Reliability of a Risk-Factor Questionnaire for Osteoporosis: A Primary Care Survey Study with Dual Energy X-ray Absorptiometry Ground Truth
Abstract
1. Introduction
2. Materials and Methods
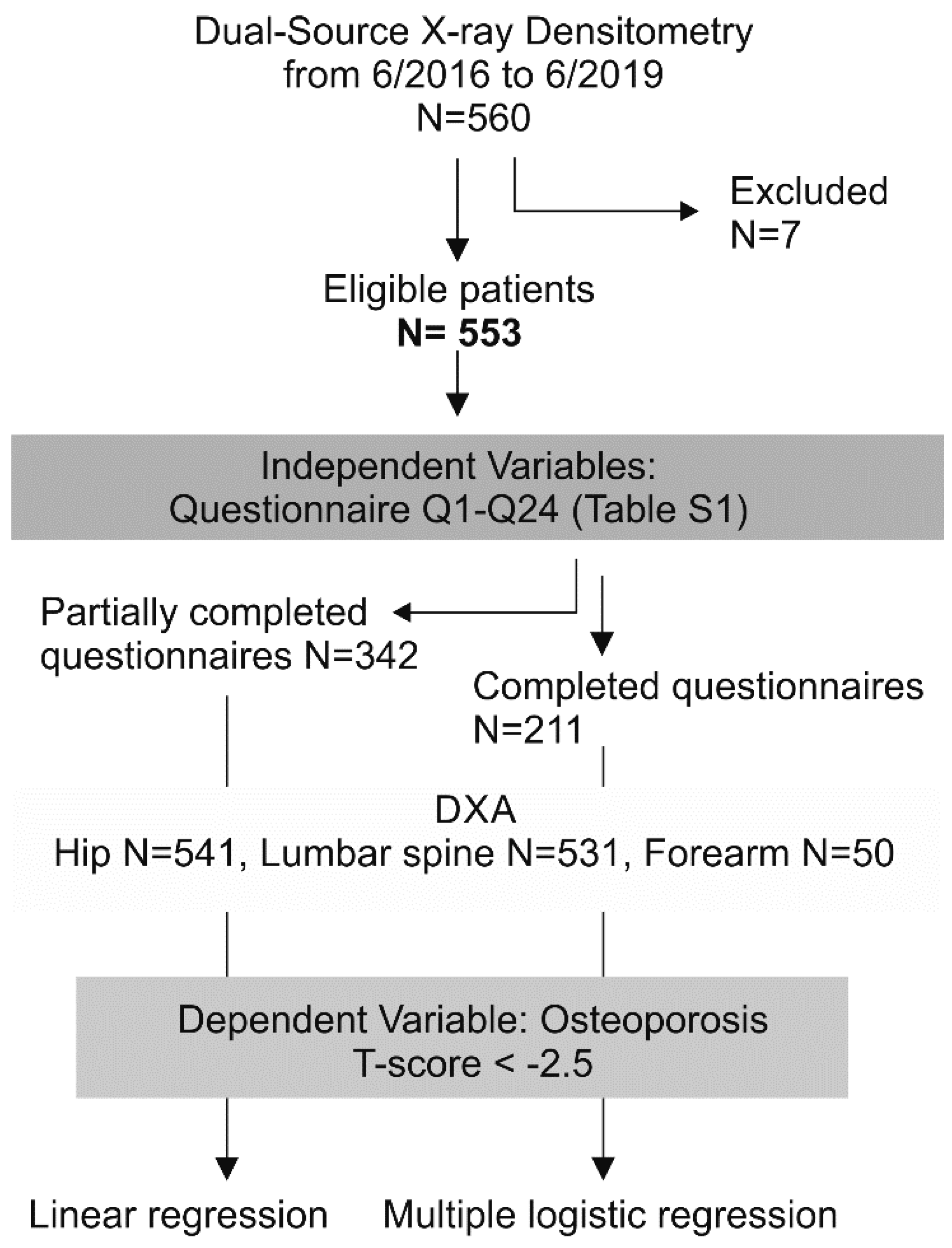
2.1. Study Design and Participant Flow
2.2. Questionnaire Design and Questionnaire Filling
2.3. Dual-Source X-ray Absorptiometry Imaging, Reference Database, and T-Score Interpretation
2.4. Ground Truth
2.5. Statistical Analysis and Handling of Missing Data
2.6. Ethical Statement
3. Results
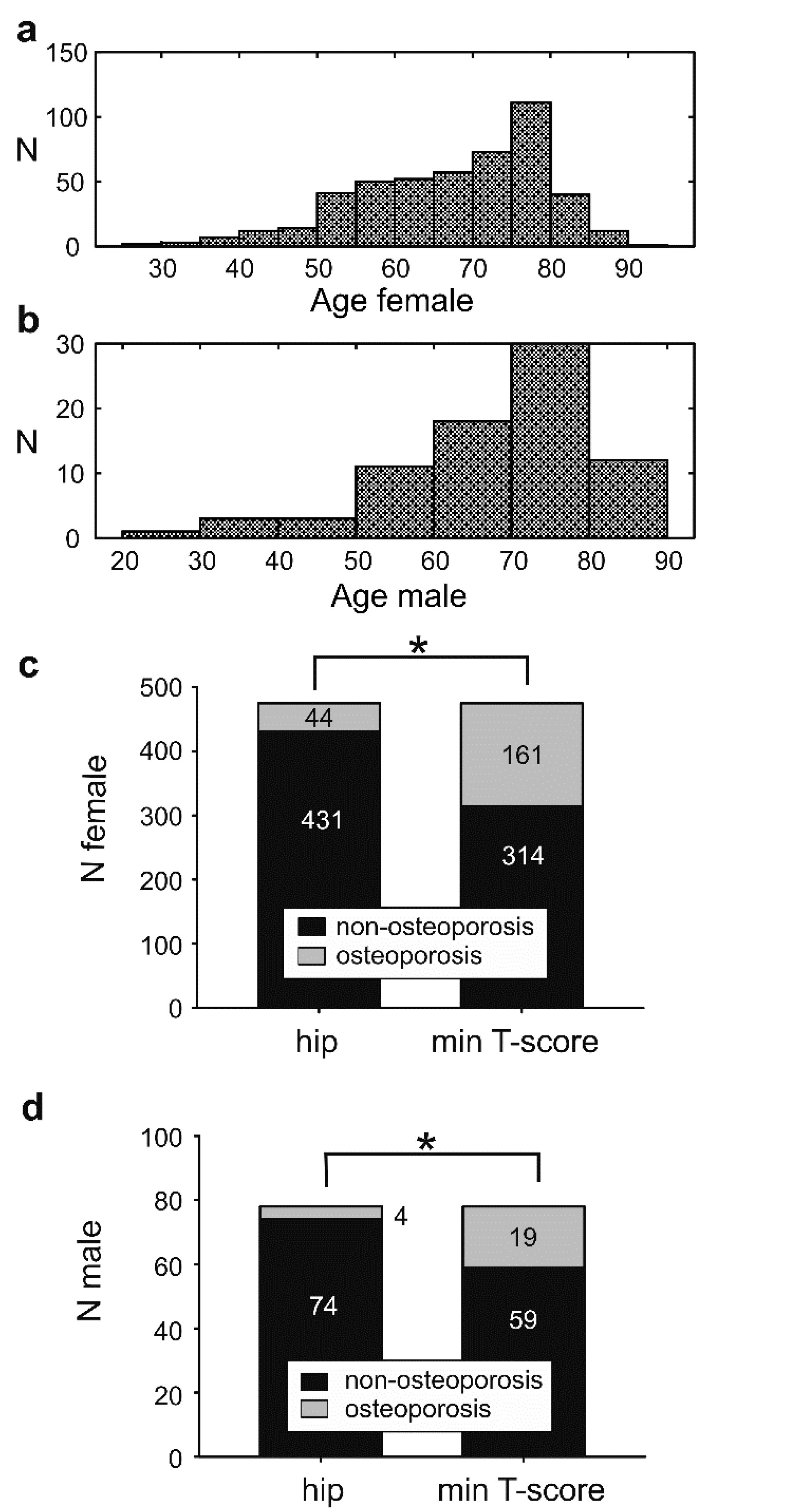
3.1. Response Rate, Dealing with Missing Data, and Profile of Non-Responders
3.2. The Osteoporosis Incidence Is Affected by the Bone Mineral Density Measuring Site
3.3. Assessment of the Patient’s Awareness Regarding Osteoporosis
3.4. Association between Risk Factor Survey and DXA as Ground Truth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Anticoag | Anticoagulation therapy |
| ACR | American College of Radiology |
| BMI | Body mass index |
| BD | Bone or connective tissue diseases |
| BMD | Bone mineral density |
| CA | Cancer |
| DXA | Dual-energy x-ray absorptiometry |
| ED | Eating disorders |
| FDA | Food and Drug Administration |
| IAEA | International Atomic Energy Agency |
| IOF | International Osteoporosis Foundation |
| ISCD | International Society for Clinical Densitometry |
| NOF | National Osteoporosis Foundation |
| n.r. | Non-responders |
| OR | Odds ratio |
| OP | Osteoporosis |
| RA | Rheumatoid arthritis |
| SexHorm | Sex hormone disorders |
| ThPTh | Hormonal disorders of the thyroid or parathyroid glands |
| RA | Rheumatoid arthritis |
| WHO | World Health Organization |
References
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- 2019 ISCD Official Positions-Adult-International Society for Clinical Densitometry (ISCD). Available online: https://iscd.org/learn/official-positions/adult-positions/ (accessed on 27 January 2021).
