Abstract
Some studies have described that when the hemoglobin levels of chronic kidney disease (CKD) patients change, especially in those taking erythropoiesis-stimulating agents (ESA), they are associated with unfavorable outcomes such as increased morbidity and mortality, mainly due to cardiovascular events. This prospective cohort study included patients with end-stage renal disease currently undergoing hemodialysis. The initial 6-month clinical evaluation provided data of the variability in hemoglobin, associated blood parameters, and the use of erythropoietin. Subsequently, the patients were followed up for 78 months to evaluate mortality-associated factors. In total, 133 patients completed the 6-month follow-up with a mean age of 47.1 (±13.2) years. The majority were women (51.9%). Six-month hemoglobin levels were as follows: always low (18.0%), intermediate/target (1.5%), always high (0.8%), low-amplitude fluctuation/Hb low (n = 37; 27.8%), low-amplitude fluctuation/Hb high (13.53%), and high-amplitude fluctuation (38.6%), among end-stage renal disease patients. At the end of 78 months, 50 (37.6%) patients died; 70% of deaths were attributed to cardiovascular etiologies. A high variability was observed in hemoglobin levels, which was not associated with mortality. Among all the variables evaluated, age, erythropoietin dose, and transferrin saturation were associated with a higher mortality. Thus, this study suggests that greater attention to erythropoietin doses and transferrin saturation levels may improve the survival of dialysis patients.
1. Introduction
The management of anemia has become central to the treatment of patients with chronic kidney disease (CKD) under dialysis. Observational studies show that patients experience considerable changes in hemoglobin levels over time, with only 5% of them being able to maintain normal levels (Hb 11–12 g/dL) during a 6-month period [1,2,3]. Ebben et al. reported that only 10.3% of patients with stage 5 chronic kidney disease had stable hemoglobin levels during a 6-month study duration and only 6.5% of patients had their hemoglobin levels in the target range of 11–12 g/dL [4].
When the hemoglobin levels of CKD patients change, especially in those taking erythropoiesis-stimulating agents (ESA), they are associated with unfavorable outcomes such as increased morbidity and mortality, mainly due to cardiovascular events [5,6], as described in a meta-analysis published by Phrommintikul et al. who observed a significant increase in the risk of mortality when the hemoglobin level was between 12 and 16 g/dL [7].
In a prospective cohort study with 432 patients with CKD on dialysis, Foley et al. demonstrated that lowering the hemoglobin level by 1 g/dL was independently associated with the dilation of the left ventricle, and with the development or recurrence of heart failure [8]. Left ventricular hypertrophy is a survival factor for CKD and is present in the majority of patients who start on dialysis [9]. Low serum hemoglobin level is an independent predictor of left ventricular hypertrophy even in patients with mild to moderate renal dysfunction [10].
A high risk of death and/or adverse events in patients with hemoglobin levels outside the target range and in those with high-amplitude hemoglobin fluctuations was confirmed by the research of Kuragano et al. [11], who also found an association between consistently high serum ferritin levels and high-amplitude ferritin fluctuations and bad prognosis. More recently, a prospective study of 169 patients over a period of 12 months demonstrated that high hemoglobin variability is an independent risk factor for cardiovascular mortality in hemodialysis patients and might influence cardiac function [12]. Major predictors of hemoglobin variability seem to be inflammation and duration of anemia [13].
Hence, in accordance with the above associations between changes in hemoglobin levels and the risks for cardiovascular events, we aimed to evaluate the variability of hemoglobin and the factors associated with the mortality of patients on hemodialysis during a 78-month follow-up period.
2. Materials and Methods
2.1. Study Protocol
This is a prospective cohort study, which included patients with end-stage renal disease (ESRD) undergoing hemodialysis at two centers in São Luís, Maranhão, Brazil, from January 2009 to January 2016. The study was divided into two stages: the 1st stage (inclusion and follow-up for six months to assess hemoglobin variability and associated factors), and the 2nd stage (follow-up for seventy-eight months to assess patient survival and identifying mortality-related factors). In the first stage, the patients answered a standardized questionnaire with sociodemographic data and clinical history, after which they underwent monthly laboratory assessments (January to June 2009). In the 2nd stage (July 2009 to January 2016), the initial group was monitored for outcome assessment (survival × mortality), with visits to the centers every 6 months.
