Gestational Diabetes Mellitus in Southeast Asia: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection and Data Extraction
- articles published in scientific journals between 1 January 2010 to 25 May 2020.
- articles published in English due to the limitation of resources for translation.
- used randomized-controlled trial, observational, cross-sectional, retrospective, or prospective study designs.
- outcome measures included prevalence, screening methods, risk factors, complications, or management of disease related to GDM.
- pharmacological or non-pharmacological interventions
3. Results
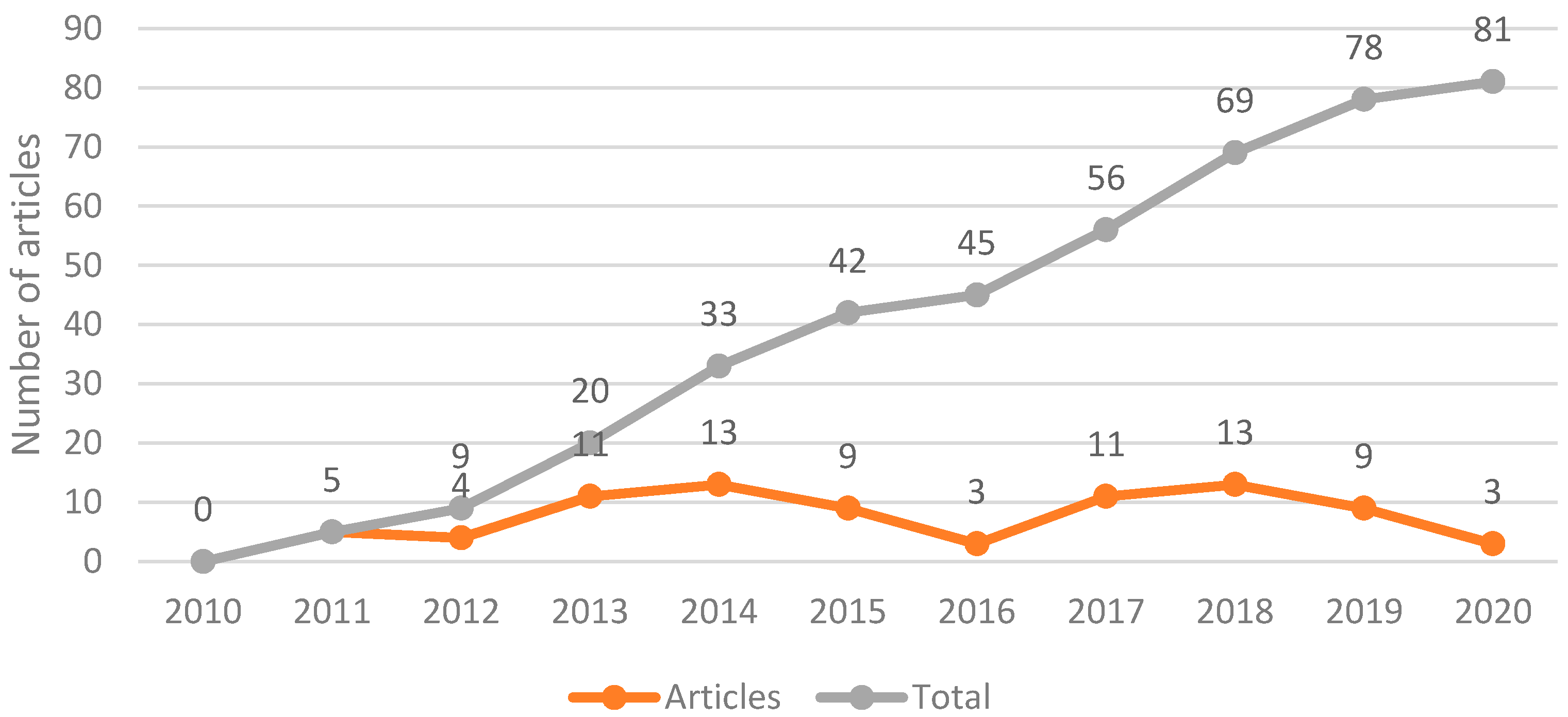
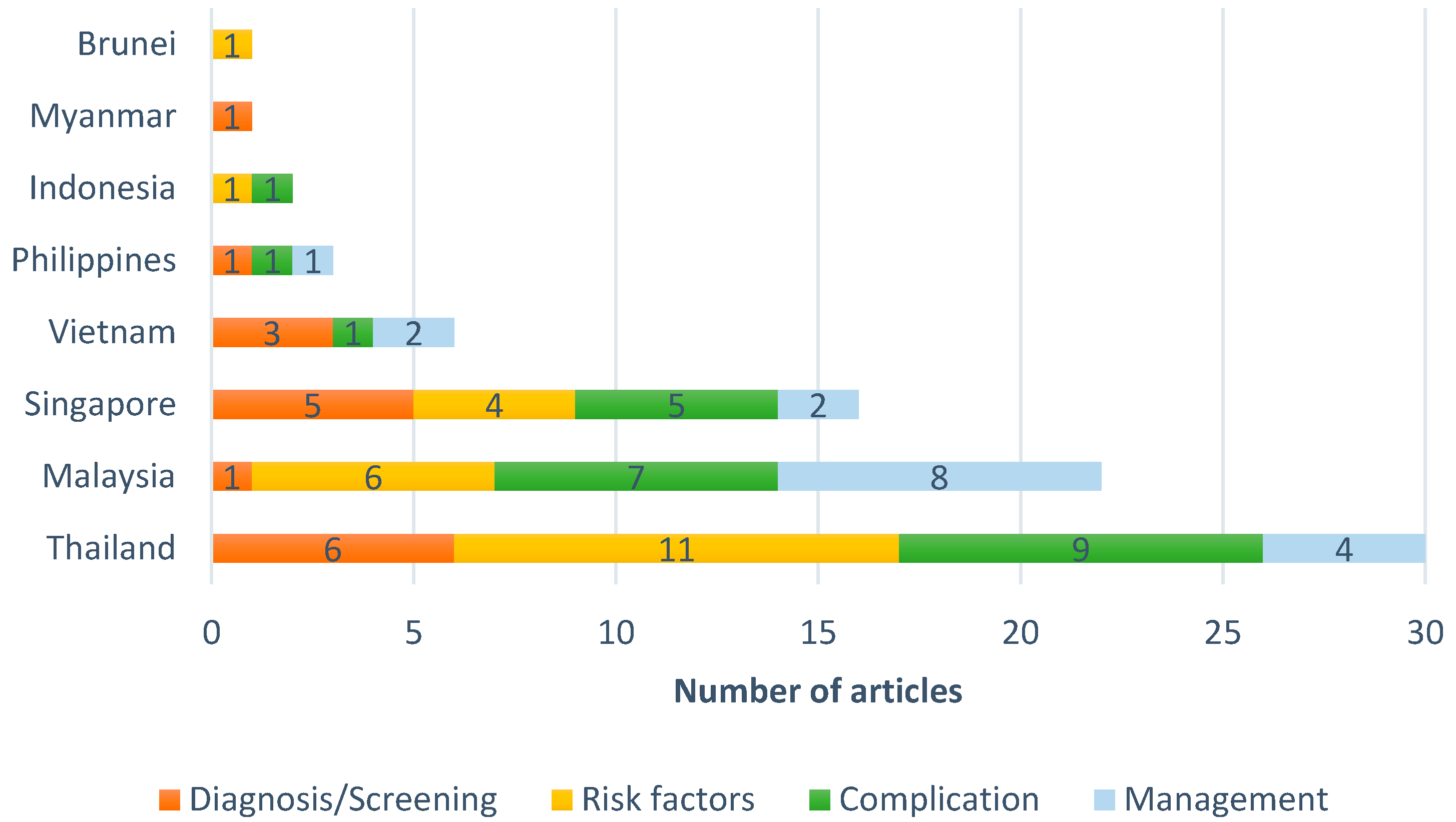
3.1. Study Selection and Characteristics
3.2. Research Domains
3.2.1. GDM Screening and Diagnosis
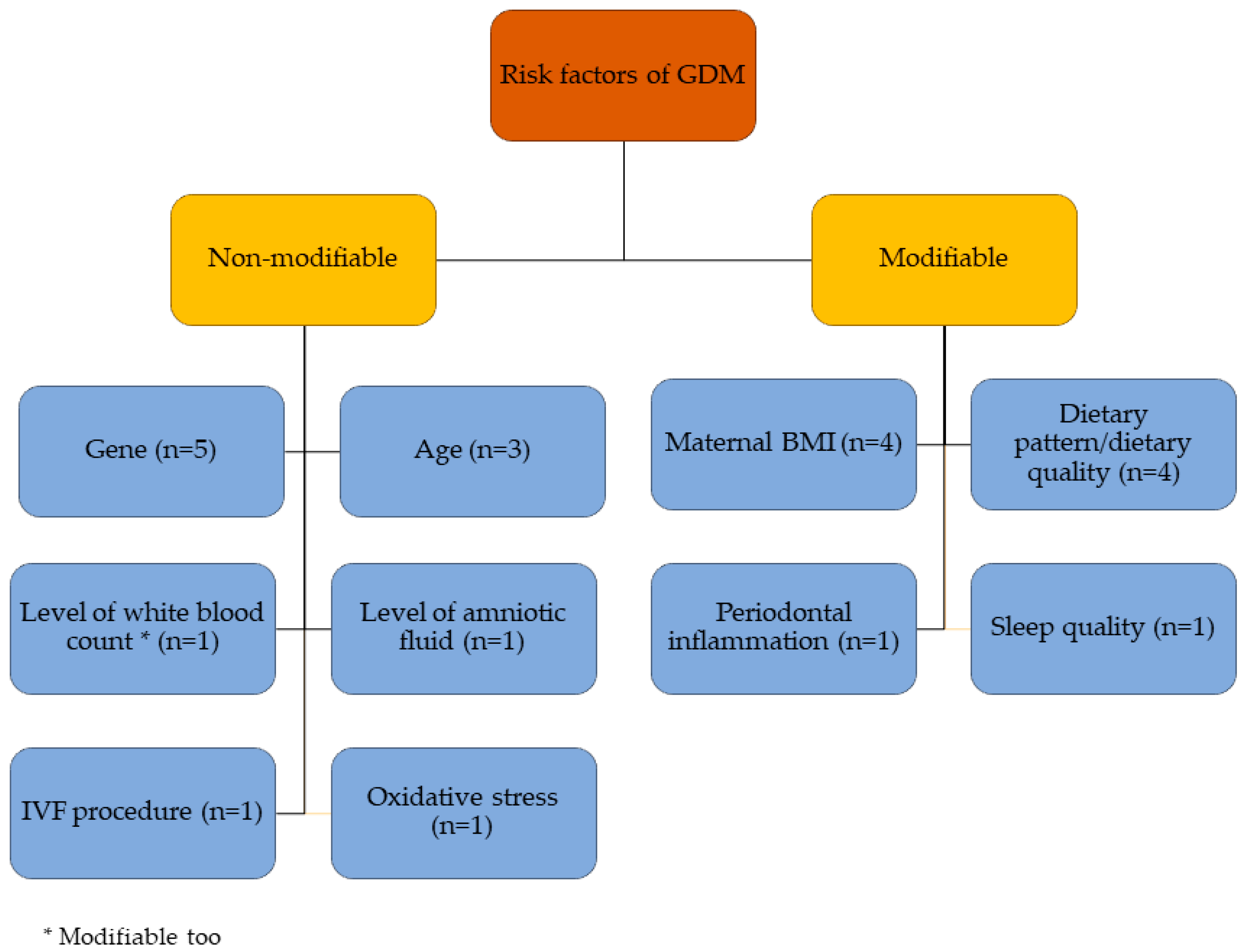
3.2.2. Risk Factors
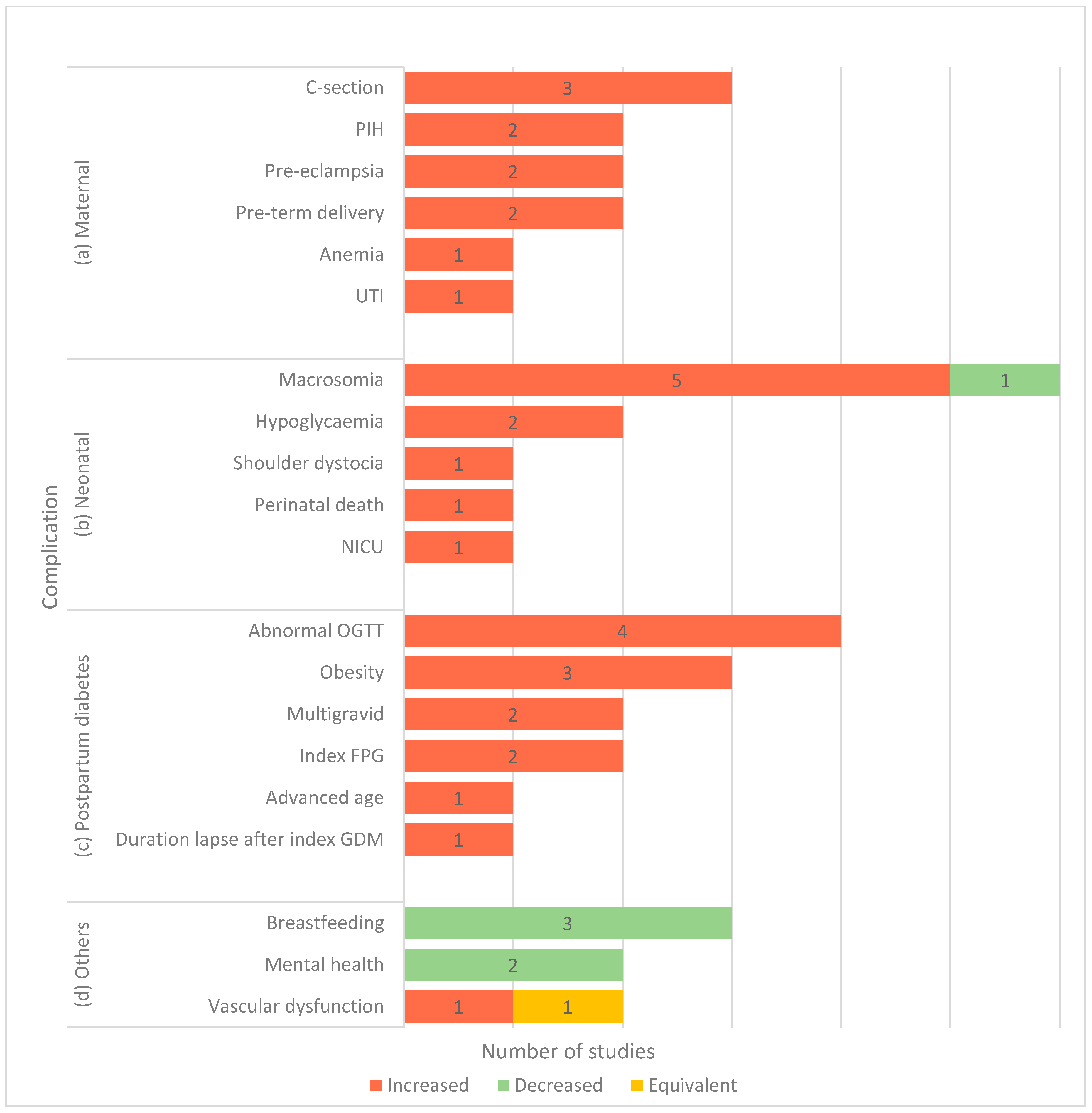
3.2.3. GDM-Related Complications
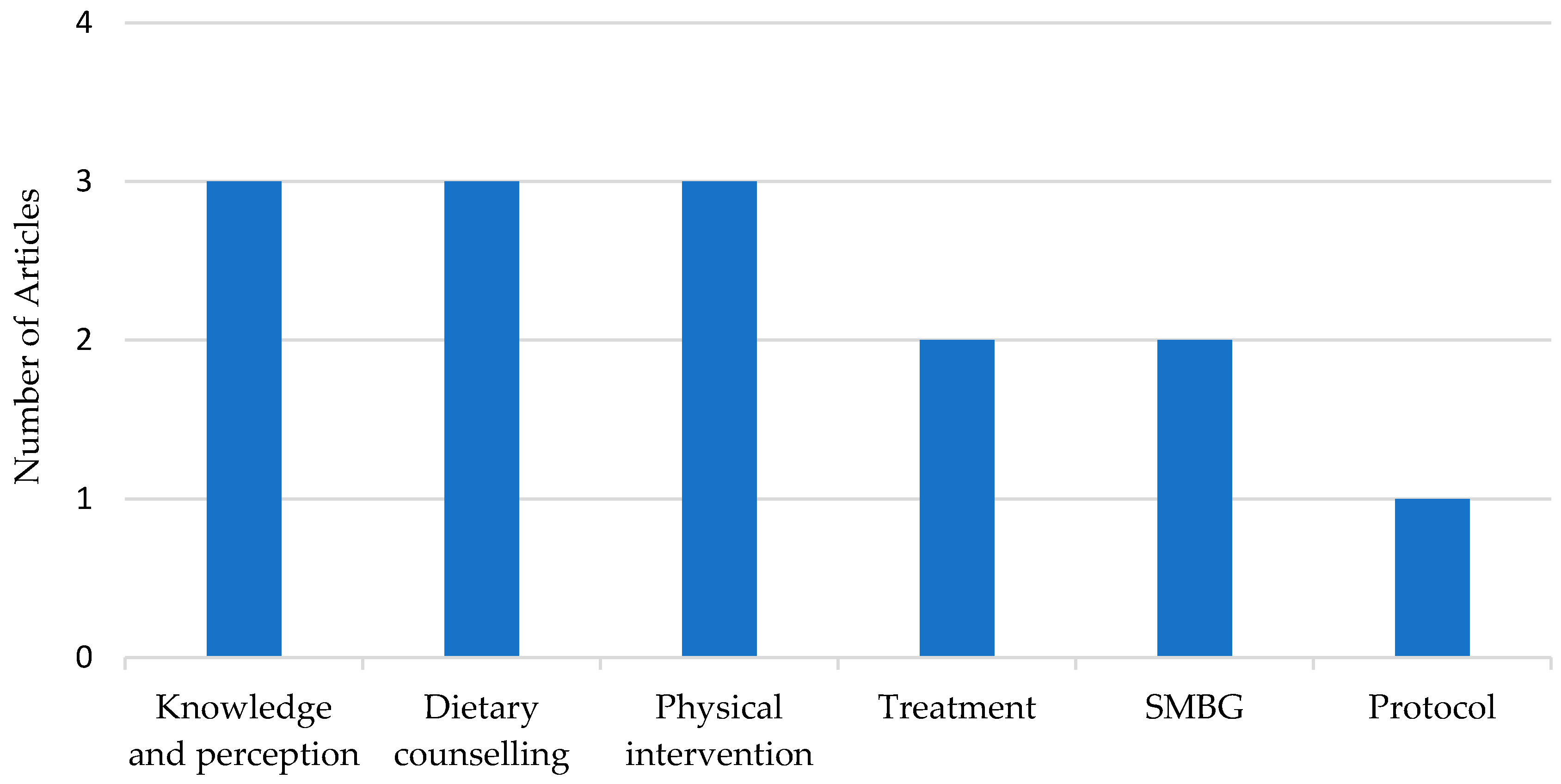
3.2.4. Management
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019. [Google Scholar]
- Tan, J.W.; Koh, S.C.D.; Tan, K.H. Challenges of implementation of universal screening for gestational diabetes mellitus in a Singapore tertiary hospital. J. Matern. Fetal Neonatal Med. 2016, 29, 95. [Google Scholar]
- Metzger, B.E.; Coustan, D.R. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998, 21, B161–B167. [Google Scholar]
- Tutino, G.E.; Tam, W.H.; Yang, X.; Chan, J.C.; Lao, T.T.; Ma, R.C. Diabetes and pregnancy: Perspectives from Asia. Diabet. Med. 2014, 31, 302–318. [Google Scholar]
- Economic UNDOs ASUNDY, February 2017. Available online: https://unstats.un.org/unsd/demographic/products/dyb/dyb2015/Table01.pdf (accessed on 30 January 2021).
