Spatial Variation of Survival for Colorectal Cancer in Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Statistical Modeling
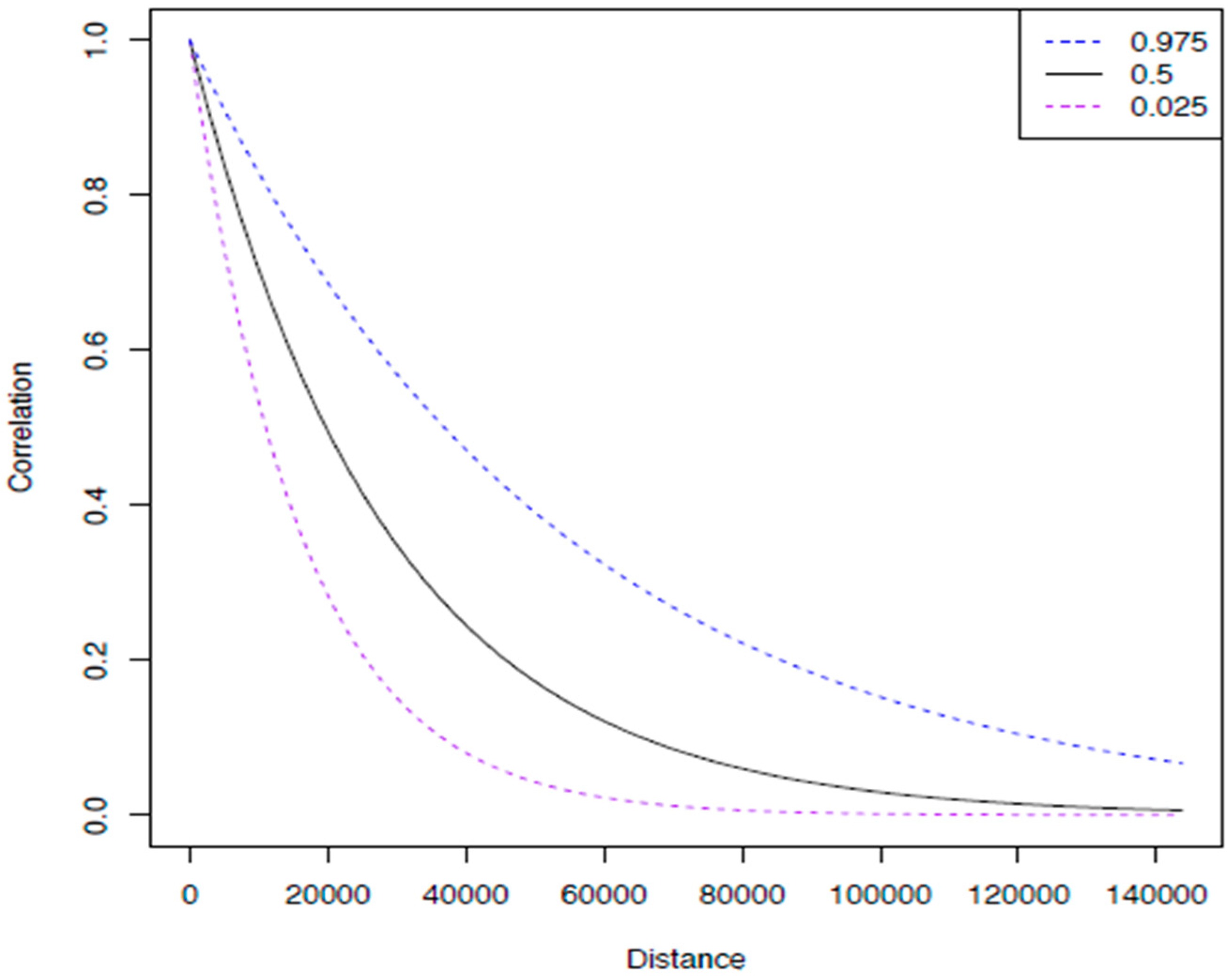
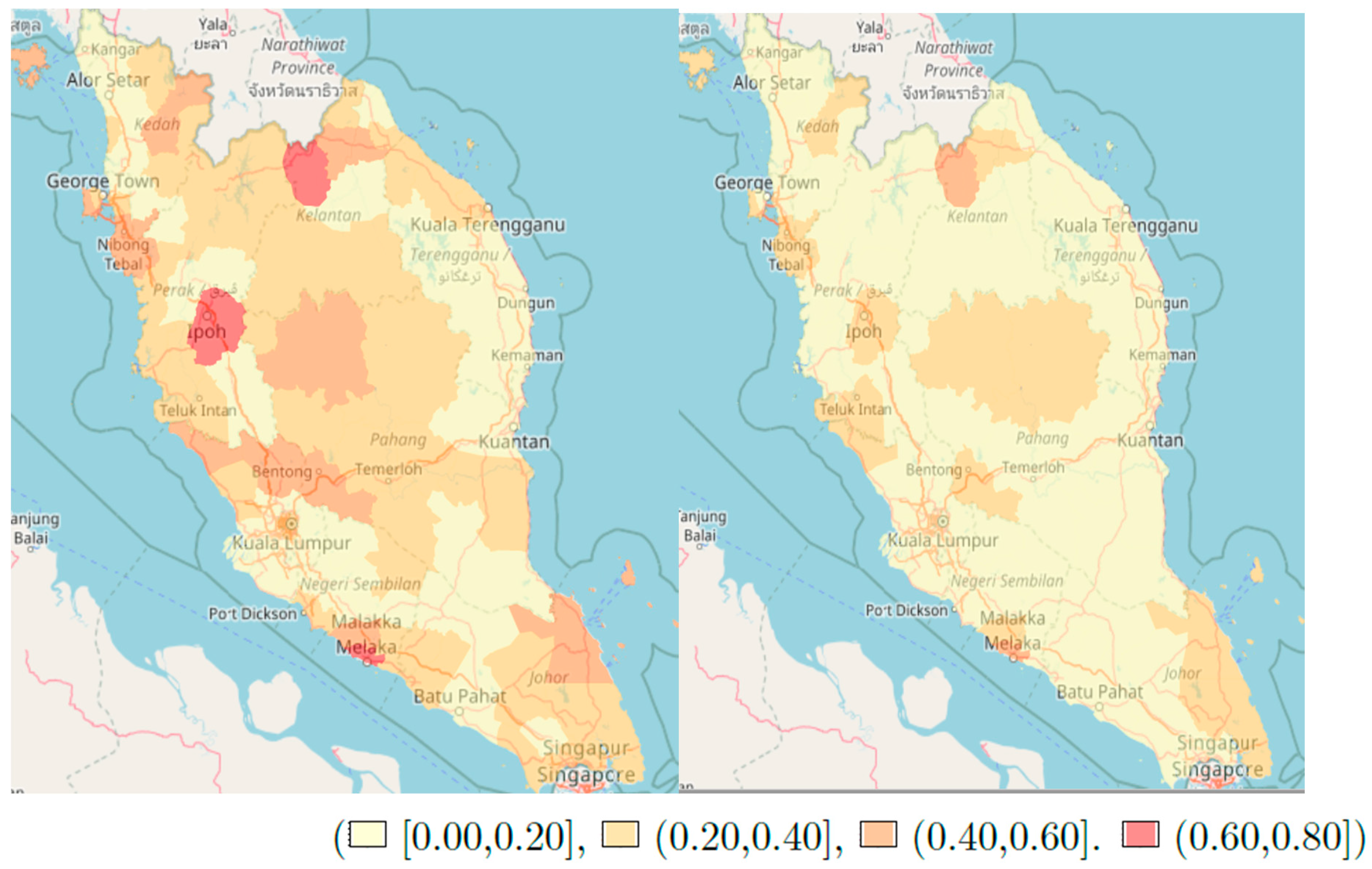
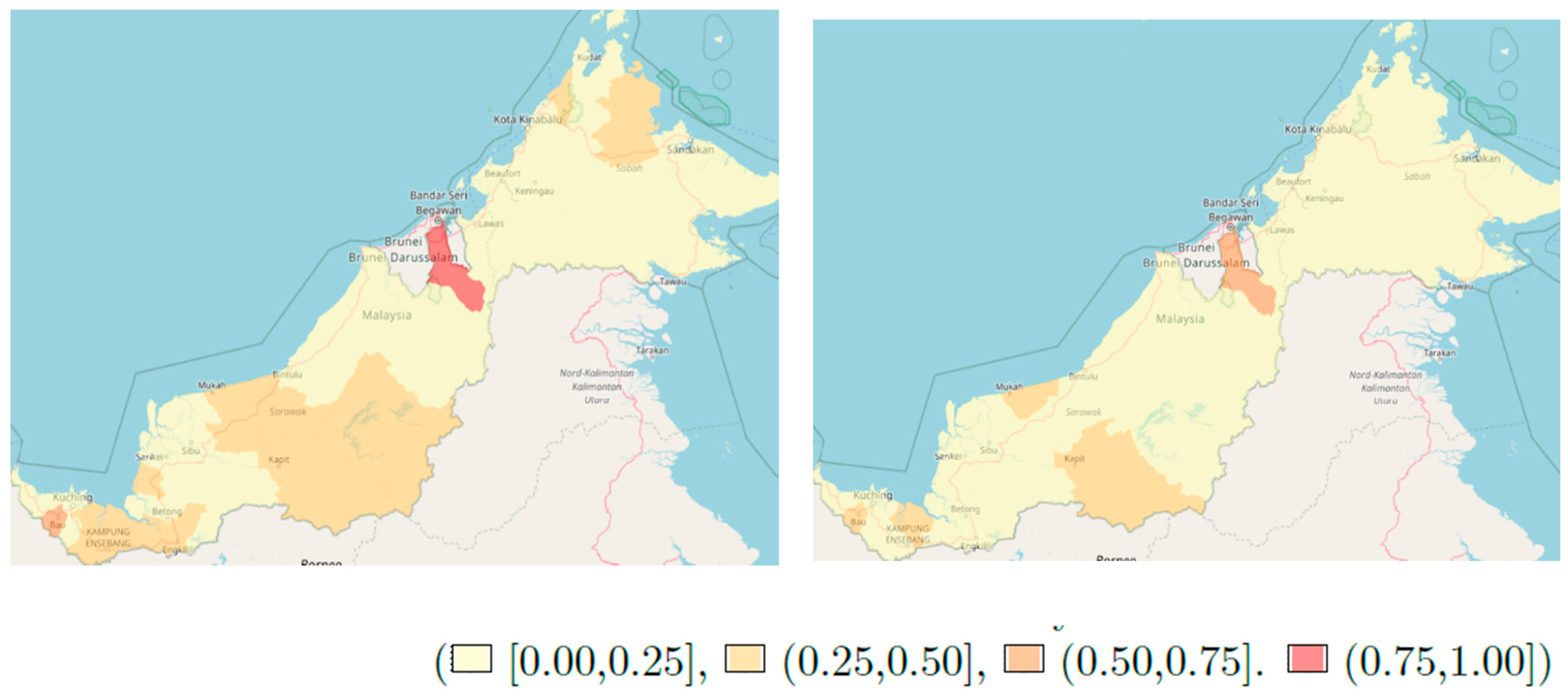
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douaiher, J.; Ravipati, A.; Grams, B.; Chowdhury, S.; Alatise, O.; Are, C. Colorectal cancer-global burden, trends, and geographical variations. J. Surg. Oncol. 2017, 115, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Cramb, S.M.; Moraga, P.; Mengersen, K.; Baade, P.D. Spatial variation in cancer incidence and survival over time across Queensland, Australia. Spat. Spatio-Temporal Epidemiol. 2017, 23, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.J.; Lawson, A.B.; Jackson, C.L.; Zhao, S. Assessment of spatial variation in breast cancer-specific mortality using Louisiana SEER data. Soc. Sci. Med. 2017, 193, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Freeman, V.L.; Naylor, K.B.; Boylan, E.E.; Booth, B.J.; Pugach, O.; Barrett, R.E.; Campbell, R.T.; McLafferty, S.L. Spatial access to primary care providers and colorectal cancer-specific survival in Cook County, Illinois. Cancer Med. 2020, 9, 3211–3223. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.; Shimakura, S.; Gorst, D. Modeling Spatial Variation in Leukemia Survival Data. J. Am. Stat. Assoc. 2002, 97, 965–972. [Google Scholar] [CrossRef]
- Fairley, L.; Forman, D.; West, R.; Manda, S.O.M. Spatial variation in prostate cancer survival in the Northern and Yorkshire region of England using Bayesian relative survival smoothing. Br. J. Cancer 2008, 99, 1786–1793. [Google Scholar] [CrossRef]
- Hsieh, J.C.-F.; Cramb, S.M.; McGree, J.; Dunn, N.A.M.; Baade, P.D.; Mengersen, K. Spatially Varying Coefficient Inequalities: Evaluating How the Impact of Patient Characteristics on Breast Cancer Survival Varies by Location. PLoS ONE 2016, 11, e0155086. [Google Scholar] [CrossRef]
- Henry, K.A.; Niu, X.; Boscoe, F.P. Geographic Disparities in Colorectal Cancer Survival. Int. J. Health Geogr. 2009, 8, 48. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2724436&tool=pmcentrez&rendertype=abstract (accessed on 3 November 2014). [CrossRef] [PubMed]
- Baade, P.D.; Dasgupta, P.; Aitken, J.F.; Turrell, G. Geographic remoteness, area-level socioeconomic disadvantage and inequalities in colorectal cancer survival in Queensland: A multilevel analysis. BMC Cancer 2013, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, J.; Ugarte, M.D.; Goicoa, T.; Militino, A.F. Age- and sex-specific spatio-temporal patterns of colorectal cancer mortality in Spain (1975–2008). Popul. Heal. Metrics 2014, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Dejardin, O.; Jones, A.; Rachet, B.; Morris, E.; Bouvier, V.; Jooste, V.; Coombes, E.; Forman, D.; Bouvier, A.; Launoy, G. The influence of geographical access to health care and material deprivation on colorectal cancer survival: Evidence from France and England. Heal. Place 2014, 30, 36–44. [Google Scholar] [CrossRef]
- Kweon, S.-S.; Kim, M.-G.; Kang, M.-R.; Shin, M.-H.; Choi, J.-S. Difference of stage at cancer diagnosis by socioeconomic status for four target cancers of the National Cancer Screening Program in Korea: Results from the Gwangju and Jeonnam cancer registries. J. Epidemiol. 