The Growth Factors in Advanced Platelet-Rich Fibrin (A-PRF) Reduce Postoperative Complications after Mandibular Third Molar Odontectomy
Abstract
:1. Introduction
2. Materials and Methods
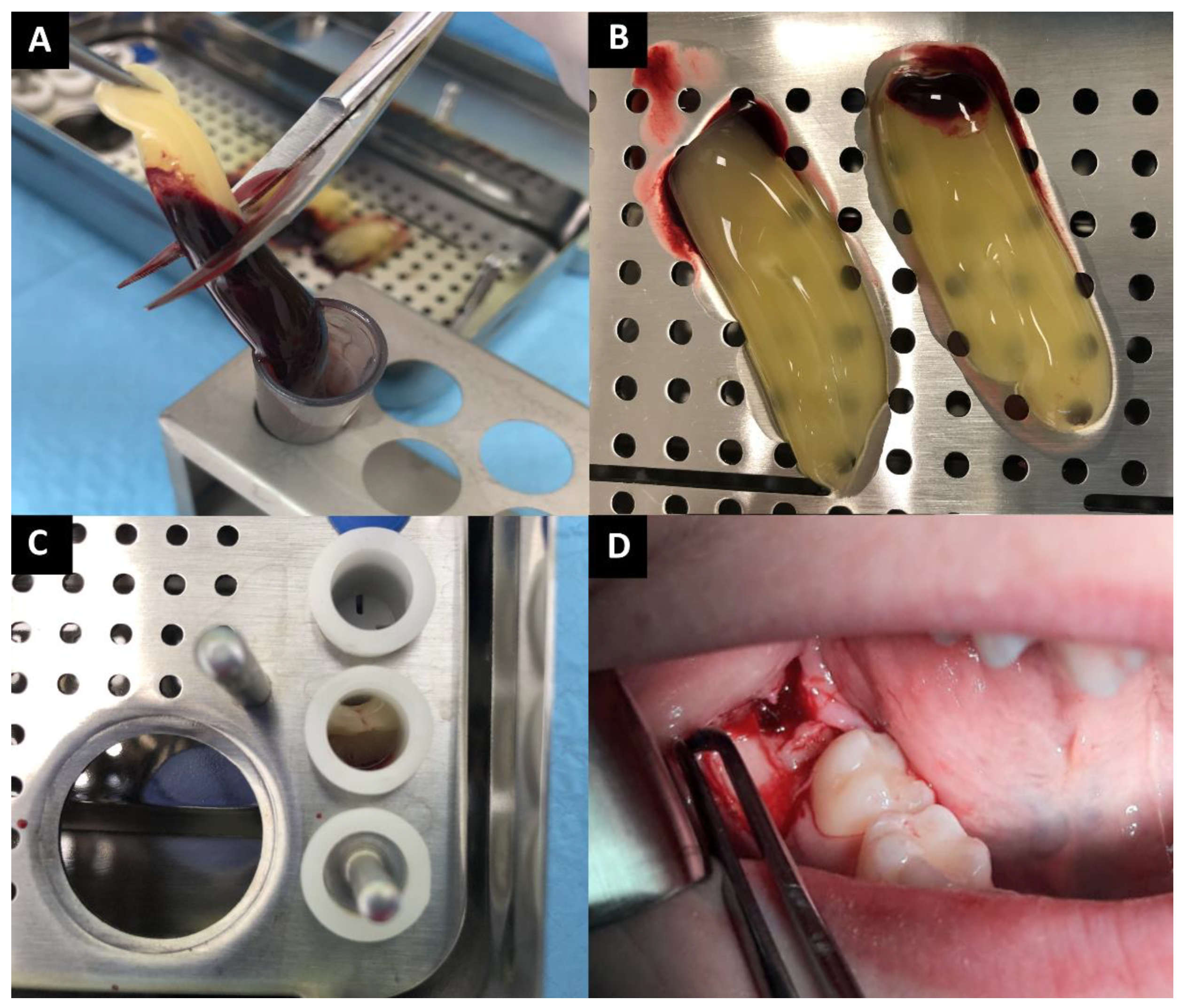
2.1. PRF Preparation
2.2. Surgical Procedure
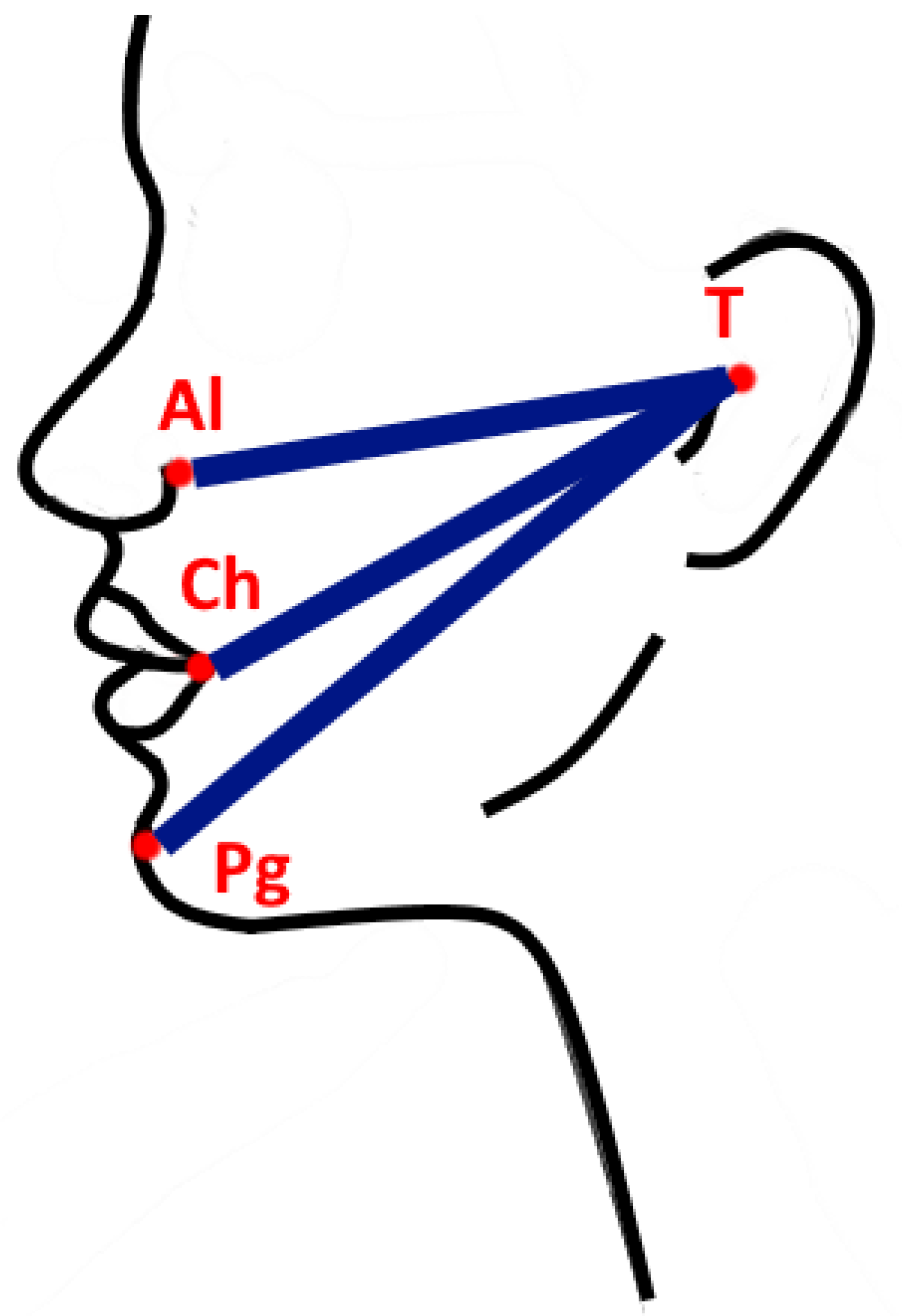
2.3. Postoperative Evaluations
2.4. Statistical Analysis
3. Results
3.1. Characteristic of Patients
3.2. Influence of A-PRF Application on Postoperative Complications
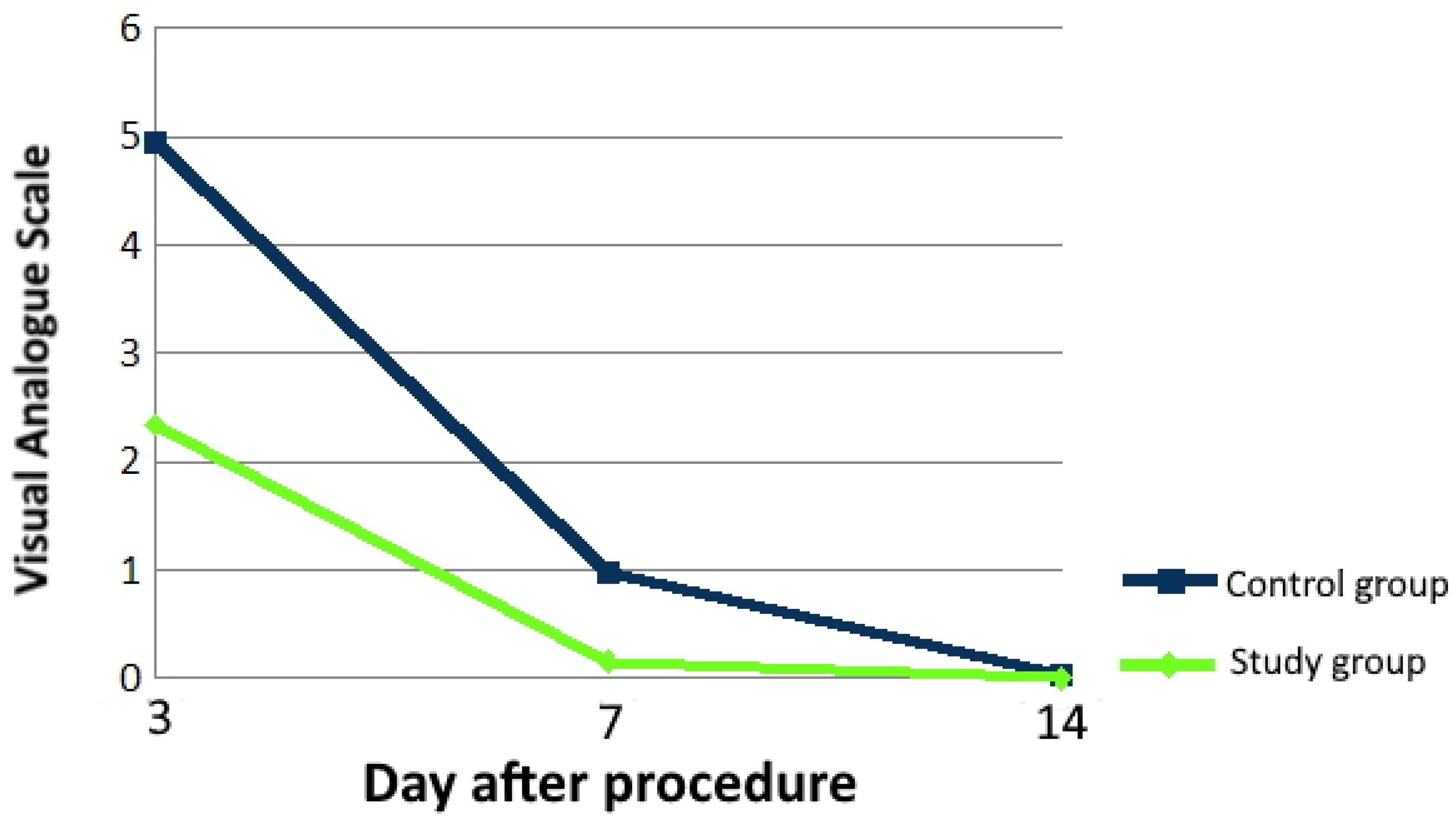
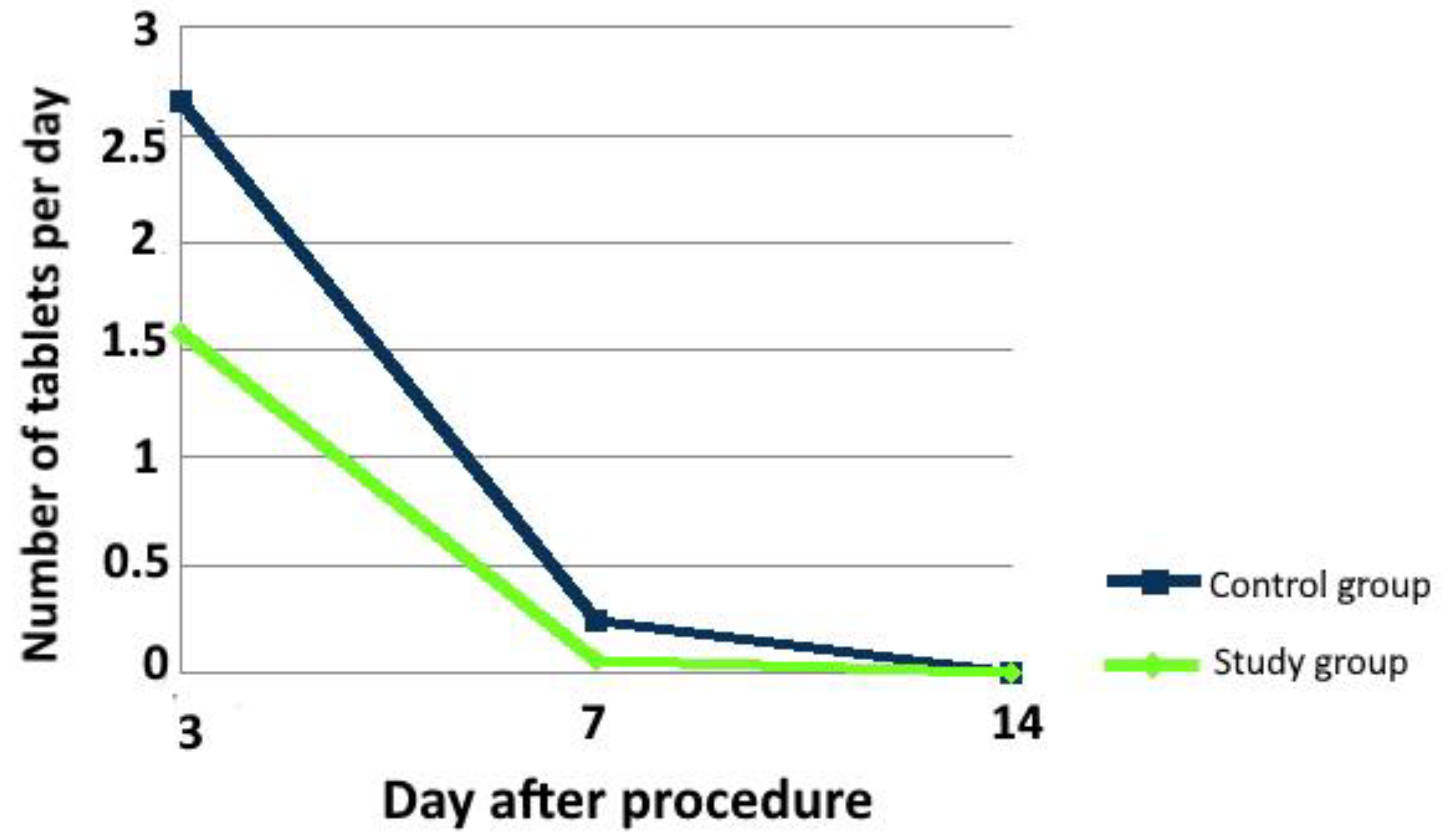
3.2.1. Pain and Analgesics Intake
3.2.2. Trismus
3.2.3. Edema
3.2.4. Followings Connected with Bleeding
3.2.5. Prevalence of Dry Socket
3.2.6. Prevalence of Pyrexia
3.2.7. Skin Warmth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-PRF | advanced platelet-rich fibrin |
| Al | Alare |
| BMP-2 | bone morphogenic factor 2 |
| BMP-7 | bone morphogenic factor 7 |
| Ch | chelion |
| DF | degrees of freedom |
| F | F ratio |
| FGF | fibroblast growth factor |
| IL-1β | interleukin 1β |
| IL-4 | interleukin 4 |
| IL-6 | interleukin 6 |
| L-PRF | leukocyte and platelet-rich fibrin |
| MS | mean squares |
| p | p value |
| PD-EGF | platelet-derived epidermal growth factor |
| PDGF | platelet-derived growth factor |
| Pg | pogonion |
| PRP | platelet-rich plasma |
| SS | sum of squares |
| T | tragus |
| T0 | time ‘zero’ |
| TGF | transforming growth factor |
| TGFβ-1 | tumor necrosis factor β-1 |
| TNFα | tumor necrosis factor α |
| TSP-1 | thrombospondin-1 |
| VAS | visual analog scale |
| VEGF | vascular-endothelial growth factor |
References
- Zhang, W.; Dai, Y.B.; Wan, P.C.; Xu, D.D.; Guo, Y.; Li, Z. Relationship between post-extraction pain and acute pulpitis: A randomised trial using third molars. Int. Dent. J. 2016, 66, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Jaroń, A.; Trybek, G. The Pattern of Mandibular Third Molar Impaction and Assessment of Surgery Difficulty: A Retrospective Study of Radiographs in East Baltic Population. Int. J. Environ. Res. Public Health 2021, 18, 6016. [Google Scholar] [CrossRef] [PubMed]