2.2. Patients
Patients were selected from two hemodialysis centers (an outpatient and a hospital center) at São Luís, Maranhão, Brazil, which treated 390 patients. Patients were selected from a nominal list supplied by each center, after applying the inclusion and exclusion criteria, which are presented in Table 1.

Table 1.
Inclusion and exclusion criteria.
Patients who met the inclusion criteria (n = 165) were informed about the study and those who agreed to participate signed an informed consent form. In the first 6-month follow-up, patient exclusions happened due to death, withdrawals from the study, entry into clinical studies, transfer from the treatment center, or transplantation. One hundred and thirty-three patients completed the 1st study stage and entered into the follow-up stage for 78 months.
2.3. Laboratory Assessments
In the second session of hemodialysis for the first week of each month, biological samples were collected to prevent the interference of hemodilution in the interpretation of laboratory tests. During these 6 months, high-sensitivity C-reactive protein (hsCRP), complete blood count, iron, ferritin, transferrin, and reticulocytes were measured every month. The samples were processed in the clinical research laboratory of our institution.
2.4. Definitions
The target level of hemoglobin was taken to be 10–12 g/dL. For identifying the factors influencing the change in hemoglobin levels, we used the Kidney Disease Improving Global Outcomes (KDIGO) definitions [11]. The monthly Hb values for each patient were classified as: low (<10 g/dL), intermediate (10 to 12 g/dL), or high (>12 g/dL). To evaluate the variation, patients were separated into six groups: always low; intermediate/target; always high; low-amplitude fluctuation/low Hb (BA/HbB); low-amplitude fluctuation/high Hb (BA/HbA); and high-amplitude fluctuation (AA), as described by Ebben et al. [4] (Appendix A). Transferrin saturation levels of 30–55% were considered normal. Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) were evaluated by average measures of each first register of blood pressure of the first hemodialysis session of the month during the follow up.
2.5. Statistical Analysis
In the descriptive analysis, the numerical variables were presented as mean and standard deviation (mean ± SD) or median (minimum and maximum values), and the categorical variables as frequency and percentage. The Shapiro–Wilk test was used to verify the normality of the numerical variables, and the Chi-square test was used for comparison of the proportions. The Cox regression model was used to identify mortality-associated factors. The multivariate model included the variables with p < 0.10 in the univariate analysis. Only the variables with p ≤ 0.05 remained in the final model. Data were processed using Stata 12.0 (StataCorp, College Station, TX, USA).
2.6. Ethical Considerations
This study was approved by the Research Ethics Committee of the University Hospital of the Federal University of Maranhão (CEP/HUUFMA) under protocol number 004150/08-50, in accordance with the norms for research on human beings.
3. Results
One hundred and thirty-three patients completed the initial study period and entered into the monitoring stage. The mean age of this group was 47.1 (±13.2) years, with women occupying the majority (51.9%). Hypertension was the most common etiology of ESRD patients followed by chronic glomerulonephritis. The average time of hemodialysis was 41.8 (±41.3) months. Approximately 27% of the patients had a cardiovascular diagnosis. The baseline characteristics of the sample are shown in Table 2.

Table 2.
Characteristics of the studied sample (n = 133).
There was no difference between doses of erythropoietin used between patients aged 60 years and over (22 patients, 16.5%) and patients under 60 years (111 patients, 83.5%).
During the initial 6-month follow-up, the levels of hemoglobin observed in the study cohort were: always low (n = 24; 18.0%); intermediate/target (n = 2; 1.5%); always high (n = 1; 0.8%); low-amplitude fluctuation/Hb low (n = 37; 27.8%); low-amplitude fluctuation/Hb high (n = 18; 13.53%); and high amplitude fluctuation (n = 51; 38.6%).