- Jiwani, A.; Marseille, E.; Lohse, N.; Damm, P.; Hod, M.; Kahn, J.G. Gestational diabetes mellitus: Results from a survey of country prevalence and practices. J. Matern. Fetal Neonatal Med. 2012, 25, 600–610. [Google Scholar]
- Pullinger, C.R.; Goldfine, I.D.; Tanyolaç, S.; Movsesyan, I.; Faynboym, M.; Durlach, V.; Chiefari, E.; Foti, D.P.; Frost, P.H.; Malloy, M.J.; et al. Evidence that an HMGA1 gene variant associates with type 2 diabetes, body mass index, and high-density lipoprotein cholesterol in a Hispanic-American population. Metab. Syndr. Relat. Disord. 2014, 12, 25–30. [Google Scholar]
- Yan, J.; Su, R.; Ao, D.; Wang, Y.; Wang, H.; Yang, H. Genetic variants and clinical relevance associated with gestational diabetes mellitus in Chinese women: A case-control study. J. Matern. Fetal Neonatal Med. 2018, 31, 2115–2121. [Google Scholar]
- Yuen, L.; Wong, V.W. Gestational diabetes mellitus: Challenges for different ethnic groups. World J. Diabetes 2015, 6, 1024–1032. [Google Scholar]
- Chong, Y.S.; Cai, S.; Lin, H.; Soh, S.E.; Lee, Y.S.; Leow, M.K.; Chan, Y.H.; Chen, L.; Holbrook, J.D.; Tan, K.H.; et al. Ethnic differences translate to inadequacy of high-risk screening for gestational diabetes mellitus in an Asian population: A cohort study. BMC Pregnancy Childbirth 2014, 14, 345. [Google Scholar]
- Ferrara, A.; Kahn, H.S.; Quesenberry, C.P.; Riley, C.; Hedderson, M.M. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet. Gynecol. 2004, 103, 526–533. [Google Scholar]
- Hunsberger, M.; Rosenberg, K.D.; Donatelle, R.J. Racial/ethnic disparities in gestational diabetes mellitus: Findings from a population-based survey. Womens Health Issues 2010, 20, 323–328. [Google Scholar]
- Kieffer, E.C.; Martin, J.A.; Herman, W.H. Impact of maternal nativity on the prevalence of diabetes during pregnancy among U.S. ethnic groups. Diabetes Care 1999, 22, 729–735. [Google Scholar]
- Peters, M.D.J.; Godfrey, M.C.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Methodology for JBI scoping reviews. In The Joanna Briggs Institute Reviewers Manual; Joanna Briggs Institute: Adelaide, Australia, 2015; 24p. [Google Scholar]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar]
- Benjamin, F.; Wilson, S.J.; Deutsch, S.; Seltzer, V.L.; Droesch, K.; Droesch, J. Effect of advancing pregnancy on the glucose tolerance test and on the 50-g oral glucose load screening test for gestational diabetes. Obstet. Gynecol. 1986, 68, 362–365. [Google Scholar]
- Arora, D.; Arora, R.; Sangthong, S.; Leelaporn, W.; Sangratanathongchai, J. Universal screening of gestational diabetes mellitus: Prevalence and diagnostic value of clinical risk factors. J. Med. Assoc. Thail. 2013, 96, 266–271. [Google Scholar]
- Gilder, M.E.; Zin, T.W.; Wai, N.S.; Ner, M.; Say, P.S.; Htoo, M.; Say, S.; Htay, W.W.; Simpson, J.A.; Pukrittayakamee, S.; et al. Gestational diabetes mellitus prevalence in Maela refugee camp on the Thai-Myanmar border: A clinical report. Glob. Health Action 2014, 7, 23887. [Google Scholar]
- Muniswaran, G.; Soelar, S.A.; Karalasingam, S.D.; Bujang, M.A.; Jeganathan, R.; Suharjono, H. Effectiveness of selective risk based screening for Gestational Diabetes (GDM) in Malaysia: A retrospective cohort study based on the National Obstetric Registry (NOR) of Malaysia. Med. J. Malays. 2017, 72, 46–49. [Google Scholar]
- Tran, T.S.; Hirst, J.E.; Do, M.A.T.; Morris, J.M.; Jeffery, H.E. Early prediction of gestational diabetes mellitus in Vietnam: Clinical impact of currently recommended diagnostic criteria. Diabetes Care 2013, 36, 618–624. [Google Scholar]
- Chen, P.Y.; Finkelstein, E.A.; Kruger, E.; Ng, M.J.; Tan, K.H. Cost-effectiveness analysis on gestational diabetes mellitus screening strategies. J. Matern. Fetal Neonatal Med. 2014, 27, 69–70. [Google Scholar]
- Poo, Z.X.; Wright, A.; Ruochen, D.; Singh, R. Optimal first trimester HbA1c threshold to identify Singaporean women at risk of gestational diabetes mellitus and adverse pregnancy outcomes: A pilot study. Obstet. Med. 2019, 12, 79–84. [Google Scholar]
- Luewan, S.; Bootchaingam, P.; Tongsong, T. Comparison of the Screening Tests for Gestational Diabetes Mellitus between “one-Step” and “two-Step” Methods among Thai Pregnant Women. Obstet. Gynecol. Int. 2018, 2018, 1521794. [Google Scholar] [CrossRef]
- De Luna, K.S.; Cunanan, E.C. Diagnosis of gestational diabetes mellitus using the international association of the diabetes and pregnancy study groups criteria and adverse pregnancy outcomes among a cohort of Filipino women: An association analysis. Phillippine J. Intern. Med. 2017, 55., 1–8. [Google Scholar]
- Nguyen, C.L.; Lee, A.H.; Pham, N.M.; Nguyen, P.T.H.; Van Ha, A.V.; Chu, T.K.; Van Duong, D.; Duong, H.T.; Binns, C.W. Prevalence and pregnancy outcomes of gestational diabetes mellitus by different international diagnostic criteria: A prospective cohort study in Vietnam. J. Matern. Fetal Neonatal Med. 2020, 33, 3706–3712. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039. [Google Scholar] [CrossRef]
- O’Sullivan, J.B.; Mahan, C.M. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964, 13, 278–285. [Google Scholar]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obs. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- Boriboonhirunsarn, D.; Lertbunnaphong, T.; Tientong, K. Diagnosis of gestational diabetes mellitus: Comparison between national diabetes data group and carpenter- coustan Criteria. Asian Biomed. 2014, 8, 505–509. [Google Scholar] [CrossRef]
- Luengmettakul, J.; Sunsaneevithayakul, P.; Talungchit, P. Pregnancy outcome in women with gestational diabetes mellitus according to the Carpenter-Coustan criteria in Thailand. J. Obstet. Gynaecol. Res. 2015, 41, 1345–1351. [Google Scholar] [CrossRef]
- Hansarikit, J.; Manotaya, S. Sensitivity and specificity of modified 100-g oral glucose tolerance tests for diagnosis of gestational diabetes mellitus. J. Med. Assoc. Thail. 2011, 94, 540–544. [Google Scholar]