- Materni, A.; De Angelis, N.; Di Tullio, N.; Colombo, E.; Benedicenti, S.; Amaroli, A. Flapless Surgical Approach to Extract Impacted Inferior Third Molars: A Retrospective Clinical Study. J. Clin. Med. 2021, 10, 593. [Google Scholar] [CrossRef]
- Trybek, G.; Rydlińska, J.; Aniko-Włodarczyk, M.; Jaroń, A. Effect of Platelet-Rich Fibrin Application on Non-Infectious Complications after Surgical Extraction of Impacted Mandibular Third Molars. Int. J. Environ. Res. Public Health 2021, 18, 8249. [Google Scholar] [CrossRef]
- Ansari, M.A.M.F.; Mutha, A. Digital Assessment of Difficulty in Impacted Mandibular Third Molar Extraction. J. Maxillofac. Oral Surg. 2020, 19, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Antonelli, A.; Averta, F.; Diodati, F.; Muraca, D.; Bennardo, F.; Giudice, A. Does Mandibular Gonial Angle Influence the Eruption Pattern of the Lower Third Molar? A Three-Dimensional Study. J. Clin. Med. 2021, 10, 4057. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, R.C.d.S.; Mourão, C.F.d.A.B.; Geremias, T.C.; Sacco, R.; Guimarães, L.S.; Montemezzi, P.; Cardarelli, A.; Moraschini, V.; Calasans-Maia, M.D.; Louro, R.S. Does the Coronectomy a Feasible and Safe Procedure to Avoid the Inferior Alveolar Nerve Injury during Third Molars Extractions? A Systematic Review. Healthcare 2021, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, A.; Soler-Capdevila, J.; Ustrell-Barral, M.; Gay-Escoda, C. Patient, radiological, and operative factors associated with surgical difficulty in the extraction of third molars: A systematic review. Int. J. Oral Maxillofac. Surg. 2020, 49, 655–665. [Google Scholar] [CrossRef]