After 78 months of follow-up, 18 transplants were recorded and 50 (37.6%) patients died, with cardiovascular causes being responsible for 70% of deaths. Sudden death was the main cause (34.3%) of cardiovascular death (Table 3).

Table 3.
Outcomes observed over 78 months in the sample studied (n = 133).
The data of the patients maintaining hemoglobin in the target range and their association with mortality are shown in Figure 1. Despite a longer duration of maintaining target hemoglobin, the association with mortality was not statistically significant (p = 0.12). Similarly, the hemoglobin variability patterns described by Ebben et al. were not associated with overall group mortality [4], as shown in Figure 2 (p = 0.39).
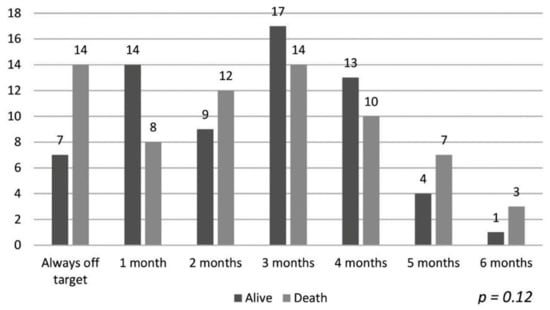
Figure 1.
Target hemoglobin time and mortality.
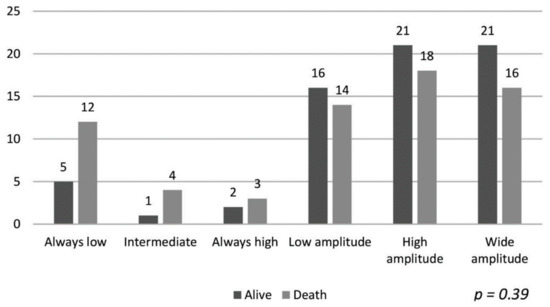
Figure 2.
Patterns of variability in hemoglobin levels (according to Ebben et al. [4]) and mortality.
Overall mortality was associated with age (HR 1.02, p = 0.01), diabetes mellitus (HR 2.01, p < 0.01), systolic blood pressure (SBP) >140 mmHg (HR 1.78, p = 0.04), hemoglobin levels above median (HR 0.57, p = 0.03), and erythropoietin dose above the median (HR 1.94, p = 0.01) in the univariate analysis (Table 4). There was no association between ferritin level and death in this study. The multivariate Cox regression model analysis showed that only age and erythropoietin dose above the median were significantly associated with overall mortality (Table 5).

Table 4.
Factors associated with mortality in the long term (78 months): univariate Cox regression model.

Table 5.
Factors associated with the overall mortality over the long term (78 months): multivariate Cox regression analysis.
Regarding cardiovascular mortality, there was a significant association with diabetes mellitus (HR 2.69, p = 0.01), SBP >140 mmHg (HR 2.88, p = 0.01), hemoglobin levels above the median (HR 0.41, p = 0.02), and transferrin saturation index <30% or >55% (HR 2.55, p = 0.01) in the univariate analysis (Table 6). In the multivariate model, only transferrin saturation index <30% or >55% maintained an association with statistical significance (HR 2.39, p = 0.04).

Table 6.
Risk factors associated with cardiovascular mortality in the long term (78 months): univariate Cox regression analysis.
4. Discussion
This study aimed to evaluate the variability of hemoglobin and the factors associated with the mortality of patients on hemodialysis and found that among all the variables evaluated, age, erythropoietin dose, and transferrin saturation were associated with a higher mortality in patients with stage 5 chronic kidney disease. Patients with ESRD have an increased risk of mortality, about 10–20 times higher than other patient populations [14], and cardiovascular complications are the main cause of death of patients under dialysis [15]. Factors such as anemia, mineral bone disorder, inflammation, systemic arterial hypertension, hypervolemia, and malnutrition, alone or together, are responsible for the high cardiovascular mortality [16]. The last census of the Brazilian Society of Nephrology (Sociedade Brasileira de Nefrologia) reported a progressive increase in the mortality of patients under dialysis, reaching 18.2% in 2016 [17], close to the values observed in the USA [18] and Europe [19], where these rates were always higher than those in Brazil. In this study, during a 78-month follow-up period, 50 patients died (mortality rate of 6.9% patients/year), lower than that found in the annual survey of the BSN, probably related to the quality of care provided to patients.