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Bates, D.; Black, D.M. Clinical Use of Bone Densitometry. JAMA 2002, 288, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E. Overdiagnosis of osteoporosis: Fact or fallacy? Osteoporos. Int. 2015, 26, 2051–2054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watts, N.B. Insights from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Nat. Rev. Endocrinol. 2014, 10, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Schwenkglenks, M.; Lippuner, K.; Häuselmann, H.J.; Szucs, T.D. A model of osteoporosis impact in Switzerland 2000–2020. Osteoporos. Int. 2004, 16, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Winzenberg, T.M.; Jiang, Q.; Chen, M.; Palmer, A. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos. Int. 2015, 26, 1929–1937. [Google Scholar] [CrossRef]
- Anderson, P.A.; Morgan, S.L.; Kreuger, D.; Zapalowski, C.; Tanner, B.; Jeray, K.J.; Krohn, K.D.; Lane, J.P.; Yeap, S.S.; Shuhart, C.R.; et al. Use of Bone Health Evaluation in Orthopedic Surgery: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 517–543. [Google Scholar] [CrossRef]
- Adams, J.E. Advances in bone imaging for osteoporosis. Nat. Rev. Endocrinol. 2012, 9, 28–42. [Google Scholar] [CrossRef]
- Dual Energy X ray Absorptiometry for Bone Mineral Density and Body Composition Assessment. Available online: https://www.iaea.org/publications/8459/dual-energy-x-ray-absorptiometry-for-bone-mineral-density-and-body-composition-assessment (accessed on 2 August 2020).
- Schousboe, J.T.; Shepherd, J.A.; Bilezikian, J.P.; Baim, S. Executive Summary of the 2013 International Society for Clinical Densitometry Position Development Conference on Bone Densitometry. J. Clin. Densitom. 2013, 16, 455–466. [Google Scholar] [CrossRef]
- Hans, D.; Downs, R.W.; Duboeuf, F.; Greenspan, S.; Jankowski, L.G.; Kiebzak, G.M.; Petak, S.M. Skeletal Sites for Osteoporosis Diagnosis: The 2005 ISCD Official Positions. J. Clin. Densitom. 2006, 9, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; Kanis, J.A.; Baim, S.; Bilezikian, J.P.; Binkley, N.; Cauley, J.A.; Compston, J.E.; Cooper, C.; Dawson-Hughes, B.; Fuleihan, G.E.-H.; et al. Joint Official Positions of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. J. Clin. Densitom. 2011, 14, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Brecht, J.G.; Kruse, H.P.; Möhrke, W.; Oestreich, A.; Huppertz, E. Health-Economic Comparison of Three Recom-mended Drugs for the Treatment of Osteoporosis. Int. J. Clin. Pharmacol. Res. 2004, 24, 1–10. [Google Scholar] [PubMed]
- Miller, P.D. Management of severe osteoporosis. Expert Opin. Pharmacother. 2015, 17, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef]
- McLendon, A.N.; Woodis, C.B. A Review of Osteoporosis Management in Younger Premenopausal Women. Women’s Health 2014, 10, 59–77. [Google Scholar] [CrossRef]
- Naylor, K.; Jacques, R.M.; Paggiosi, M.; Gossiel, F.; Peel, N.F.A.; McCloskey, E.V.; Walsh, J.S.; Eastell, R. Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: The TRIO study. Osteoporos. Int. 2016, 27, 21–31. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 2095–2097. [Google Scholar] [CrossRef]
- Ward, R.J.; Roberts, C.C.; Bencardino, J.T.; Arnold, E.; Baccei, S.J.; Cassidy, R.C.; Chang, E.Y.; Fox, M.G.; Greenspan, B.S.; Gyftopoulos, S.; et al. ACR Appropriateness Criteria ® Osteoporosis and Bone Mineral Density. J. Am. Coll. Radiol. 2017, 14, S189–S202. [Google Scholar] [CrossRef]
- Welcome to FRAX. Available online: https://www.sheffield.ac.uk/FRAX/index.aspx (accessed on 23 August 2020).