- Adamska, P.; Adamski, Ł.J.; Musiał, D.; Tylek, K.; Studniarek, M.; Wychowański, P.; Kaczoruk-Wieremczuk, M.; Pyrzowska, D.; Jereczek-Fossa, B.A.; Starzyńska, A. Panoramic radiograph—A useful tool to assess the difficulty in extraction of third molars. Eur. J. Transl. Clin. Med. 2020, 3, 44–52. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 10, 37–44. [Google Scholar] [CrossRef]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral Investig. 2021, 25, 115–1167. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, V.; Arora, A.; Goyal, A. Bioactive Platelet Aggregates: Prp, Prgf, Prf, Cgf And Sticky Bone. J. Dent. Med. Sci. 2017, 16, 5–11. [Google Scholar] [CrossRef]
- Okuda, K.; Kawase, T.; Momose, M.; Murata, M.; Saito, Y.; Suzuki, H.; Wolff, L.F.; Yoshie, H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J. Periodontol. 2003, 74, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Ezzatt, O.M. Autologous Platelet Concentrate Preparations in Dentistry. Biomed J. Sci. Tech. Res. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-Rich Plasma: Evidence to Support Its Use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Neph, A.; Schroeder, A.; Enseki, K.R.; Everts, P.A.; Wang, J.H.; Onishi, K. Role of Mechanical Loading for Platelet-Rich Plasma-Treated Achilles Tendinopathy. Curr. Sports Med. Rep. 2020, 19, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunite en paro-implantologie: Le PRF. Implantodontie 2000, 42, 55–62. [Google Scholar]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cole, J.; Deutsch, D.P.; Everts, P.A.; Niedbalski, R.P.; Panchaprateep, R.; Rinaldi, F.; Rose, P.T.; Sinclair, R.; Vogel, J.E.; et al. Platelet-Rich Plasma as a Treatment for Androgenetic Alopecia. Dermatol. Surg. 2019, 45, 1262–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everts, P.A.; Knape, J.T.; Weibrich, G.; Schönberger, J.P.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.; van Zundert, A. Platelet-rich plasma and platelet gel: A review. J. Extra Corpor. Technol. 