs, below 2000 bp, with 100% identification. A conversed transposase DDE domain was found in front of the blaCTX-M-27 on the two contigs, which may suggest the blaCTX-M-27 was located on a widely distributed transferable element, shared between these two isolates.
The gyrA p. S83L mutation point appeared in all 6 isolates of this clade, parC p.S80I and gyrA p. D87N mutations of J2E1 were also detected in Mer-304 and Mer-335. Both Mer-304 and J2E1 were harboring unique mutations on parE as p.L445H and pE460D, respectively; all the other 4 isolates on this branch were harboring parE p.I529L mutation instead, which suggested the common existence of quinolone related mutations in ESBL-producing isolates of ST131 clade A from both clinic and environment.
3.5. Location Determined of Selected AMR Genes and Genetic Environment Analysis of Blactx-Ms
To predict the location of resistance genes with sequencing data, the contigs contain resistance genes were BLAST with BLASTn to find the best hits (Table S4). Four of the nine contigs harboring blaCTX-M genes were found highly similar to the chromosomes sequence, all four isolates were collected from sampling point 1. Further DNA alignment and annotation have investigated the similar genetic environment of the blaCTX-M gene cluster (Table 4). The same type of insert sequence ISEcp1 was found in the forward direction of the blaCTX-M gene, with a distance of either 48 or 42 bp (Figure 2). A WbuC family cupin fold metalloprotein was found that followed the blaCTX-M-15 in a reverse direction. Interestingly, we found this gene cluster highly converses, as also present on the other two contigs carry blaCTX-M-15, which predicted to locate on the plasmid. This may reflect an important hypothesis that this gene cluster can jump from the plasmid to the chromosome. As the genes before and after this cluster are highly different, it is more likely this cluster is randomly inserted into the chromosome.

Table 4.
Insert sequence and their location detected by ISFinder.
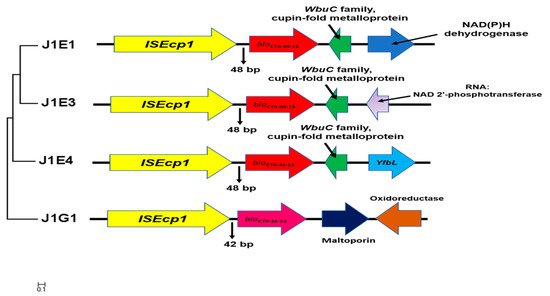
Figure 2.
The genetic environment surrounding blaCTX-M genes located on the chromosome. The maximum likelihood tree was built based on the alignment of 5000 bp fragments contain insert sequence and blaCTX-M gene extract from the chromosome. The gene annotation and direction were generated by the RAST server and further corrected with NCBI blast. The conversed gene cluster contains ISEcp1, blaCTX-M-15, and WubC family metalloprotein.
All of the four qnrS1 genes were found to be located on plasmids, and three of them were co-located on the same contigs with blaCTX-M genes. Interestingly, the qnrS1 gene in J2E2 was followed with another insert sequence ISKpn19; besides, the fragment between two inserts is the same as the sequence of the fragment from J2E4, which also contain ISEcp1, blaCTX-M-15, and qnrS1. This may raise the hypothesis that the fragment between two inserts potentially forms a co-transfer cluster.
Both of the mcr genes were predicted to locate on the plasmid. No perfect hit was found for the contig carrying mcr-1.1 in J1E4, the hit with the highest coverage (52%) found by Blastn was an IncFIA(HI1)/IncHI1A/IncHI1B(R27) type plasmid from E. coli. Only the first 7000 bp of the contig showed 100% identity. This prediction has been further proved with the conjugation test. The contig carrying mcr-3.1 of strain J2E3 showed a high similarity to a plasmid of a sewage isolate from Japan, which may suggest the mcr-3.1 of J2E3 is also located on a plasmid.