- Kelley, K.; Clark, B.; Brown, V.; Sitzia, J. Good practice in the conduct and reporting of survey research. Int. J. Qual. Health Care 2003, 15, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Khangura, S.; Brehaut, J.C.; Graham, I.D.; Moher, D.; Potter, B.K.; Grimshaw, J.M. Reporting Guidelines for Survey Research: An Analysis of Published Guidance and Reporting Practices. PLoS Med. 2011, 8, e1001069. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, C.; Adler, R.A.; Blake, G.M.; Caudill, J.P.; Khan, A.; Leib, E.; Maricic, M.; Prior, J.C.; Eis, S.R.; Rosen, C.; et al. Dual-Energy X-Ray Absorptiometry Technical Issues: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z.; Wein, M.N. Osteoporosis: Pathophysiology and Clinical Management, 3rd ed.; Humana: Louisville, KY, USA, 2020; ISBN 978-3-319-69286-9. [Google Scholar]
- NHANES III (1988–1994). Available online: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/Default.aspx (accessed on 2 August 2020).
- Agency, I.A.E. Dual Energy X ray Absorptiometry for Bone Mineral. Density and Body Composition Assessment: IAEA Human Health Series No. 15; International Atomic Energy Agency: Austria, Vienna, 2011; ISBN 978-92-0-110610-0. [Google Scholar]
- Lewiecki, E.M.; Binkley, N.; Morgan, S.L.; Shuhart, C.R.; Camargos, B.; Carey, J.J.; Gordon, C.M.; Jankowski, L.G.; Lee, J.-K.; Leslie, W.D. Best Practices for Dual-Energy X-ray Absorptiometry Measurement and Reporting: International Society for Clinical Densitometry Guidance. J. Clin. Densitom. 2016, 19, 127–140. [Google Scholar] [CrossRef]
- Krohn, K.; Schwartz, E.N.; Chung, Y.-S.; Lewiecki, E.M. Dual-energy X-ray Absorptiometry Monitoring with Trabecular Bone Score: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 501–505. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Schousboe, J.T.; Broy, S.B.; Engelke, K.; Leslie, W.D. Executive Summary of the 2015 ISCD Position Development Conference on Advanced Measures From DXA and QCT: Fracture Prediction Beyond BMD. J. Clin. Densitom. 2015, 18, 274–286. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Watts, N.B.; McClung, M.R.; Petak, S.M.; Bachrach, L.K.; Shepherd, J.A.; Downs, R.W. Official Positions of the International Society for Clinical Densitometry. J. Clin. Endocrinol. Metab. 2004, 89, 3651–3655. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J.; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Langer, F.W.; Codevilla, A.A.D.S.; Bringhenti, R.; Osto, L.C.D.; Campos, T.R.S.; Martins, T.T.; Barin, A.E.; Rigo, P.H.; Boufleuer, N.D.; Santinon, S.F.; et al. Low self-awareness of osteoporosis and fracture risk among postmenopausal women. Arch. Osteoporos. 2016, 11, 27. [Google Scholar] [CrossRef]
- Cram, P.; Schlechte, J.A.; Rosenthal, G.E.; Christensen, A.J. Patient Preference for Being Informed of Their DXA Scan Results. J. Clin. Densitom. 2004, 7, 275–280. [Google Scholar] [CrossRef]
- Zeeuws, D.; Cocquereaux, K.; Santermans, L. Patient’s and General Practitioner’s Perspectives Regarding Dis-turbed Eating. Psychiatr. Danub. 2019, 31, 416–417. [Google Scholar] [PubMed]