The relationship between hemoglobin levels, use of ESA, and mortality has been studied previously, especially among patients with chronic kidney disease [20,21,22]. The TREAT [23] study showed no statistical association between cardiovascular and renal outcomes with the correction of anemia using darbepoetin or placebo. The CREATE [24] and CHOIR [25] studies also showed no reduction in the mortality of ESRD patients who received an erythropoietin dose to maintain hemoglobin levels above 11–12 g/dL, which are currently recommended by the KDIGO [14]. Moreover, the CHOIR [25] study was finalized before the period laid down by the higher tendency for mortality in the group with higher hemoglobin. In contrast, a meta-analysis of nine studies with 5143 patients, performed by Phrommintikul et al. [7], indicated an increased risk of all-cause mortality among patients with high hemoglobin levels treated with EPO; the same study showed similar incidences of acute myocardial infarction in the patients. Ogawa et al. studied the relationship between responsiveness to ESA and outcome in 320 patients undergoing hemodialysis [26] and found a higher risk of mortality among patients with hemoglobin levels <10 g/dL and a dose of ESA >120 IU/kg/week. Likewise, our study showed that the use of erythropoietin at doses above the median of the group could be associated with overall mortality.
Epidemiological studies revealed an increase in the elderly population on dialysis [27]. Regardless of the presence of renal dysfunction, elderly individuals have a high risk of cardiovascular complications and mortality [28]. A recent meta-analysis of 23 studies performed by Ma et al. including 86,915 individuals undergoing hemodialysis [29] reported that age increased the all-cause mortality risk. Similarly, Myers et al. concluded that age was also associated with the higher mortality of patients on dialysis [30], but there is a strong influence from blood pressure levels, as lower systolic and diastolic blood pressure were associated with higher mortality in patients aged 50 years and above. In our study, age was associated with increased all-cause mortality, regardless of other factors. It is worth mentioning that the mean age of the Brazilian patients who initiate hemodialysis was considerably lower (41 years) when compared to, for example, European patients, who initiate hemodialysis mostly at an age of over 65 years [31]. Thereby, the younger age of our patients has probably played an important role in the good survival observed.
Although the life expectancy of women is higher than that of the general population [32], several studies show similar survival rates for both genders with respect to ESRD patients on hemodialysis [33]. The difference in life expectancy for women with ESRD on hemodialysis as compared to those without the disease is attributed to the low doses of erythropoietin administered during dialysis, which increases their chances of developing anemia along with comorbidities such as low bone mineral densities that necessitate treatment with temporary vascular access [33]. A retrospective cohort study conducted with 28,971 Canadian patients showed that women aged <45 years presented a 31% higher risk of death when compared to men of the same age [34]. The relationship between female sex and risk of mortality in patients on dialysis was also recently published by Ma et al. [29]. Comparing the sexes, the authors reported a 41% higher risk of death among women due to cardiovascular causes. These results differ from those presented in this study, which found lower mortality from all causes among women, possibly influenced by the low mean age of the group, associated with the appropriate doses of dialysis and the presence of definitive vascular access in all patients.
The association between transferrin saturation and mortality was reported previously. In a cohort study using data from the NHANES study, Mainous et al. reported that levels of transferrin saturation greater than 55% were associated with increased mortality from all causes [35]. Another study, published by Well et al. observed a combined effect of the same transferrin saturation values described above and high levels of LDL (>160 mg/dL) on mortality in a healthy North American population [36]; the authors ascertained that the increased oxidation of LDL by iron makes lipoprotein more atherogenic, which increases cardiovascular mortality. In our study, which included only CKD patients on hemodialysis, elevated (<55%) or low (<30%) transferrin saturation was associated with higher cardiovascular mortality.