3.6. Co-Conjugation of Mcr-1 Gene and Blatem-1 Gene
The mcr-1 gene was successfully conjugated to the J53 strain and confirmed with the PCR test. Interestingly, as tested with beta-lactamase genes specific primer on the transconjugants, a co-existing of the blaTEM-1 gene was found, but not the blaCTX-M-15 gene. The co-conjugation maybe because these two genes were both predicted to be located on plasmids, while the blaCTX-M-15 gene was predicted to be chromosome-located instead by Blastn. From this angle, the genetic prediction of location is agreed with the transferability phenotype.
We also compared the antimicrobial susceptibility of transconjugants and the donor strain (Table 5). The colistin resistance phenotype is successfully presented in the transconjugants and even the MIC of colistin increased twice. Due to the blaTEM-1B gene is also detected in the transconjugants, the transconjugant strain has also shown resistance to both the ampicillin and cephalosporin beta-lactams. However, the transconjugant strain is less responsive to clavulanic acid on ceftazidime resistance, which may be due to the missing blaCTX-M-15 gene. Even so, the transconjugant still showed the ESBL-producing phenotype. Interestingly, besides the resistance to beta-lactams, the transconjugant has also shown a higher MIC to ciprofloxacin, and a similar level of resistance to tetracycline and chloramphenicol, which suggest the co-conjugation of these resistances with mcr-1.

Table 5.
The minimum inhibitory concentration (MIC) comparison among J1E4, J53, and the transconjugants.
4. Discussion
An increasing prevalence of ESBL-producing bacteria has been reported from different sources including food, animal, and clinical isolates globally [18]. Besides the potential risk of transmitting ESBL-producing isolates from other sources to humans suggested [19], the spreading of ESBL genes through horizontal gene transfer (HGT) has also become a big concern to public health. Concerning this, more deep genetic studies are needed. As shown in this study, WGS has become a useful tool to study the ESBL-producing isolate from different sources. The phylogenetic analysis with WGS can help to draw the links between isolates from different sources, and further DNA alignment can help to predict the AMR gene cluster spread between different isolates. In Singapore, the WGS has been widely used for ESBL studies of isolates from human, food, and animals, but not for isolates from reservoir water [20]. One study has reported resistant bacteria from aquaculture farms in Singapore without WGS analysis [21]. To our knowledge, our study is the first WGS analysis report of reservoir ESBL-producing isolates from Singapore.
The ESBL-producing E. coli has great clinical importance as a high prevalence of MDR, which limits the options for infection control [22]. The co-existence of ESBL genes and other resistance genes has been widely reported and was also shown in our isolates. All isolates were detected to carry more than 2 classes of resistance genes, and four of the isolates were resistant to both beta-lactams and ciprofloxacin. Besides, more than one type of Ori was detected in these isolates, which may suggest their high capabilities in gene communication. Moreover, six out of the nine isolates were carrying contigs with more than one type of AMR genes, which may suggest the co-transfer potential of these AMR genes. The most significant co-location in our isolates is the fluoroquinolone resistance genes and beta-lactamase. Fluoroquinolone and beta-lactams are both important and widely used antimicrobials in the clinic. The co-transfer of these two types of genes would be a potential risk to public health.
E. coli ST131 has been recognized as one of the top contributors to urinary tract infections in the human clinic globally [4]. E. coli ST 131 has been reported to evolve with MDR especially the ESBL-producing phenotype quite often. Research in the United States has pointed out the ST131 as the major cause of serious MDR infections in 2007 [23]. The J2E1 (ST131 clade A) isolate was detected to carrying the virulence factor of gad (glutamate decarboxylase), iha (adherence protein), and senB (plasmid-encoded enterotoxin). The PathogenFinder has also suggested its significant potential as a human pathogen. J2E1 was also determined to be MDR, and resistance genes subjected to six antimicrobials were detected including a blaCTX-M-27. Unlike reported in other countries like Denmark, which have shown ST131 is highly associated with blaCTX-M-15 [24], blaCTX-M-27 has also been reported with a high prevalence among ST131 from bloodstream infections in other research in Singapore. Even though infections caused by clade C were dominant among ST131 E. coli, blood infections caused by clade A isolates carrying blaCTX-M-27 has also been reported in Singapore [25]. The isolate J2E1 collected here is O16: H5, a similar serotype that has been reported in some countries like France recently [26]. This serotype has also often been reported in clade A ST131, which is different from the abundant reports of O25: H4, mainly found in clade C. Even though no direct relationships were found between the water isolates and the isolates from the clinic, the same plasmid type, mutations, and similar resistance profiles were detected among them, and especially some conserved sequences contain the resistance genes were found, still suggesting a high potential horizontal AMR gene transfer between aquatic isolates and the clinical isolates.