- Compston, J.E.; Watts, N.B.; Chapurlat, R.D.; Cooper, C.; Boonen, S.; Greenspan, S.L.; Pfeilschifter, J.; Silverman, S.; Díez-Pérez, A.; Lindsay, R.; et al. Obesity Is Not Protective against Fracture in Postmenopausal Women: GLOW. Am. J. Med. 2011, 124, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Silman, A.; Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002, 4, S265–S272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Saag, K. Osteoporosis Pathophysiology, Epidemiology, and Screening in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2019, 21, 34. [Google Scholar] [CrossRef]
- Sapir-Koren, R.; Livshits, G. Postmenopausal osteoporosis in rheumatoid arthritis: The estrogen deficiency-immune mechanisms link. Bone 2017, 103, 102–115. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J. Epidemiology of Thyroid Disorders. In The Thyroid and Its Diseases: A Comprehensive Guide for the Clinician; Luster, M., Duntas, L.H., Wartofsky, L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 75–85. ISBN 978-3-319-72102-6. [Google Scholar]
- Darbà, J.; Marsà, A. Epidemiology and management of parathyroid gland disorders in Spain over 15 years: A retrospective multicentre analysis. PLoS ONE 2020, 15, e0230130. [Google Scholar] [CrossRef]
- Cook, R.B.; Collins, D.; Tucker, J.; Zioupos, P. Comparison of questionnaire and quantitative ultrasound techniques as screening tools for DXA. Osteoporos. Int. 2005, 16, 1565–1575. [Google Scholar] [CrossRef]
- Subramaniam, S.; Soelaiman, I.-N.; Chin, K.-Y. Performance of Osteoporosis Self-Assessment Tool (OST) in Predicting Osteoporosis—A Review. Int. J. Environ. Res. Public Health 2018, 15, 1445. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, S.; Scherz, N.; Reich, O.; Brüngger, B.; Senn, O.; Rosemann, T.; Neuner-Jehle, S. Appropriateness of bone density measurement in Switzerland: A cross-sectional study. BMC Public Health 2018, 18, 423. [Google Scholar] [CrossRef] [PubMed]
- Rubin, K.H.; Rothmann, M.J.; Holmberg, T.; Høiberg, M.; Möller, S.; Barkmann, R.; Glüer, C.C.; Hermann, A.P.; Bech, M.; Gram, J.; et al. Effectiveness of a two-step population-based osteoporosis screening program using FRAX: The randomized Risk-stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos. Int. 2018, 29, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Rubin, K.H.; Holmberg, T.; Rothmann, M.J.; Høiberg, M.; Barkmann, R.; Gram, J.; Hermann, A.P.; Bech, M.; Rasmussen, O.W.; Glüer, C.C.; et al. The Risk-Stratified Osteoporosis Strategy Evaluation study (ROSE): A Randomized Prospective Population-Based Study. Design and Baseline Characteristics. Calcif. Tissue Int. 2015, 96, 167–179. [Google Scholar] [CrossRef]
- Matthews, H.L.; Laya, M.; DeWitt, D. Rural Women and Osteoporosis: Awareness and Educational Needs. J. Rural. Health 2006, 22, 279–283. [Google Scholar] [CrossRef]
- Osteoporosis-4th Edition. Available online: https://www.elsevier.com/books/osteoporosis/marcus/978-0-12-415853-5 (accessed on 19 January 2021).