The main limitation of the present study is the small number of patients from one region; however, statistical tests were used to calculate a significant sample size of the population studied.
Another limitation of this study is that it is not possible to affirm if the presence of worse clinical conditions in older patients is the real factor determining bad prognosis in this population. Weinhandl et al. [37] examined three groups of Medicare data: prevalent dialysis patients on 1 July 2006 (n = 133,246); prevalent dialysis patients on 1 July 1996 (n = 78,602); and incident patients between 1 January 2005 and 30 June 2006 (n = 24,999). Because disease severity factors both influence Hb level variability and predict death, the objective of the study was to determine whether adjustment for disease severity would decrease or eliminate the association between Hb level variability and mortality risk described in previous studies, such as those of Yang et al. [38]. The conclusion was that after adjustment for confounding disease severity, evidence supporting an association between interpatient Hb level variability and mortality is weak and inconsistent. In this study, there was no difference between the doses of erythropoietin used between patients aged 60 years and over and patients aged under 60 years. However, we cannot say that the mortality difference between the youngest and the oldest was due only to the variability of hemoglobin.
Moreover, since inflammation seems to be one of the major predictors of hemoglobin variability [11,13] and we did not analyze the influence of hemoglobin variability, iron biomarkers values, and inflammatory markers during the observational time of the study, it is not certain that patients have had no change in hemoglobin variability categories during their 6 years of follow-up. Conversely, they could have become either iron-depleted or iron-overloaded during this period or inflamed, which could confound the results.
The strength of the study was the long period of follow-up that allowed an evaluation of the outcomes of the long-term factors related to anemia. We thereby conclude that transferrin saturation (<30% or >55%) and high doses of erythropoietin are related to increased mortality in patients undergoing hemodialysis.
5. Conclusions
No association was observed between hemoglobin levels or its variations and the mortality of patients during a 78-month follow-up period. Age and erythropoietin dose above the median were associated with increased all-cause mortality and the levels of transferrin saturation displayed an association with cardiovascular mortality. Women were associated with a lower occurrence of deaths. We also ascertain that the overlap of risk factors may affect mortality among end-stage kidney disease patients on dialysis and require strict monitoring and treatment to reduce the occurrence of adverse outcomes, such as cardiovascular events and death. More studies should be developed in order to understand the mechanisms involved in these results, so that they can be modified in clinical practice.
Author Contributions
Conceptualization, R.d.C.C.S. and N.S.F.; methodology, J.S.L.; software, V.G.G.M.; validation, D.J.d.A.B., M.P.R.M., F.L.d.S. and E.C.R.d.L.C.; formal analysis, A.M.d.S.; investigation, G.A.d.S.S.; resources, N.S.F. and J.S.L.; data curation, E.J.F.S.; writing—original draft preparation, J.S.L.; writing—review and editing, N.S.F.; supervision, R.d.C.C.S.; project administration, G.E.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Research Ethics Committee of the University Hospital of the Federal University of Maranhão (CEP/HUUFMA) (protocol code 004150/08-50, approved in 11/02/2008).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Ebben et al. (2006) [4] evaluated the frequency with which hemodialysis patients maintain stable hemoglobin levels below, within, and above the Centers for Medicare and Medicaid Services target range and assessed patterns of hemoglobin level change that resulted in large fluctuations across the target range during a 6-month period. They studied 152,846 hemodialysis patients and divided them into six patient groups, which were defined on the basis of patterns of hemoglobin level fluctuation (Table 1). Patients who were classified in the low-amplitude fluctuation with low hemoglobin levels and low-amplitude fluctuation with high hemoglobin levels groups showed fluctuations in their hemoglobin levels near the lower and upper levels of the target range, such that their hemoglobin levels crossed one of the boundaries at 11 or 12.5 g/dL during the 6-month period. Patients who were classified in the high-amplitude fluctuation group showed large fluctuations in their hemoglobin levels, such that they crossed both the upper and the lower boundaries of the target range.