- Heetchuay, T.; Punpuckdeekoon, P. Adverse outcomes of pregnancy with single abnormal value of 100-gram oral glucose tolerance test in phramongkutklao hospital. J. Med. Assoc. Thail. 2017, 100, 479–487. [Google Scholar]
- Hirst, J.E.; Tran, T.S.; Do, M.A.T.; Morris, J.M.; Jeffery, H.E. Consequences of gestational diabetes in an urban hospital in Viet Nam: A prospective cohort study. PLoS Med. Public Libr. Sci. 2012, 9, e1001272. [Google Scholar] [CrossRef]
- Chi, C.; Loy, S.L.; Chan, S.-Y.; Choong, C.; Cai, S.; Soh, S.-E.; Tan, K.H.; Yap, F.; Gluckman, P.D.; Godfrey, K.M.; et al. Impact of adopting the 2013 World Health Organization criteria for diagnosis of gestational diabetes in a multi-ethnic Asian cohort: A prospective study. BMC Pregnancy Childbirth 2018, 18, 69. [Google Scholar] [CrossRef]
- Yew, T.W.; Khoo, C.M.; Thai, A.C.; Kale, A.S.; Yong, E.L.; Tai, E.S. The prevalence of gestational diabetes mellitus among asian females is lower using the new 2013 world health organization diagnostic criteria. Endocr. Pract. 2014, 20, 1064–1069. [Google Scholar] [CrossRef]
- Ismail, N.A.M.; Aris, N.M.; Mahdy, Z.A.; Ahmad, S.; Naim, N.M.; Siraj, H.H.; Jaafar, R.; Ishak, S.; Harun, R.; Abd Jamal, A.R.; et al. Single nucleotide polymorphism for certain genes involved in gestational diabetes with risk factors and complications positive. Sains Malays. 2013, 42, 1613–1618. [Google Scholar]
- Jamalpour, S.; Zain, S.M.; Mosavat, M.; Mohamed, Z.; Omar, S.Z. A case-control study and meta-analysis confirm glucokinase regulatory gene rs780094 is a risk factor for gestational diabetes mellitus. Gene 2018, 650, 34–40. [Google Scholar] [CrossRef]
- Low, C.F.; Tohit, E.R.M.; Chong, P.P.; Idris, F. Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch. Gynecol. Obstet. 2011, 283, 1255–1260. [Google Scholar] [CrossRef]
- Adzura, S.; Muhaya, M.; Normalina, M.; Zaleha, A.M.; Ezat, W.P.S.; Tajunisah, I. Correlation of serum insulin like growth factor-1 with retinopathy in Malaysian pregnant diabetics. Int. J. Ophthalmol. 2011, 11, 573–576. [Google Scholar]
- Rueangdetnarong, H.; Sekararithi, R.; Jaiwongkam, T.; Kumfu, S.; Chattipakorn, N.; Tongsong, T.; Jatavan, P. Comparisons of the oxidative stress biomarkers levels in gestational diabetes mellitus (GDM) and non-GDM among Thai population: Cohort study. Endocr. Connect. 2018, 7, 681–687. [Google Scholar] [CrossRef]
- Khovidhunkit, W.; Pruksakorn, P.; Plengpanich, W.; Tharavanij, T. Retinol-binding protein 4 is not associated with insulin resistance in pregnancy. Metab. Clin. Exp. 2012, 61, 65–69. [Google Scholar] [CrossRef]
- Brody, S.C.; Harris, R.; Lohr, K. Screening for gestational diabetes: A summary of the evidence for the U.S. Preventive Services Task Force. Obstet. Gynecol. 2003, 101, 380–392. [Google Scholar] [CrossRef]
- Berger, H.; Crane, J.; Farine, D.; Armson, A.; De La Ronde, S.; Keenan-Lindsay, L.; LeDuc, L.; Reid, G.; Van Aerde, J. Screening for gestational diabetes mellitus. J. Obstet. Gynaecol. Can. 2002, 24, 894–912. [Google Scholar] [CrossRef]
- Scott, D.; Loveman, E.; McIntyre, L.; Waugh, N. Screening for gestational diabetes: A systematic review and economic evaluation. Health Technol. Assess. 2002, 6, 1–161. [Google Scholar] [CrossRef]
- Coustan, D.R.; Nelson, C.; Carpenter, M.W.; Carr, S.R.; Rotondo, L.; Widness, J.A. Maternal age and screening for gestational diabetes: A population-based study. Obstet. Gynecol. 1989, 73, 557–561. [Google Scholar]
- Chantrapanichkul, P.; Chawanpaiboon, S. Adverse pregnancy outcomes in cases involving extremely young maternal age. Int. J. Gynaecol. Obstet. 2013, 120, 160–164. [Google Scholar] [CrossRef]
- Traisrisilp, K.; Jaiprom, J.; Luewan, S.; Tongsong, T. Pregnancy outcomes among mothers aged 15 years or less. J. Obstet. Gynaecol. Res. 2015, 41, 1726–1731. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Pimol, K.; Sodsee, S.; Titapant, V.; Pooliam, J. Prospective cohort study of adverse pregnancy outcomes in extremely young maternal age. J. Med. Assoc. Thail. 2018, 101, 1555–1562. [Google Scholar]
- Pattanathaiyanon, P.; Phaloprakarn, C.; Tangjitgamol, S. Comparison of gestational diabetes mellitus rates in women with increased and normal white blood cell counts in early pregnancy. J. Obstet. Gynaecol. Res. 2014, 40, 976–982. [Google Scholar] [CrossRef]
- Hanprasertpong, T.; Kor-Anantakul, O.; Suwanrath, C.; Suntharasaj, T.; Pruksanusak, N.; Hanprasertpong, J.; Geater, A. Subsequent gestational diabetes mellitus prediction in advanced maternal age using amniotic fluid glucose concentration during second trimester genetic amniocentesis. J. Obstet. Gynaecol. 2016, 36, 744–747. [Google Scholar] [CrossRef]
- Htwe, O.; Coates, P.D.; Wint, Z.; Win, S.; Bidin, H.; Chong, V.H. Body mass index on outcomes of nulliparous singleton pregnancies in Brunei Darussalam. Brunei Int. Med. J. 2013, 9, 307–314. [Google Scholar]
- Lim, W.Y.; Kwek, K.; Chong, Y.S.; Lee, Y.S.; Yap, F.; Chan, Y.H.; Godfrey, K.M.; Gluckman, P.D.; Saw, S.M.; Pan, A. Maternal adiposity and blood pressure in pregnancy: Varying relations by ethnicity and gestational diabetes. J. Hypertens. 2014, 32, 857–864. [Google Scholar] [CrossRef]
- Kongubol, A.; Phupong, V. Prepregnancy obesity and the risk of gestational diabetes mellitus. BMC Pregnancy Childbirth 2011, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Somprasit, C.; Tanprasertkul, C.; Rattanasiri, T.; Saksiriwutth, P.; Wongkum, J.; Kovavisarach, E.; Jongfueangparinya, K.; Panichakul, P.; Wuthiwong, J. High pre-pregnancy body mass index and the risk of poor obstetrics outcomes among Asian women using BMI criteria for asians by world health organization western pacific region (WPRO): A large cohort study. J. Med. Assoc. Thail. 2015, 98, S101–S107. [Google Scholar]