2006, 38, 174–187. [Google Scholar] [PubMed]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Mitulović, G.; Miron, R.J.; Gruber, R. Platelet-Rich Fibrin Increases BMP2 Expression in Oral Fibroblasts via Activation of TGF-β Signaling. Int. J. Mol. Sci. 2021, 22, 7935. [Google Scholar] [CrossRef] [PubMed]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Miron, R.J.; Gruber, R. Relative Centrifugal Force (RCF; G-Force) Affects the Distribution of TGF-β in PRF Membranes Produced Using Horizontal Centrifugation. Int. J. Mol. Sci. 2020, 21, 7629. [Google Scholar] [CrossRef] [PubMed]
- Mazor, Z.; Horowitz, R.A.; Del Corso, M.; Prasad, H.S.; Rohrer, M.D.; Dohan Ehrenfest, D.M. Sinus floor augmentation with simultaneous implant placement using Choukroun’s platelet-rich fibrin as the sole grafting material: A radiologic and histologic study at 6 months. J. Periodontol. 2009, 80, 2056–2064. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Barrio, C.; del Olmo-Aguado, S.; García-Pérez, E.; de la Fuente, M.; Muruzabal, F.; Anitua, E.; Baamonde-Arbaiza, B.; Fernández-Vega-Cueto, L.; Fernández-Vega, L.; Merayo-Lloves, J. Antioxidant Role of PRGF on RPE Cells after Blue Light Insult as a Therapy for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 1021. [Google Scholar] [CrossRef] [Green Version]
- Yüce, E.; Kömerik, N. Potential effects of advanced platelet rich fibrin as a wound-healing accelerator in the management of alveolar osteitis: A randomized clinical trial. Niger J. Clin. Pract. 2019, 22, 1189–1195. [Google Scholar] [CrossRef]
- Giudice, A.; Antonelli, A.; Muraca, D.; Fortunato, L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020, 31, 813–818. [Google Scholar] [CrossRef]
- Sousa, F.; Machado, V.; Botelho, J.; Proença, L.; Mendes, J.J.; Alves, R. Effect of A-PRF Application on Palatal Wound Healing after Free Gingival Graft Harvesting: A Prospective Randomized Study. Eur. J. Dent. 2020, 14, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Xiang, X.; Shi, P.; Zhang, P.; Shen, J.; Kang, J. Impact of platelet-rich fibrin on mandibular third molar surgery recovery: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 163. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamed, F.S.; Tawfik, M.A.; Abdelfadil, E.; Al-Saleh, M.A.Q. Efficacy of Platelet-Rich Fibrin After Mandibular Third Molar Extraction: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2017, 75, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.P.; Manuel, S.; Kumar, L.K.S. Potential for Osseous Regeneration of Platelet-Rich Fibrin-A Comparative Study in Mandibular Third Molar Impaction Sockets. J. Oral Maxillofac. Surg. 2017, 75, 1322–1329. [Google Scholar] [CrossRef]
- Kapse, S.; Surana, S.; Satish, M.; Hussain, S.E.; Vyas, S.; Thakur, D. Autologous platelet-rich fibrin: Can it secure a better healing? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Prasad, K.; Ramanujam, L.K.R.; Dexith, J.; Chauhan, A. Evaluation of treatment outcome after impacted mandibular third molar surgery with the use of autologous platelet-rich fibrin: A randomized controlled clinical study. J. Oral Maxillofac. Surg. 2015, 73, 1042–1049. [Google Scholar] [CrossRef]
- Dar, M.M.; Shah, A.A.; Najar, A.L.; Younis, M.; Kapoor, M.; Dar, J.I. Healing Potential of Platelet Rich Fibrin in Impacted Mandibular Third Molar Extraction Sockets. Ann. Maxillofac. Surg. 2018, 8, 206–213. [Google Scholar] [CrossRef]
- Ozgul, O.; Senses, F.; Er, N.; Tekin, U.; Tuz, H.H.; Alkan, A.; Kocyigit, I.D.; Atil, F. Efficacy of platelet rich fibrin in the reduction of the pain and swelling after impacted third molar surgery: Randomized multicenter split-mouth clinical trial. Head Face Med. 2015, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Zahid, T.M.; Nadershah, M. Effect of Advanced Platelet-rich Fibrin on Wound Healing after Third Molar Extraction: A Split-mouth Randomized Double-blind Study. J. Contemp. Dent. Pract. 