Ebben et al. concluded that hemoglobin levels in almost 90% of patients are in some degree of flux at any point in time, and the fluctuation is highly associated with clinical complications, as shown below in Table A1.

Table A1.
Patterns of hemoglobin level fluctuation and association with clinical complications and provider practices.
Table A1.
Patterns of hemoglobin level fluctuation and association with clinical complications and provider practices.
| Patterns of Hemoglobin Level Fluctuation | Frequency (%) | Hospital Admission (%) | Admission for Infection (%) | Average Length of Hospital Stay (d) | Average Comorbidity (n) |
|---|---|---|---|---|---|
| Consistently low (<11 g/dL) | 1.8 | 69.2 | 29.5 | 12.7 | 2.4 |
| Consistently target range (11 to 12.5 g/dL) | 6.5 | 25.3 | 6.2 | 1.9 | 1.1 |
| Consistently high (≥12.5 g/dL) | 2.0 | 29.8 | 7.4 | 2.2 | 1.2 |
| Low-amplitude fluctuation with low hemoglobin levels | 21.3 | 51.1 | 17.6 | 6.5 | 1.8 |
| Low-amplitude fluctuation with high hemoglobin levels | 28.9 | 33.5 | 9.3 | 2.8 | 1.3 |
| High-amplitude fluctuation | 39.5 | 54.0 | 17.7 | 6.4 | 1.8 |
Reproduced with permission from Ebben et al. [4], American Society of Nephrology—Clinical Journal (online); published by American Society of Nephrology, 2006.
References
- Gilbertson, D.T.; Ebben, J.P.; Foley, R.N.; Weinhandl, E.D.; Bradbury, B.D.; Collins, A.J. Hemoglobin Level Variability: Associations with Mortality. Clin. J. Am. Soc. Nephrol. 2008, 3, 133–138. [Google Scholar] [CrossRef]
- Collins, A.J.; Ebben, J.P.; Gilbertson, D.T. EPO Adjustments in Patients With Elevated Hemoglobin Levels: Provider Practice Patterns Compared With Recommended Practice Guidelines. Am. J. Kidney Dis. 2007, 49, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lacson, E., Jr.; Ofsthun, N.; Lazarus, J.M. Effect of variability in anemia management on hemoglobin outcomes in ESRD. Am. J. Kidney Dis. 2003, 41, 111. [Google Scholar] [CrossRef] [PubMed]
- Ebben, J.P.; Gilbertson, D.T.; Foley, R.N.; Collins, A.J. Hemoglobin Level Variability: Associations with Comorbidity, Intercurrent Events, and Hospitalizations. Clin. J. Am. Soc. Nephrol. 2006, 1, 1205–1210. [Google Scholar] [CrossRef]
- Fishbane, S.; Berns, J.S. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int. 2005, 68, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Besararab, A.; Bolton, W.K.; Browne, J.K.; Egrie, J.C.; Nissenson, A.R.; Okamoto, D.M.; Schwab, S.J.; Goodkin, D.A. The effects of normal as compared with low hematócritos values in patients with cardiac disease who are receiving hemodialysis and epoetin. N. Engl. Med. 1998, 339, 584–590. [Google Scholar] [CrossRef]
- Phrommintikul, A.; Haas, S.J.; Elsik, M.; Krum, H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: A meta-analysis. Lancet 2007, 369, 381–388. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barre, P.E. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am. J. Kidney Dis. 1996, 28, 53–61. [Google Scholar] [CrossRef]
- Silberberg, J.S.; Barre, P.E.; Prichard, S.S.; Sniderman, A.D. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989, 36, 286–290. [Google Scholar] [CrossRef]