- Cai, S.; Natarajan, P.; Chan, J.; Wong, P.; Tan, K.H.; Godfrey, K.; Gluckman, P.D.; Shek, L.; Yap, F.; Kramer, M.; et al. Maternal hyperglycemia in singleton pregnancies conceived by IVF may be modified by first-trimester BMI. Hum. Reprod. 2017, 32, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.; Shariff, Z.M.; Yusof, B.-N.M.; Rejali, Z.; Appannah, G.; Bindels, J.; Tee, Y.Y.S.; Van Der Beek, E.M. The association between dietary patterns before and in early pregnancy and the risk of gestational diabetes mellitus (GDM): Data from the Malaysian SECOST cohort. PLoS ONE 2020, 15, e0227246. [Google Scholar] [CrossRef] [PubMed]
- de Seymour, J.; Chia, A.; Colega, M.; Jones, B.; McKenzie, E.; Shirong, C.; Godfrey, K.; Kwek, K.; Saw, S.M.; Conlon, C.; et al. Maternal dietary patterns and gestational diabetes mellitus in a multi-ethnic Asian cohort: The GUSTO study. Nutrition 2016, 8, 574. [Google Scholar] [CrossRef]
- Pang, W.W.; Colega, M.; Cai, S.; Chan, Y.H.; Padmapriya, N.; Chen, L.W.; Soh, S.E.; Han, W.M.; Tan, K.H.; Lee, Y.S.; et al. Higher Maternal Dietary Protein Intake Is Associated with a Higher Risk of Gestational Diabetes Mellitus in a Multi-ethnic Asian Cohort. J. Nutr. 2017, 147, 653–660. [Google Scholar] [CrossRef]
- Amiruddin, R.; Asrianti, T.; Abdullah, M.T. Fiber, coffee, cigarette and gestational diabetes mellitus in Makassar Indonesia. Asian J. Epidemiol. 2017, 10, 26–31. [Google Scholar] [CrossRef][Green Version]
- Lai, J.S.; Pang, W.W.; Cai, S.; Lee, Y.S.; Chan, J.K.; Shek, L.P.; Yap, F.K.; Tan, K.H.; Godfrey, K.M.; Van Dam, R.M.; et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin. Nutr. 2018, 37, 940–947. [Google Scholar] [CrossRef]
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition quality of food purchases varies by household income: The SHoPPER study. BMC Public Health 2019, 19, 231. [Google Scholar] [CrossRef]
- Chokwiriyachit, A.; Dasanayake, A.P.; Suwannarong, W.; Hormdee, D.; Sumanonta, G.; Prasertchareonsuk, W.; Wara-Aswapati, N.; Combellick, J.; Pitiphat, W. Periodontitis and gestational diabetes mellitus in non-smoking females. J. Periodontol. 2013, 84, 857–862. [Google Scholar] [CrossRef]
- Cai, S.; Tan, S.; Gluckman, P.D.; Godfrey, K.M.; Saw, S.-M.; Teoh, O.H.; Chong, Y.-S.; Meaney, M.J.; Kramer, M.S.; Gooley, J.J.; et al. Sleep quality and nocturnal sleep duration in pregnancy and risk of gestational diabetes mellitus. Sleep 2017, 40, zsw058. [Google Scholar] [CrossRef] [PubMed]
- Asvanarunat, E. Outcomes of gestational weight gain outside the Institute of Medicine Guidelines. J. Med. Assoc. Thail. 2014, 97, 1119–1125. [Google Scholar]
- Srichumchit, S.; Luewan, S.; Tongsong, T. Outcomes of pregnancy with gestational diabetes mellitus. Int. J. Gynaecol. Obstet. 2015, 131, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Aris, I.M.; Soh, S.E.; Tint, M.T.; Saw, S.M.; Rajadurai, V.S.; Godfrey, K.M.; Gluckman, P.D.; Yap, F.; Chong, Y.S.; Lee, Y.S. Associations of gestational glycemia and pre-pregnancy adiposity with offspring growth and adiposity in an Asian population. Am. J. Clin. Nutr. 2015, 102, 1104–1112. [Google Scholar] [CrossRef]
- Aris, I.M.; Soh, S.E.; Tint, M.T.; Liang, S.; Chinnadurai, A.; Saw, S.M.; Rajadurai, V.S.; Kwek, K.; Meaney, M.J.; Godfrey, K.M.; et al. Effect of maternal glycemia on neonatal adiposity in a multi-ethnic asian birth cohort. J. Clin. Endocrinol. Metab. 2014, 99, 240–247. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, N. Factors influencing macrosomia in pregnant women in a tertiary care hospital in Malaysia. J. Obstet. Gynaecol. Res. 2014, 40, 439–444. [Google Scholar] [CrossRef]
- Lee, K.W.; Ching, S.M.; Kee, H.F.; Ramachandran, V.; Chong, S.C.; Tusimin, M.; Nordin, N.M.; Devaraj, N.K.; Cheong, A.T.; Chia, Y. Neonatal outcomes and its association among gestational diabetes mellitus with and without depression, anxiety and stress symptoms in Malaysia: A cross-sectional study. Midwifery 2020, 81, 102586. [Google Scholar] [CrossRef]
- Sunjaya, A.P.; Sunjaya, A.F. Diabetes in pregnancy and infant mortality: Link with glycemic control. Diabetes Metab. Syndr. 2018, 12, 1031–1037. [Google Scholar] [CrossRef]
- Youngwanichsetha, S.; Phumdoung, S. Association between neonatal hypoglycaemia and prediabetes in postpartum women with a history of gestational diabetes. J. Clin. Nurs. 2014, 23, 2181–2185. [Google Scholar] [CrossRef]
- Fatin, A.A.B.; Alina, T.I. Proportion of women with history of gestational diabetes mellitus who performed an oral glucose test at six weeks postpartum in Johor Bahru with abnormal glucose tolerance. Malays. Fam. Physician 2019, 14, 2–9. [Google Scholar]
- Hanprasertpong, T.; Hanprasertpong, J. Pregnancy outcomes in Southeast Asian migrant workers at Southern Thailand. J. Obstet. Gynaecol. 2015, 35, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Aris, I.M.; Su, L.L.; Chong, Y.S.; Wong, T.Y.; Tan, K.H.; Wang, J.J. Effect of gestational diabetes and hypertensive disorders of pregnancy on postpartum cardiometabolic risk. Endocr. Connect. 2018, 7, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.G.; Seely, E.W. Brief review: Hypertension in pregnancy: A manifestation of the insulin resistance syndrome? Hypertension 2001, 37, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Malong, C.L.; Sia-Atanacio, A.Y.; Andag-Silva, A.; Cunanan, E.C. Incidence of postpartum diabetes and glucose intolerance among women with gestational diabetes mellitus seen at university of santo tomas hospital—A preliminary study. J. ASEAN Fed. Endocr. Soc. 2013, 28, 56. [Google Scholar]
- Youngwanichsetha, S.; Phumdoung, S. Factors related to prediabetes among postpartum Thai women with a history of gestational diabetes mellitus. Nurs. Health Sci. 2013, 15, 449–453. [Google Scholar] [CrossRef]
- Ruksasakul, R.; Tharavanij, T.; Sritipsukho, P. Metabolic syndrome in Thai women previously diagnosed with gestational diabetes. J. Med. Assoc. Thail. 2016, 99, S195–S202. [Google Scholar]