2017, 27, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.; Markossian, T.; Johnson, A.; Dong, F.; Bayakly, R. Geographic Residency Status and Census Tract Socioeconomic Status as Determinants of Colorectal Cancer Outcomes. Am. J. Public Heal. 2014, 104, e63–e71. [Google Scholar] [CrossRef] [PubMed]
- Samat, N.; Shattar, A.K.A. Spatial accessibility of colorectal. World Appl. Sci. J. 2014, 31, 1772–1782. [Google Scholar]
- Samat, N.; Shattar, A.K.A.; Sulaiman, Y.; Ab Manan, A.; Weng, C.N. Investigating Geographic Distribution of Colorectal Cancer Cases: An Example from Penang State, Malaysia. Asian Soc. Sci. 2013, 9, 38–46. [Google Scholar] [CrossRef]
- Shah, S.A.; Neoh, H.-M.; Rahim, S.S.S.A.; Azhar, Z.I.; Hassan, M.R.; Safian, N.; Jamal, R. Spatial analysis of colorectal cancer cases in Kuala Lumpur. Asian Pac. J. Cancer Prev. 2014, 15, 1149–1154. [Google Scholar] [CrossRef][Green Version]
- National Cancer Patient Registry (NCPR). Available online: http://www.acrm.org.my/ncpr/ (accessed on 14 January 2021).
- Portal Rasmi Kementerian Kesihatan Malaysia. Available online: https://www.moh.gov.my/ (accessed on 14 January 2021).