- Levin, A.; Thompson, C.R.; Ethier, J.; Carlisle, E.J.; Tobe, S.; Mendelssohn, D.; Burgess, E.; Jindal, K.; Barrett, B.; Singer, J.; et al. Left ventricular mass index increase in early renal disease: Impact of decline in hemoglobin. Am. J. Kidney Dis. 1999, 34, 125–134. [Google Scholar] [CrossRef]
- Kuragano, T.; Matsumura, O.; Matsuda, A.; Hara, T.; Kiyomoto, H.; Murata, T.; Kitamura, K.; Fujimoto, S.; Hase, H.; Joki, N.; et al. Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. 2014, 86, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.-J.; Zhang, X.; Huang, L.-S.; Ji, G.; Huang, H.-D.; Xie, Y.; Jiang, G.-R.; Zhou, X.; Lu, W. Impact of hemoglobin variability on cardiovascular mortality in maintenance hemodialysis patients. Int. Urol. Nephrol. 2018, 50, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Bal, Z.; Demirci, B.G.; Karakose, S.; Tutal, E.; Uyar, M.E.; Acar, N.O.; Sezer, S. Factors Influencing Hemoglobin Variability and Its Association with Mortality in Hemodialysis Patients. Sci. World J. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Parfrey, P.S.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Teixeira, V.P.C.; Abreu, P.F.; Deus, R.B.; Kirsztajn, G.M. Progressão de doença renal e sua prevenção. In Atualização Terapêutica: Manual Prático de Diagnóstico e Tratamento, 22th ed.; Prado, F.C., Ramos, J.A., Valle, J.R., Eds.; Artes Médicas: São Paulo, Brazil, 2005; pp. 904–905. [Google Scholar]
- London, G.M. The Clinical Epidemiology of Cardiovascular Diseases in Chronic Kidney Disease: Cardiovascular Disease in Chronic Renal Failure: Pathophysiologic Aspects. Semin. Dial. 2003, 16, 85–94. [Google Scholar] [CrossRef]
- SBN. Sociedade Brasileira de Nefrologia, Censo. 2016. Available online: http://www.sbn.org.br/censos.htm (accessed on 8 December 2016).
- U.S. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United State; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2011. [Google Scholar]
- ERA-EDTA Registry: ERA-EDTA Registry Annual Report 2010; Academic Medical Center, Department of Medical Informatics: Amsterdam, The Netherlands, 2012.
- Fukuma, S.; Yamaguchi, T.; Hashimoto, S.; Nakai, S.; Iseki, K.; Tsubakihara, Y.; Fukuhara, S. Erythropoiesis-Stimulating Agent Responsiveness and Mortality in Hemodialysis Patients: Results from a Cohort Study From the Dialysis Registry in Japan. Am. J. Kidney Dis. 2012, 59, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Rosati, A.; Bigazzi, R.; Paoletti, S.; Mantuano, E.; Beati, S.; Marchetti, V.; Bernabini, G.; Grazi, G.; Rizza, G.M.; et al. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: Results from the RISCAVID study. Nephrol. Dial. Transplant. 2011, 26, 2641–2648. [Google Scholar] [CrossRef]
- Lau, J.H.; Gangji, A.S.; Rabbat, C.G.; Brimble, K.S. Impact of haemoglobin and erythropoietin dose changes on mortality: A secondary analysis of results from a randomized anaemia management trial. Nephrol. Dial. Transplant. 2010, 25, 4002–4009. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Burdmann, E.A.; Chen, C.-Y.; Cooper, M.E.; De Zeeuw, D.; Eckardt, K.-U.; Feyzi, J.M.; Ivanovich, P.; KewalRamani, R.; Levey, A.S.; et al. A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2009, 361, 2019–2032. [Google Scholar] [CrossRef]