- Chew, W.F.; Rokiah, P.; Chan, S.P.; Chee, W.S.S.; Lee, L.F.; Chan, Y.M. Prevalence of glucose intolerance, and associated antenatal and historical risk factors among Malaysian women with a history of gestational diabetes mellitus. Singap. Med. J. 2012, 53, 814–820. [Google Scholar]
- Wanthong, S.; Lertwattanarak, R.; Sunsaneevithayakul, P.; Sriussadaporn, S.; Vannasaeng, S.; Sriwijitkamol, A. High prevalence of diabetes and abnormal glucose tolerance in Thai women with previous gestational diabetes mellitus. J. Clin. Transl. Endocrinol. 2017, 9, 21–24. [Google Scholar] [CrossRef]
- Li, L.-J.; Kramer, M.; Tapp, R.J.; Man, R.E.K.; Lek, N.; Cai, S.; Yap, F.; Gluckman, P.; Tan, K.H.; Chong, Y.S.; et al. Gestational diabetes mellitus and retinal microvasculature. BMC Ophthalmol. 2017, 17, 4. [Google Scholar] [CrossRef]
- Tengku-Fatishah, A.; Nik-Ahmad-Zuky, N.L.; Shatriah, I. Macular and retinal nerve fibre layer thickness in pregnant women with gestational diabetes mellitus. Clin. Ophthalmol. 2020, 14, 1215–1221. [Google Scholar] [CrossRef]
- Youngwanichsetha, S. Factors related to exclusive breastfeeding among postpartum Thai women with a history of gestational diabetes mellitus. J. Reprod. Infant Psychol. 2013, 31, 208–217. [Google Scholar] [CrossRef]
- Jirakittidul, P.; Panichyawat, N.; Chotrungrote, B.; Mala, A. Prevalence and associated factors of breastfeeding in women with gestational diabetes in a University Hospital in Thailand. Int. Breastfeed. J. 2019, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.H.; Binns, C.; Nguyen, C.L.; Ha, A.V.V.; Chu, T.K.; Duong, D.V.; Do, D.V.; Lee, A.H. Gestational Diabetes Mellitus Reduces Breastfeeding Duration: A Prospective Cohort Study. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2019, 14, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.S.; Kacmar, J.E.; Nothnagle, M.; Lawrence, R.A. A systematic review of the literature associating breastfeeding with type 2 diabetes and gestational diabetes. J. Am. Coll. Nutr. 2005, 24, 320–326. [Google Scholar] [CrossRef]
- De Bortoli, J.; Amir, L.H. Is onset of lactation delayed in women with diabetes in pregnancy? A systematic review. Diabet. Med. 2016, 33, 17–24. [Google Scholar] [CrossRef]
- Much, D.; Beyerlein, A.; Roßbauer, M.; Hummel, S.; Ziegler, A.G. Beneficial effects of breastfeeding in women with gestational diabetes mellitus. Mol. Metab. 2014, 3, 284–292. [Google Scholar] [CrossRef]
- Lee, K.W.; Ching, S.M.; Hoo, F.K.; Ramachandran, V.; Chong, S.C.; Tusimin, M.; Nordin, N.M. Prevalence and factors associated with depressive, anxiety and stress symptoms among women with gestational diabetes mellitus in tertiary care centres in Malaysia: A cross-sectional study. BMC Pregnancy Childbirth 2019, 19, 367. [Google Scholar] [CrossRef]
- Lee, K.W.; Ching, S.M.; Ramachandran, V.; Tusimin, M.; Nordin, N.M.; Chong, S.C.; Kee, H.F. Association Analysis of 14 Candidate Gene Polymorphism with Depression and Stress among Gestational Diabetes Mellitus. Genes 2019, 10, 988. [Google Scholar] [CrossRef]
- Hussain, Z.; Yusoff, Z.M.; Sulaiman, S.A.S. Evaluation of knowledge regarding gestational diabetes mellitus and its association with glycaemic level: A Malaysian study. Prim. Care Diabetes 2015, 9, 184–190. [Google Scholar] [CrossRef]
- Hussain, Z.; Yusoff, Z.M.; Sulaiman, S.A.S. A study exploring the association of attitude and treatment satisfaction with glycaemic level among gestational diabetes mellitus patients. Prim. Care Diabetes 2015, 9, 275–282. [Google Scholar] [CrossRef]
- Youngwanichsetha, S.; Phumdoung, S.; Ingkathawornwong, T. The effects of mindfulness eating and yoga exercise on blood sugar levels of pregnant women with gestational diabetes mellitus. Appl. Nurs. Res. 2014, 27, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Yusoff, Z.M.; Sulaiman, S.A. Gestational diabetes mellitus: Pilot study on patient’s related aspects. Arch. Pharm. Pract. 2014, 5, 84–90. [Google Scholar] [CrossRef]
- Hirst, J.E.; Tran, T.; Do, M.A.T.; Forsyth, R.; Morris, J.M.; Jeffery, H.E. Women with gestational diabetes in Vietnam: A qualitative study to determine attitudes and health behaviours. BMC Pregnancy Childbirth 2012, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Hewage, S.S.; Singh, S.R.; Chi, C.; Chan, J.K.Y.; Yew, T.W.; Han, W.M.; Yoong, J. Health care providers’ perceptions of responsibilities and resources to reduce Type 2 diabetes risk after gestational diabetes mellitus. Clin. Diabetes 2018, 36, 160–167. [Google Scholar] [CrossRef]
- Sangeetha, S.; Fatimah, A.; Rohana, A.G.; Norasyikin, A.W.; Karuthan, C.; Nik, S.S.; Mohd, Y.B.; Nor, A.K. Lowering dietary glycaemic index through nutrition education among Malaysian women with a history of gestational diabetes mellitus. Malays. J. Nutr. 2013, 19, 9–23. [Google Scholar]
- Shuhaimi, F.A.; Nor, N.M.; Buhari, S.S.; Ikram, E.H.K.; Zamri, U.S.M. Research article carbohydrate sources preferences of women with past medical history of gestational diabetes mellitus in puncak alam, malaysia. Pak. J. Nutr. 2019, 18, 1028–1035. [Google Scholar] [CrossRef]
- Farhanah, A.S.; Fatin Nasirah, M.D.; Barakatun Nisak, M.Y.; Nor Azlin, M.I.; Zalilah, M.S. Current dietetic practices in the management of gestational diabetes mellitus: A survey of Malaysian dietitians. Asian J. Clin. Nutr. 2014, 6, 67–74. [Google Scholar] [CrossRef][Green Version]
- Youngwanichsetha, S.; Phumdoung, S. Lived experience of blood glucose self-monitoring among pregnant women with gestational diabetes mellitus: A phenomenological research. J. Clin. Nurs. 2017, 26, 2915–2921. [Google Scholar] [CrossRef]
- Paramasivam, S.S.; Chinna, K.; Singh, A.K.K.; Ratnasingam, J.; Ibrahim, L.; Lim, L.; Tan, A.T.B.; Chan, S.P.; Tan, P.C.; Omar, S.Z.; et al. Continuous glucose monitoring results in lower HbA1c in Malaysian women with insulin-treated gestational diabetes: A randomized controlled trial. Diabet. Med. 2018, 35, 1118–1129. [Google Scholar] [CrossRef]