- Omar, Z.A.; Ibrahim Tamin, N.S. National Cancer Registry Report Malaysia Cancer Statistics Data and Figure 2007. Malays. Natl. Cancer Regist. 2011, 85–87. [Google Scholar]
- Hassan, M.R.A.; Khazim, W.K.W.; Mustapha, N.R.N.; Said, R.M.; Tan, W.L.; Suan, M.A.M. The Second Report of the National Cancer Patient Registry-Colorectal Cancer, 2008–2013; CRC: Kedah, Malaysia, 2014. [Google Scholar]
- Rahman, N.A.; Zakaria, S. The household-based socio-economic index for every district in Peninsular Malaysia. World Acad. Sci. Eng. Technol. 2012, 6, 1389–1395. [Google Scholar]
- PGräler, B.; Pebesma, E.; Heuvelink, G. Spatio-Temporal Interpolation using gstat. RFID J. 2016, 8, 204–218. [Google Scholar]
- Taylor, B.M.; Rowlingson, B. Spatsurv: An R Package for Bayesian Inference with Spatial Survival Models. J. Stat. Softw. 2017, 77, 1–32. [Google Scholar] [CrossRef]
- Barclay, K.L.; Goh, P.-J.; Jackson, T.J. Socio-economic disadvantage and demographics as factors in stage of colorectal cancer presentation and survival. ANZ J. Surg. 2014, 85, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Manser, C.N.; Bauerfeind, P. Impact of socioeconomic status on incidence, mortality, and survival of colorectal cancer patients: A systematic review. Gastrointest. Endosc. 2014, 80, 42–60.e9. [Google Scholar] [CrossRef]
- Ministry of Housing, Communities & Local Government UK. In English Indices of Deprivation; MHCLG: London, UK, 2015.
- Canfield, M.A.; Ramadhani, T.A.; Langlois, P.H.; Waller, D.K. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Zhan, F.B.; Zou, B.; Wilson, J.G. Spatial Access to Health Care Services and Disparities in Colorectal Cancer Stage at Diagnosis in Texas. Prof. Geogr. 2013, 65, 527–541. [Google Scholar] [CrossRef]
- Xu, F.; Rimm, A.A.; Fu, P.; Krishnamurthi, S.S.; Cooper, G.S. The Impact of Delayed Chemotherapy on Its Completion and Survival Outcomes in Stage II Colon Cancer Patients. PLoS ONE 2014, 9, e107993. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, M.; Thomsen, R.W.; Bossen, K.S.; Sørensen, H.H.T.; Nørgaard, M. The impact of comorbidity on cancer survival: A review. Clin. Epidemiol. 2013, 5, 3–29. [Google Scholar] [CrossRef]

| Covariates | n | Peninsular Malaysia Median HR (95% CRI) ** | East Malaysia Median HR (95% CRI) ** |
|---|---|---|---|
| Age | 4412 | 1.01 (1.01, 1.02) * | 1.01 (1.01, 1.02) * |
| Sex | |||
| Female | 1894 | Reference | |
| Male | 2470 | 1.05 (0.93, 1.19) | 0.96 (0.76, 1.24) |
| Missing | 48 | 0.81 (0.40, 1.42) | 0.60 (0.02, 5.29) |
| Ethnicity | |||
| Non Malay | 2489 | Reference | |
| Malay | 1901 | 1.26 (1.13, 1.43) * | 1.47 (1.05, 2.01) * |
| Missing | 22 | 1.24 (0.20, 4.58) | 1.53 (0.27, 5.87) |
| Education | |||
| Nil | 399 | Reference | |
| Primary | 553 | 0.92 (0.72, 1.17) | 1.00 (0.68, 1.46) |
| Secondary | 651 | 1.00 (0.78, 1.27) | 1.01 (0.68, 1.47) |
| Tertiary | 195 | 0.63 (0.43, 0.88) * | 0.60 (0.32, 1.10) |