- Drueke, T.B.; Locatelli, F.; Clyne, N.; Eckardt, K.-U.; MacDougall, I.C.; Tsakiris, D.; Burger, H.-U.; Scherhag, A. Normalization of Hemoglobin Level in Patients with Chronic Kidney Disease and Anemia. N. Engl. J. Med. 2006, 355, 2071–2084. [Google Scholar] [CrossRef]
- Singh, A.K.; Szczech, L.; Tang, K.L.; Barnhart, H.; Sapp, S.; Wolfson, M.; Reddan, D. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N. Engl. J. Med. 2006, 355, 2085–2098. [Google Scholar] [CrossRef]
- Ogawa, T.; Shimizu, H.; Kyono, A.; Sato, M.; Yamashita, T.; Otsuka, K.; Nitta, K. Relationship between responsiveness to erythropoiesis-stimulating agent and long-term outcomes in chronic hemodialysis patients: A single-center cohort study. Int. Urol. Nephrol. 2013, 46, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Foley, R.N.; Chavers, B.; Gilbertson, D.; Herzog, C.; Johansen, K.; Kasiske, B.; Kutner, N.; Liu, J.; Peter, W.S.; et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am. J. Kidney Dis. 2012, 59, e1-420. [Google Scholar]
- Nakai, S.; Iseki, K.; Itami, N.; Ogata, S.; Kazama, J.J.; Kimata, N.; Shigematsu, T.; Shinoda, T.; Shoji, T.; Suzuki, K.; et al. An Overview of Regular Dialysis Treatment in Japan (As of 31 December 2010). Ther. Apher. Dial. 2012, 16, 483–521. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Myers, O.B.; Adams, C.; Rohrscheib, M.R.; Servilla, K.S.; Miskulin, D.; Bedrick, E.J.; Zager, P.G. Age, Race, Diabetes, Blood Pressure, and Mortality among Hemodialysis Patients. J. Am. Soc. Nephrol. 2010, 21, 1970–1978. [Google Scholar] [CrossRef]
- Luxardo, R.; Kramer, A.; González-Bedat, M.C.; Massy, Z.A.; Jager, K.J.; Rosa-Diez, G.; Noordzij, M. Collaborators The epidemiology of renal replacement therapy in two different parts of the world: The Latin American Dialysis and Transplant Registry versus the European Renal Association-European Dialysis and Transplant Association Registry. Rev. Panam. Salud Pública 2018, 42, e87. [Google Scholar] [CrossRef]
- Zarulli, V.; Jones, J.A.B.; Oksuzyan, A.; Lindahl-Jacobsen, R.; Christensen, K.; Vaupel, J.W. Women live longer than men even during severe famines and epidemics. Proc. Natl. Acad. Sci. USA 2018, 115, E832–E840. [Google Scholar] [CrossRef]
- Sehgal, A.R. Impact of Quality Improvement Efforts on Race and Sex Disparities in Hemodialysis. JAMA 2003, 289, 996–1000. [Google Scholar] [CrossRef]
- Sood, M.M.; Rigatto, C.; Komenda, P.; Mojica, J.; Tangri, N. Mortality Risk for Women on Chronic Hemodialysis Differs by Age. Can. J. Kidney Heal. Dis. 2014, 3, 10. [Google Scholar] [CrossRef]
- Mainous, A.G., III; Gill, J.M.; Carek, P.J. Elevated serum transferrin saturation and mortality. Ann. Fam. Med. 2004, 2, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Wells, B.J.; Mainous, A.G., III; King, D.E.; Gill, J.M.; Carek, P.J.; Geesey, M.E. The combined effect of transferrin saturation and low-density lipoprotein on mortality. Fam. Med. 2004, 36, 324–329. [Google Scholar] [PubMed]
- Weinhandl, E.D.; Peng, Y.; Gilbertson, D.T.; Bradbury, B.D.; Collins, A.J. Hemoglobin Variability and Mortality: Confounding by Disease Severity. Am. J. Kidney Dis. 2011, 57, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Israni, R.K.; Brunelli, S.M.; Joffe, M.M.; Fishbane, S.; Feldman, H.I. Hemoglobin Variability and Mortality in ESRD. J. Am. Soc. Nephrol. 2007, 18, 3164–3170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).