The chromosome located blaCTX-M-15 has been widely reported already [27]. The highly conserved structure has been found in many different species, as contained ISEcp1, following with resistance genes and a reversed WbuC family, cupin fold metalloprotein. The WbuC family protein likely contains a histidine-residue as a metal-binding ligand and has been reported to relate to O-antigen biosynthesis [28]. The WbuC gene at this special position was also frequently annotated as Tryptophan synthase, due to the similar sequence protein that has been reported in Klebsiella spp. However, as the functions of the protein in the WbuC family were highly varied in different species, the role of it here with blaCTX-M-15 is still not well documented. The resistance genes in this unique gene cluster are not limited to blaCTX-M, and mcr-9 has also been reported to be followed by WbuC genes recently [29]. Therefore, the functional studies of the WbuC genes will be very interesting in the future. Even though the five isolates with blaCTX-M-15 are carrying the same gene cluster, their MIC of different beta-lactams and their response to beta-lactamase inhibitor is still different. The regulation and cooperation of ESBL genes are still important topics in the future.
Two of the isolates were detected with mobile-colistin-resistance genes, as mcr-1.1 and mcr-3.1, respectively, but only J1E4 with mcr-1.1 has shown resistance to colistin. The mcr-type genes were firstly reported in 2015. Colistin is also known as polymyxin E and has been taken as the last resort to treat MDR infections; therefore, the transferable colistin resistance has been related to a high risk in the clinic. The mcr-type genes in E. coli isolates have been reported in Singapore from other sources like patients in hospitals [30] and food [31]. The mcr-type genes from environmental water sources have also been reported in other countries like China [32] and Switzerland [33]. However, to our knowledge, no genetic report of mcr-type genes in environmental water from Singapore has been published yet. The co-existence of mcr-type genes and ESBL genes have already been reported in other Asian countries like China [34]. However, based on the Blastn prediction, the mcr-1 and blaCTX-M-15 genes are very unlikely to be co-located. Further conjugation studies failed to show their co-transferability, which has been suggested in other studies [35]. Instead, other types of resistance correspond with the colistin resistance including the blaTEM-1B gene detected with PCR and shown with the phenotype test. This also reflects the HGT of a group of AMR genes instead of only one.
The disagreement between genotype and phenotype is quite significant for fluoroquinolone resistance among our isolates as well as other resistance that have been shown in the phenotype and genotype comparison. Three out of seven isolates were sensitive to fluoroquinolone, disagreeing with the prediction by resistance genes and mutations. This phenomenon may be attributed to the “turn off” of the expression by transcriptional regulation or other gene silencing mechanisms. Further studies are needed to investigate the mechanism. However, non-resistant phenotype does not mean there is no risk at all. The qnrS1 genes still have the potential to spread and meet a suitable host without the regulation to express. The site mutation is also important for the fluoroquinolone resistance as shown in the two isolates. Hopefully, with more WGS data update, the mutation database will increase to improve the accuracy of resistance detection.
Even though no isolates were directly linked to clinical cases in this research, the same sequence type has been found in both reservoir isolates and clinical isolates, including ST131, ST38, and ST10. This may also be because the water collection period was one-two years later compared to clinic isolates collections. However, ST131 is highly evolved with humans and very unlikely to be the natural inhabited strain in water; this may suggest potential contamination from humans to the reservoir water. The core genome alignment has increased the accuracy of isolate tracking compared to only using MLSTs; therefore, it can show the detailed difference between isolates. So far there is quite limited information on AMR genes from the water source in Singapore, which also means there is a great need to apply NGS in future AMR studies of the aquatic environment. Theoretically, the separated water systems in Singapore should show advantages in avoiding waste contamination. However, as the sequencing data are limited, we cannot draw this conclusion from this research. However, in the future, with a more complete AMR surveillance system, we can further evaluate the benefit of this system and provide more scientifically-based suggestions to control the AMR spreading in reservoir water.