2019, 20, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Agarwal, S. Advanced-PRF: Clinical evaluation in impacted mandibular third molar sockets. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Caymaz, M.G.; Uyanik, L.O. Comparison of the effect of advanced platelet-rich fibrin and leukocyte- and platelet-rich fibrin on outcomes after removal of impacted mandibular third molar: A randomized split-mouth study. Niger J. Clin. Pract. 2019, 22, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Asutay, F.; Yolcu, Ü.; Geçör, O.; Acar, A.H.; Öztürk, S.A.; Malkoç, S. An evaluation of effects of platelet-rich-fibrin on postoperative morbidities after lower third molar surgery. Niger J. Clin. Pract. 2017, 20, 1531–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülşen, U.; Şentürk, M.F. Effect of platelet rich fibrin on edema and pain following third molar surgery: A split mouth control study. BMC Oral Health 2017, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Torul, D.; Omezli, M.M.; Kahveci, K. Evaluation of the effects of concentrated growth factors or advanced platelet rich-fibrin on postoperative pain, edema, and trismus following lower third molar removal: A randomized controlled clinical trial. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Eshghpour, M.; Dastmalhi, P.; Nekooei, A.H.; Nejat, A. Effect of platelet-rich fibrin on frequency of alveolar osteitis following mandibular third molar surgery: A double-blinded randomized clinical trial. J. Oral Maxillofac. Surg. 2014, 72, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Unsal, H.; Erbasar, G.N. Evaluation of the Effect of Platelet-Rich Fibrin on the Alveolar Osteitis Incidence and Periodontal Probing Depth after Extracting Partially Erupted Mandibular Third Molars Extraction. Niger J. Clin. Pract. 2018, 21, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
| Clinical and Pathological Patient Characteristics | Control Group | Study Group | p | ||
|---|---|---|---|---|---|