- Johnell, O.; Kanis, J.A.; Oden, A.; Johansson, H.; De Laet, C.; Delmas, P.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; Mellstrom, D.; et al. Predictive Value of BMD for Hip and Other Fractures. J. Bone Miner. Res. 2005, 20, 1185–1194. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; De Laet, C.; Johansson, H.; Oden, A.; Delmas, P.; Eisman, J.; Fujiwara, S.; Garnero, P.; Kroger, H.; et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004, 35, 375–382. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johansson, H.; Oden, A.; Johnell, O.; De Laet, C.; Eisman, J.; McCloskey, E.; Mellstrom, D.; Melton, L.; Pols, H.; et al. A family history of fracture and fracture risk: A meta-analysis. Bone 2004, 35, 1029–1037. [Google Scholar] [CrossRef]
- Cauley, J.A.; Fuleihan, G.E.-H.; Luckey, M.M. FRAX® International Task Force of the 2010 Joint International Society for Clinical Densitometry & International Osteoporosis Foundation Position Development Conference. J. Clin. Densitom. 2011, 14, 237–239. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Compston, J.E.; Miller, P.D.; Adachi, J.D.; Adams, J.E.; Leslie, W.D.; Kanis, J.A.; Moayyeri, A.; Adler, R.A.; Hans, D.; et al. Official Positions for FRAX® Bone Mineral Density and FRAX® Simplification. J. Clin. Densitom. 2011, 14, 226–236. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Binkley, N. FRAX® Clinical Task Force of the 2010 Joint International Society for Clinical Densitometry & International Osteoporosis Foundation Position Development Conference. J. Clin. Densitom. 2011, 14, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Harvey, N.C.; McCloskey, E.V. A brief history of FRAX. Arch. Osteoporos. 2018, 13, 118. [Google Scholar] [CrossRef] [PubMed]

| n Total (Male/Female) = 553 (78/475), Age (68 ± 12/68 ± 13) y.o. | Ground Truth Hip, Index Osteoporosis | Ground Truth Min T Score, Index Osteoporosis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survey Question | Univariate Analysis, Linear Regression (Spearman) | Multiple Logistic Regression, Feed All 24 Covariates | Univariate Analysis, Linear Regression (Spearman) | Multiple Logistic Regression | ||||||||||||||
| Hosmer-Lemeshow Statistic ALL: 2.700 (p = 0.952) Hosmer-Lemeshow Statistic FEM: 0.626 (p = 1.000) | Hosmer-Lemeshow Statistic ALL: 9.345 (p = 0.314) Hosmer-Lemeshow Statistic: 3.484 (p = 0.900) | |||||||||||||||||
| n | P | R | R2 | n | Coefficient | SE | Wald Statistic, Chi-Squared | p | Odds Ratio | p | R | R2 | Coefficient | SE | Wald Statistic, Chi-Squared | p | Odds Ratio | |
| Q2 RA total | 523 | 0.5100 | 0.0288 | 0.0008 | 211 | −0.6370 | 0.8720 | 0.5340 | 0.4650 | 0.5290 | 0.2550 | 0.4990 | 0.0025 | −1.0730 | 0.5480 | 3.8370 | 0.0500 | 0.3420 |
| Q2 RA female | 450 | 0.6730 | 0.0199 | 0.0004 | 174 | −19.4040 | >1000 | 0.0000 | 0.9970 | 0.0000 | 0.5090 | 0.0312 | 0.0010 | −2.4340 | 0.9780 | 6.1920 | 0.0130 | 0.0877 |
| Q6 ED total | 545 | <0.001 | 0.1970 | 0.0390 | 5.2650 | 1.4960 | 12.3810 | <0.001 | 193.5130 | 0.1530 | 0.0163 | 0.0038 | 2.3380 | 1.0700 | 4.7760 | 0.0290 | 10.3600 | |
| Q6 ED female | 469 | <0.001 | 0.2190 | 0.0479 | 6.2800 | 2.3800 | 6.9600 | 0.0080 | 533.9250 | 0.0870 | 0.0790 | 0.0062 | 2.9230 | 1.2900 | 5.1330 | 0.0230 | 18.5980 | |
| Q12 OP total | 504 | <0.001 | 0.1620 | 0.0264 | 1.4460 | 0.7150 | 4.0870 | 0.0430 | 4.2440 | <0.001 | 0.3570 | 0.1270 | 1.9030 | 0.4190 | 20.6720 | <0.001 | 6.7060 | |