5. Conclusions
In a summary, this study has presented the existence of ESBL-producing E. coli in the surface water (reservoir water) in Singapore, including high-risk strain like ST131 isolate, and proved they are carrying conversed blaCTX-M genes clusters present on both plasmid and chromosome, as well as conjugatable resistance genes as the mcr-1.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/3/937/s1, Table S1: MIC of selected antimicrobials; Table S2: Metadata of isolates applied for phylogenetic analysis; Table S3. Resistance gene list of ST131 clade A isolates. Table S4. Best hits of the contigs harboring AMR genes found by Blastn. Table S5: Diameter of the inhibit zone for disc diffusion. Table S6. Primers used for the PCR test. Figure S1. The number of AMR genes subject to different classes detected by ResFinder. Figure S2. The comparison of Disc diffusion result between the donor J1E4 and the transconjugant J53coli. Figure S3. The results of the double-disc synergy test.
Author Contributions
Y.Z. and J.S. designed the research; Y.Z. performed the main experiment, including isolation, identification, whole genome sequencing analysis, antimicrobial susceptibility tests, and drafted the manuscript; J.S. helped with editing the manuscript; S.G. supported with the WGS analysis and editing the manuscript; K.L.G.S. helped with DNA extraction; G.O.H.M. helped with the disc diffusion test. All authors have approved the final version for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Nanyang Technological University, Singapore.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
All authors declare that there is no conflict of interest.
References
- Rupp, M.E.; Fey, P.D. Extended spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. Drugs 2003, 63, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.H.A.M.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H. Acquired Antibiotic Resistance Genes: An Overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef] [PubMed]
- Merino, I.; Hernández-García, M.; Turrientes, M.-C.; Pérez-Viso, B.; López-Fresneña, N.; Diaz-Agero, C.; Maechler, F.; Fankhauser-Rodriguez, C.; Kola, A.; Schrenzel, J.; et al. Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J. Antimicrob. Chemother. 2018, 73, 2973–2980. [Google Scholar] [CrossRef]
- Petty, N.K.; Ben Zakour, N.L.; Stanton-Cook, M.; Skippington, E.; Totsika, M.; Forde, B.M.; Phan, M.-D.; Moriel, D.G.; Peters, K.M.; Davies, M.; et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc. Natl. Acad. Sci. USA 2014, 111, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.A.L.-V.; Dierikx, C.; Stuart, J.C.; Voets, G.; Munckhof, M.V.D.; Van Essen-Zandbergen, A.; Platteel, T.; Fluit, A.; Van De Sande-Bruinsma, N.; Scharinga, J.; et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 2011, 17, 873–880. [Google Scholar] [CrossRef]
- Jemima, S.; Verghese, S. Multiplex PCR for blaCTX-M & blaSHV in the extended spectrum beta lactamase (ESBL) producing gram-negative isolates. Indian J. Med. Res. 2008, 128, 313. [Google Scholar]
- Haque, A.; Yoshizumi, A.; Saga, T.; Ishii, Y.; Tateda, K. ESBL-producing Enterobacteriaceae in environmental water in Dhaka, Bangladesh. J. Infect. Chemother. 2014, 20, 735–737. [Google Scholar] [CrossRef]
- Said, L.B.; Jouini, A.; Alonso, C.A.; Klibi, N.; Dziri, R.; Boudabous, A.; Slama, K.B.; Torres, C. Characteristics of extended-spectrum β-lactamase (ESBL)-and pAmpC beta-lactamase-producing Enterobacteriaceae of water samples in Tunisia. Sci. Total Environ. 2016, 550, 1103–1109. [Google Scholar] [CrossRef]