| Age Median ± SD (Range) [Years] | 28.5 ± 5.7 (18–42) | 29.3 ± 7.4 (19–47) | - | ||
| Gender | Female | 32 (64%) | 33 (66%) | 0.216 | |
| Male | 18 (36%) | 17 (34%) | |||
| Grade of tooth retention | Complete retention | 9 (18%) | 3 (6%) | 0.003 | |
| Partial retention | 41 (82%) | 47 (94%) | |||
| Tooth position | Vertical | 25 (50%) | 33 (66%) | 0.839 | |
| Horizontal | 25 (50%) | 17 (34%) | |||
| Time of surgery procedure | <30 min | 41 (82%) | 40 (80%) | 0.114 | |
| ≥30 min | 9 (19%) | 10 (20%) | |||
| Pain (VAS scale) | 3rd day | 0–3 | 11 (22%) | 42 (84%) | <0.001 |
| 4–6 | 25 (50%) | 8 (16%) | |||
| 7–10 | 14 (28%) | 0 (0%) | |||
| 7th day | 0–3 | 48 (96%) | 49 (98%) | <0.001 | |
| 4–6 | 2 (4%) | 1 (2%) | |||
| 7–10 | 0 (0%) | 0 (0%) | |||
| 14th day | 0–3 | 50 (100%) | 50 (100%) | >0.050 | |
| 4–6 | 0 (0%) | 0 (0%) | |||
| 7–10 | 0 (0%) | 0 (0%) | |||
| Painkillers intake | 3rd day | median (range) | 3 (0–4) doses | 2 (0–4) | <0.001 |
| 7th day | median (range) | 0 (0–2) | 0 (0–2) | 0.048 | |
| 14th day | median (range) | 0 (0) | 0 (0) | 1.000 | |
| Trismus | 3rd day | Lack of trismus | 5 (10%) | 23 (46%) | <0.001 |
| First grade | 13 (26%) | 22 (44%) | |||
| Second grade | 21 (42%) | 3 (6%) | |||
| Third grade | 11 (22%) | 1 (2%) | |||
| 7th day | Lack of trismus | 29 (58%) | 45 (90%) | <0.001 | |
| First grade | 18 (36%) | 5 (10%) | |||
| Second grade | 3 (6%) | 0 (%) | |||
| Third grade | 0 (%) | 0 (%) | |||
| 14th day | Lack of trismus | 50 (100% | 50 (100% | 0.495 | |
| First grade | 0 (%) | 0 (%) | |||
| Second grade | 0 (%) | 0 (%) | |||
| Third grade | 0 (%) | 0 (%) | |||
| Edema | Texture of edema | ||||
| 3rd day | Lack of edema | 4 (8%) | 12 (24%) | 0.006 | |
| Soft edema | 33 (66%) | 35 (70%) | |||
| Hard edema | 13 (26%) | 13 (26%) | |||
| 7th day | Lack of edema | 24 (48%) | 45 (90%) | <0.001 | |
| Soft edema | 18 (36%) | 5 (10%) | |||
| Hard edema | 8 (16%) | 0 (0%) | |||