| Missing | 2614 | 1.03 (0.85, 1.28) | 0.82 (0.61, 1.18) |
| Smoking | |||
| Non Smoker | 1543 | Reference | |
| Former Smoker | 528 | 1.17 ( 0.97, 1.40) | 1.45 (0.99, 2.00) |
| Active smoker | 423 | 1.13 ( 0.93, 1.37) | 1.12 (0.76, 1.63) |
| Missing | 1918 | 1.03 (0.90, 1.19) | 1.09 (0.80, 1.50) |
| Staging | |||
| Stage I | 215 | Reference | |
| Stage II | 600 | 1.65 (1.09, 2.57) * | 2.04 (0.81, 6.85) |
| Stage II | 802 | 3.17 (2.13, 4.83) * | 3.63 (1.43, 10.90) * |
| Stage IV | 647 | 6.37 (4.00, 9.75) * | 7.33 (2.99, 24.00) * |
| Not staged | 620 | 2.79 (1.88, 4.46) * | 5.06 (2.03, 16.00) * |
| Missing | 1528 | 4.14 (2.82, 6.45) * | 3.91 (1.56, 12.70) * |
| ICDSite | |||
| Colon | 2394 | Reference | |
| Rectum | 631 | 1.05 (0.90, 1.23) | 1.32 (0.95, 1.84) |
| Rectosigmoid | 1379 | 1.06 (0.94, 1.19) | 1.16 (0.89, 1.49) |
| Missing | 8 | 0.29 (0.01, 1.52) | |
| TumourDiff | |||
| Well | 358 | Reference | |
| Moderate | 2497 | 1.17 ( 0.94, 1.46) | 1.77 (1.08, 3.01) * |
| Poor | 161 | 2.45 (1.81, 3.40) * | 2.99 (1.46, 5.88) * |
| Missing | 1396 | 1.59 (1.28, 1.99) * | 2.84 (1.73, 4.97) * |
| Metastasis | |||
| No | 1922 | Reference | |
| Yes | 1521 | 1.40 (1.21, 1.62) | 1.59 (1.19, 2.13) |
| Missing | 969 | 1.47 (1.21, 1.77) | 1.39 (0.93, 2.11) |
| Treatment | |||
| Surgery Alone | 1658 | Reference | |
| Surg, Chemo, radio | 1454 | 0.87 (0.76, 0.99) | 0.98 (0.75, 1.27) |
| Radio, Chemo | 437 | 1.11 (0.91, 1.35) | 1.22 (0.83, 1.81) |
| Other | 140 | 1.84 (1.41, 2.38) | 2.36 (0.99, 4.96) |
| Unknown | 723 | 1.13 (0.95, 1.33) | 1.56 (0.99, 2.35) |
| Diabetes | |||
| No | 3021 | Reference | |
| Yes | 982 | 1.12 (0.99, 1.28) | 1.08 (0.77, 1.43) |
| Missing | 409 | 0.88 (0.72, 1.06) | 0.73 (0.28, 1.56) |
| Hosp. density | 0.97 (0.81, 1.143) | 0.69 (0.03, 13.70) | |
| SE Index | 0.85 (0.53, 1.39) | ||
| Ø | 16384 (5828, 44570) | 44602 (24370, 78650) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazali, A.K.; Keegan, T.; Taylor, B.M. Spatial Variation of Survival for Colorectal Cancer in Malaysia. Int. J. Environ. Res. Public Health 2021, 18, 1052. https://doi.org/10.3390/ijerph18031052
Ghazali AK, Keegan T, Taylor BM. Spatial Variation of Survival for Colorectal Cancer in Malaysia. International Journal of Environmental Research and Public Health. 2021; 18(3):1052. https://doi.org/10.3390/ijerph18031052
Chicago/Turabian StyleGhazali, Anis Kausar, Thomas Keegan, and Benjamin M. Taylor. 2021. "Spatial Variation of Survival for Colorectal Cancer in Malaysia" International Journal of Environmental Research and Public Health 18, no. 3: 1052. https://doi.org/10.3390/ijerph18031052
APA StyleGhazali, A. K., Keegan, T., & Taylor, B. M. (2021). Spatial Variation of Survival for Colorectal Cancer in Malaysia. International Journal of Environmental Research and Public Health, 18(3), 1052. https://doi.org/10.3390/ijerph18031052