- Wu, D.; Su, Y.; Xi, H.; Chen, X.; Xie, B. Urban and agriculturally influenced water contribute differently to the spread of antibiotic resistance genes in a mega-city river network. Water Res. 2019, 158, 11–21. [Google Scholar] [CrossRef]
- Manaia, C.M. Assessing the Risk of Antibiotic Resistance Transmission from the Environment to Humans: Non-Direct Proportionality between Abundance and Risk. Trends Microbiol. 2017, 25, 173–181. [Google Scholar] [CrossRef]
- Zhong, Y. Optimised protocol of QIAamp® DNA mini Kit for bacteria genomic DNA extraction from both pure and mixture sample. Protoc. Exch. 2019. [Google Scholar] [CrossRef]
- Guo, S.; Tay, M.Y.; Aung, K.T.; Seow, K.L.; Ng, L.C.; Purbojati, R.W.; Drautz-Moses, D.I.; Schuster, S.C.; Schlundt, J. Phenotypic and genotypic characterization of antimicrobial resistant Escherichia coli isolated from ready-to-eat food in Singapore using disk diffusion, broth microdilution and whole genome sequencing methods. Food Control. 2019, 99, 89–97. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontén, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus Sequence Typing of Total-Genome-Sequenced Bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genom. 2008, 9, 1–15. [Google Scholar] [CrossRef]
- Siguier, P. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34 (Suppl. 1), D32–D36. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Chong, Y.; Shimoda, S.; Shimono, N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2018, 61, 185–188. [Google Scholar] [CrossRef]
- Franco, A.; Leekitcharoenphon, P.; Feltrin, F.; Alba, P.; Cordaro, G.; Iurescia, M.; Tolli, R.; D’Incau, M.; Staffolani, M.; Di Giannatale, E.; et al. Emergence of a Clonal Lineage of Multidrug-Resistant ESBL-Producing Salmonella Infantis Transmitted from Broilers and Broiler Meat to Humans in Italy between 2011 and 2014. PLoS ONE 2015, 10, e0144802. [Google Scholar] [CrossRef]
- Guo, S.; Aung, K.T.; Leekitcharoenphon, P.; Tay, M.Y.F.; Seow, K.L.G.; Zhong, Y.; Ng, L.C.; Aarestrup, F.M.; Schlundt, J. Prevalence and genomic analysis of ESBL-producing Escherichia coli in retail raw meats in Singapore. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef]
- Ng, C.; Chen, H.; Goh, S.G.; Haller, L.; Wu, Z.; Charles, F.R.; Trottet, A.; Gin, K.Y.-H. Microbial water quality and the detection of multidrug resistant E. coli and antibiotic resistance genes in aquaculture sites of Singapore. Mar. Pollut. Bull. 2018, 135, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, A.; Bazaj, A.; Cornaglia, G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: A review. J. Chemother. 2017, 29, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Johnston, B.; Clabots, C.; Kuskowski, M.A.; Castanheira, M. Escherichia coliSequence Type ST131 as the Major Cause of Serious Multidrug-ResistantE. coliInfections in the United States. Clin. Infect. Dis. 2010, 51, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Olesen, B.; Hansen, D.S.; Nilsson, F.; Frimodt-Møller, J.; Leihof, R.F.; Struve, C.; Scheutz, F.; Johnston, B.; Krogfelt, K.A.; Johnson, J.R. Prevalence and Characteristics of the Epidemic Multiresistant Escherichia coli ST131 Clonal Group among Extended-Spectrum Beta-Lactamase-Producing E. coli Isolates in Copenhagen, Denmark. J. Clin. Microbiol. 2013, 51, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.N.; Ben Zakour, N.L.; Roberts, L.W.; Wailan, A.M.; Zowawi, H.M.; Tambyah, P.A.; Lye, D.C.; Jureen, R.; Lee, T.H.; Yin, M.; et al. Whole genome analysis of cephalosporin-resistant Escherichia coli from bloodstream infections in Australia, New Zealand and Singapore: High prevalence of CMY-2 producers and ST131 carrying blaCTX-M-15 and blaCTX-M-27. J. Antimicrob. Chemother. 2017, 73, 634–642. [Google Scholar] [CrossRef]