- Panpitpat, P.; Thipaporn, T.; Somprasit, C.; Tanprasertkul, C.; Suwannarurk, K. The effects of systematic management on maternal and neonatal complications in gestational diabetes subjects. J. Med. Assoc. Thail. 2015, 98, 451–456. [Google Scholar]
- Padmapriya, N.; Bernard, J.Y.; Liang, S.; Loy, S.L.; Cai, S.; Zhe, I.S.; Kwek, K.; Godfrey, K.M.; Gluckman, P.D.; Saw, S.M.; et al. Associations of physical activity and sedentary behavior during pregnancy with gestational diabetes mellitus among Asian women in Singapore. BMC Pregnancy Childbirth 2017, 17, 364. [Google Scholar] [CrossRef] [PubMed]
- Arasoo, V.J.T.; Dominic, N.A.; Ramadas, A.; Lim, K.H.; Tionga, C.W.; Liew, E.; Tang, J.L.; Jeganathan, R. Perceived barriers to exercise in women with gestational diabetes mellitus. Int. Med. J. Malays. 2018, 17, 49–58. [Google Scholar]
- Nguyen, C.L.; Pham, N.M.; Lee, A.H.; Nguyen, P.T.H.; Chu, T.K.; Ha, A.V.V.; Duong, D.V.; Duong, T.H.; Binns, C.W. Physical activity during pregnancy is associated with a lower prevalence of gestational diabetes mellitus in Vietnam. Acta Diabetol. 2018, 55, 955–962. [Google Scholar] [CrossRef] [PubMed]
- de Luna, K.S.; Gomez, M.H.S. Insulin analog use and pregnancy outcomes among women with gestational diabetes mellitus (GDM): A retrospective analysis at the university of Santo Tomas Hospital. Phillippine J. Intern. Med. 2018, 56, 62–70. [Google Scholar]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation (WHO Technical Report Series 894); World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Dans, A.; Ng, N.; Varghese, C.; Tai, E.S.; Firestone, R.; Bonita, R. The rise of chronic non-communicable diseases in southeast Asia: Time for action. Lancet 2011, 377, 680–689. [Google Scholar] [CrossRef]
- Rana, J.S.; Li, T.Y.; Manson, J.E.; Hu, F.B. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care 2007, 30, 53–58. [Google Scholar] [CrossRef][Green Version]
- Ford, E.S.; Williamson, D.F.; Liu, S. Weight change and diabetes incidence: Findings from a national cohort of US adults. Am. J. Epidemiol. 1997, 146, 214–222. [Google Scholar] [CrossRef]
- Shai, I.; Jiang, R.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Colditz, G.A.; Hu, F.B. Ethnicity, obesity, and risk of type 2 diabetes in women: A 20-year follow-up study. Diabetes Care 2006, 29, 1585–1590. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 2020, 43, S14. [Google Scholar] [CrossRef]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation; Part 1, Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Eades, C.E.; Cameron, D.M.; Evans, J.M. Prevalence of gestational diabetes mellitus in Europe: A meta-analysis. Diabetes Res. Clin. Pract. 2017, 129, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Hartling, L.; Dryden, D.M.; Guthrie, A.; Muise, M.; VanderMeer, B.; Donovan, L. Diagnostic thresholds for gestational diabetes and their impact on pregnancy outcomes: A systematic review. Diabet. Med. 2014, 31, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.M.; Damm, P.; Dyer, A.R.; Hod, M.; Kitzmiller, J.L.; Lowe, L.P.; et al. The diagnosis of gestational diabetes mellitus: New paradigms or status quo? J. Matern. Fetal Neonatal Med. 2012, 25, 2564–2569. [Google Scholar] [PubMed]
- Beka, Q.; Bowker, S.; Savu, A.; Kingston, D.; Johnson, J.A.; Kaul, P. Development of Perinatal Mental Illness in Women with Gestational Diabetes Mellitus: A Population-Based Cohort Study. Can. J. Diabetes 2018, 42, 350–355.e1. [Google Scholar] [CrossRef] [PubMed]
- Walmer, R.; Huynh, J.; Wenger, J.; Ankers, E.; Mantha, A.B.; Ecker, J.L.; Thadhani, R.; Park, E.; Bentley-Lewis, R. Mental health disorders subsequent to gestational diabetes mellitus differ by race/ethnicity. Depress. Anxiety 2015, 32, 774–782. [Google Scholar] [CrossRef] [PubMed]


| No | Query | Results |
|---|---|---|
| 1 | Laotian.mp. or Laos | 2015 |
| 2 | Philippines/or filipino.mp | 9174 |
| 3 | Malaysia/or Malaysian mp. | 15,948 |
| 4 | Thai.mp | 12,496 |
| 5 | Thailand.mp. or Thailand/ | 32,699 |
| 6 | Singapore/or Singaporean.mp | 13,121 |
| 7 | Cambodian.mp | 1376 |
| 8 | Cambodia.mp. or Cambodia | 4234 |
| 9 | Indonesia/or Indonesian.mp | 11,202 |
| 10 | Brunei/or Bruneian.mp. | 220 |
| 11 | Vietnamese.mp | 4633 |
| 12 | Vietnam/or Vietnam.mp | 16,231 |
| 13 | Burma.mp. or Myanmar | 2784 |
| 14 | Burmese.mp | 767 |
| 15 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 | 105,265 |
| 16 | Asia, Southeastern/or southeast Asian.mp | 9951 |
| 17 | Southeast Asia.mp. | 7817 |
| 18 | 16 or 17 | 15,766 |
| 19 | Gestational diabetes mellitus.mp or Diabetes, Gestational/ | 11,497 |
| 20 | 15 or 18 | 114,813 |
| 21 | 19 or 20 | 147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. Gestational Diabetes Mellitus in Southeast Asia: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 1272. https://doi.org/10.3390/ijerph18031272
Kunasegaran T, Balasubramaniam VRMT, Arasoo VJT, Palanisamy UD, Ramadas A. Gestational Diabetes Mellitus in Southeast Asia: A Scoping Review. International Journal of Environmental Research and Public Health. 2021; 18(3):1272. https://doi.org/10.3390/ijerph18031272
Chicago/Turabian StyleKunasegaran, Thubasni, Vinod R. M. T. Balasubramaniam, Valliammai Jayanthi Thirunavuk Arasoo, Uma Devi Palanisamy, and Amutha Ramadas. 2021. "Gestational Diabetes Mellitus in Southeast Asia: A Scoping Review" International Journal of Environmental Research and Public Health 18, no. 3: 1272. https://doi.org/10.3390/ijerph18031272
APA StyleKunasegaran, T., Balasubramaniam, V. R. M. T., Arasoo, V. J. T., Palanisamy, U. D., & Ramadas, A. (2021). Gestational Diabetes Mellitus in Southeast Asia: A Scoping Review. International Journal of Environmental Research and Public Health, 18(3), 1272. https://doi.org/10.3390/ijerph18031272