| Q12 OP female | 436 | 0.0010 | 0.1570 | 0.0246 | 1.4200 | 0.9580 | 2.1980 | 0.1380 | 4.1390 | <0.001 | 0.3550 | 0.1260 | 1.9340 | 0.5020 | 14.8590 | <0.001 | 6.9190 | |
| Q13 BD total | 467 | 0.1030 | 0.0715 | 0.0057 | 0.6060 | 0.7340 | 0.6810 | 0.4090 | 1.8330 | <0.001 | 0.2013 | 0.0454 | −0.0888 | 0.4760 | 0.0348 | 0.8520 | 0.9150 | |
| Q13 BD female | 407 | 0.0030 | 0.0735 | 0.0054 | 0.9270 | 1.0290 | 0.8120 | 0.3680 | 2.5270 | <0.001 | 0.2950 | 0.4640 | −0.2090 | 0.6130 | 0.1170 | 0.7330 | 0.8110 | |
| Q20 ThPTh total | 533 | 0.1990 | 0.0557 | 0.0031 | 1.4490 | 0.6220 | 5.4330 | 0.0200 | 4.2580 | 0.1560 | 0.0615 | 0.0038 | 0.0997 | 0.3800 | 0.0688 | 0.7930 | 1.1050 | |
| Q20 ThPTh female | 457 | 0.1470 | 0.0680 | 0.0046 | 0.7340 | 0.9110 | 0.6490 | 0.4210 | 2.0830 | 0.3490 | 0.0439 | 0.0019 | −0.1850 | 0.4660 | 0.1570 | 0.6920 | 0.8310 | |
| Q21 SexHorm total | 477 | 0.0420 | 0.0930 | 0.0085 | −3.1020 | 1.3640 | 5.1720 | 0.0230 | 0.0450 | 0.0210 | 0.1060 | 0.0112 | −1.9950 | 0.6810 | 8.5820 | 0.0030 | 0.1360 | |
| Q21 SexHorm female | 414 | 0.0400 | 0.1010 | 0.0102 | −4.3590 | 1.9880 | 4.8060 | 0.0280 | 0.0128 | 0.0150 | 0.1200 | 0.0144 | −2.1990 | 0.8360 | 6.9260 | 0.0080 | 0.1110 | |
| Q22 Anticoag total | 536 | 0.1080 | 0.0695 | 0.0048 | 1.8000 | 0.6630 | 7.3690 | 0.0070 | 6.0470 | 0.9330 | 0.0036 | 0.0000 | 0.0989 | 0.4060 | 0.0595 | 0.8070 | 1.1040 | |
| Q22 Anticoag female | 462 | 0.1350 | 0.0697 | 0.0049 | 1.2960 | 0.8970 | 2.0870 | 0.1490 | 3.6550 | 0.9280 | 0.0042 | 0.0000 | −0.2680 | 0.5180 | 0.2670 | 0.6060 | 0.7650 | |
| Q23 CA total | 536 | 0.2770 | 0.0470 | 0.0022 | 2.1240 | 0.9040 | 5.5170 | 0.0190 | 8.3610 | 0.3960 | 0.0367 | 0.0014 | 0.9810 | 0.5660 | 3.0010 | 0.0830 | 2.6670 | |
| Q23 CA female | 462 | 0.3180 | 0.0466 | 0.0022 | 3.3730 | 1.2560 | 7.2100 | 0.0070 | 29.1600 | 0.4590 | 0.0345 | 0.0012 | 1.5260 | 0.6440 | 5.6150 | 0.0180 | 4.6020 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeva, M.; Predel, D.; Winzler, S.; Teichgräber, U.; Pfeil, A.; Malich, A.; Papageorgiou, I. Reliability of a Risk-Factor Questionnaire for Osteoporosis: A Primary Care Survey Study with Dual Energy X-ray Absorptiometry Ground Truth. Int. J. Environ. Res. Public Health 2021, 18, 1136. https://doi.org/10.3390/ijerph18031136
Radeva M, Predel D, Winzler S, Teichgräber U, Pfeil A, Malich A, Papageorgiou I. Reliability of a Risk-Factor Questionnaire for Osteoporosis: A Primary Care Survey Study with Dual Energy X-ray Absorptiometry Ground Truth. International Journal of Environmental Research and Public Health. 2021; 18(3):1136. https://doi.org/10.3390/ijerph18031136
Chicago/Turabian StyleRadeva, Maria, Dorothee Predel, Sven Winzler, Ulf Teichgräber, Alexander Pfeil, Ansgar Malich, and Ismini Papageorgiou. 2021. "Reliability of a Risk-Factor Questionnaire for Osteoporosis: A Primary Care Survey Study with Dual Energy X-ray Absorptiometry Ground Truth" International Journal of Environmental Research and Public Health 18, no. 3: 1136. https://doi.org/10.3390/ijerph18031136
APA StyleRadeva, M., Predel, D., Winzler, S., Teichgräber, U., Pfeil, A., Malich, A., & Papageorgiou, I. (2021). Reliability of a Risk-Factor Questionnaire for Osteoporosis: A Primary Care Survey Study with Dual Energy X-ray Absorptiometry Ground Truth. International Journal of Environmental Research and Public Health, 18(3), 1136. https://doi.org/10.3390/ijerph18031136