- Blanc, V.; Leflon-Guibout, V.; Blanco, J.; Haenni, M.; Madec, J.-Y.; Rafignon, G.; Bruno, P.; Mora, A.; Lopez, C.; Dahbi, G.; et al. Prevalence of day-care centre children (France) with faecal CTX-M-producing Escherichia coli comprising O25b:H4 and O16:H5 ST131 strains. J. Antimicrob. Chemother. 2014, 69, 1231–1237. [Google Scholar] [CrossRef]
- Dhanji, H.; Patel, R.; Wall, R.; Doumith, M.; Hope, R.; Livermore, D.M.; Woodford, N. Variation in the genetic environments of blaCTX-M-15 in Escherichia coli from the faeces of travellers returning to the United Kingdom. J. Antimicrob. Chemother. 2011, 66, 1005–1012. [Google Scholar] [CrossRef]
- Jarvis, K.G.; Grim, C.J.; Franco, A.A.; Gopinath, G.; Sathyamoorthy, V.; Hu, L.; Sadowski, J.A.; Lee, C.S.; Tall, B.D. Molecular Characterization of Cronobacter Lipopolysaccharide O-Antigen Gene Clusters and Development of Serotype-Specific PCR Assays. Appl. Environ. Microbiol. 2011, 77, 4017–4026. [Google Scholar] [CrossRef]
- Khedher, M.B.; Baron, S.A.; Riziki, T.; Ruimy, R.; Diene, S.M.; Rolain, J.M. Massive analysis of 64′628 bacterial genomes to decipher a water reservoir and origin of mobile colistin resistance (mcr) gene variants: Is there another role for this family of enzymes? BioRxiv 2019, 763474. [Google Scholar] [CrossRef]
- La, M.-V.; Lee, B.; Hong, B.Z.; Yah, J.Y.; Koo, S.-H.; Jiang, B.; Ng, L.S.; Tan, T.-Y. Prevalence and antibiotic susceptibility of colistin-resistance gene (mcr-1) positive Enterobacteriaceae in stool specimens of patients attending a tertiary care hospital in Singapore. Int. J. Infect. Dis. 2019, 85, 124–126. [Google Scholar] [CrossRef]
- Guo, S.; Tay, M.Y.F.; Aung, K.T.; Seow, K.L.G.; Zhong, Y.; Ng, L.C.; Schlundt, J. Conjugative IncX1 Plasmid Harboring Colistin Resistance Gene mcr-5.1 in Escherichia coli Isolated from Chicken Rice Retailed in Singapore. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Tuo, H.; Yang, Y.; Tao, X.; Liu, D.; Li, Y.; Xie, X.; Li, P.; Gu, J.; Kong, L.; Xiang, R.; et al. The Prevalence of Colistin Resistant Strains and Antibiotic Resistance Gene Profiles in Funan River, China. Front. Microbiol. 2018, 9, 3094. [Google Scholar] [CrossRef] [PubMed]
- Zurfuh, K.; Poirel, L.; Nordmann, P.; Nüesch-Inderbinen, M.; Hächler, H.; Stephan, R. Occurrence of the plasmid-borne mcr-1 colistin resistance gene in extended-spectrum-β-lactamase-producing Enterobacteriaceae in river water and imported vegetable samples in Switzerland. Antimicrob. Agents Chemother. 2016, 60, 2594–2595. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Hurley, D.; Li, J.; Meng, Q.; Wang, J.; Fanning, S.; Xiong, Y. Characterisation of multidrug-resistant Shiga toxin-producing Escherichia coli cultured from pigs in China: Co-occurrence of extended-spectrum β-lactamase- and mcr-1-encoding genes on plasmids. Int. J. Antimicrob. Agents 2016, 48, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, X.P.; Yang, R.S.; Fang, L.X.; Huo, W.; Li, S.M.; Jiang, P.; Liao, X.P.; Liu, Y.H. Complete nucleotide sequence of an IncI2 plasmid coharboring blaCTX-M-55 and mcr-1. Antimicrob. Agents Chemother. 2016, 60, 5014–5017. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).