| 14th day | Lack of edema | 47 (94%) | 50 (100%) | 0.242 | |
| Soft edema | 1 (2%) | 0 (0%) | |||
| Hard edema | 2 (4%) | 0 (0%) | |||
| Size of edema | |||||
| 3rd day | Lack of edema | 4 (8%) | 12 (24%) | <0.001 | |
| Small edema | 17 (34%) | 35 (70%) | |||
| Medium edema | 27 (54%) | 3 (6%) | |||
| Large edema | 2 (4%) | 0 (0%) | |||
| 7th day | Lack of edema | 24 (48%) | 45 (90%) | <0.001 | |
| Small edema | 25 (50%) | 5 (10%) | |||
| Medium edema | 1 (2%) | 0 (0%) | |||
| Large edema | 0 (0%) | 0 (0%) | |||
| 14th day | Lack of edema | 47 (94%) | 50 (100%) | 0.242 | |
| Small edema | 3 (6%) | 0 (0%) | |||
| Medium edema | 0 (0%) | 0 (0%) | |||
| Large edema | 0 (0%) | 0 (0%) | |||
| Bleeding time | <30 min | 2 (4%) | 21 (42%) | <0.001 | |
| Up to 1 h | 36 (72%) | 22 (44%) | |||
| 2–3 h | 6 (12%) | 5 (10%) | |||
| 4–6 h | 6 (12%) | 2 (4%) | |||
| Hematoma | 3rd day | Lack of hematoma | 35 (70%) | 50 (100%) | <0.001 |
| Buccal hematoma | 9 (18%) | 0 (0%) | |||
| Submandible hematoma | 5 (10%) | 0 (0%) | |||
| Neck hematoma | 1 (2%) | 0 (0%) | |||
| 7th day | Lack of hematoma | 42 (84%) | 47 (94%) | 0.453 | |
| Buccal hematoma | 3 (6%) | 1 (2%) | |||
| Submandible hematoma | 3 (6%) | 1 (2%) | |||
| Neck hematoma | 2 (4%) | 1 (2%) | |||
| 14th day | Lack of hematoma | 50 (100%) | 50 (100%) | 1.000 | |
| Buccal hematoma | 0 (0%) | 0 (0%) | |||
| Submandible hematoma | 0 (0%) | 0 (0%) | |||
| Neck hematoma | 0 (0%) | 0 (0%) | |||
| Secondary bleeding | 3rd day | No | 48 (96%) | 50 (100%) | 0.495 |
| Yes | 2 (4%) | 0 (0%) | |||
| 7th day | No | 50 (100%) | 50 (100%) | 1.000 | |
| Yes | 0 (0%) | 0 (0%) | |||
| 14th day | No | 50 (100%) | 50 (100%) | 1.000 | |
| Yes | 0 (0%) | 0 (0%) | |||
| Dry socket | 3rd day | No | 49 (98%) | 50 (100%) | 1.000 |
| Yes | 1 (2%) | 0 (0%) | |||
| 7th day | No | 49 (98%) | 49 (98%) | 1.000 | |
| Yes | 1 (2%) | 1 (2%) | |||
| 14th day | No | 50 (100%) | 50 (100%) | 1.000 | |
| Yes | 0 (0%) | 0 (0%) | |||
| Pyrexia | 3rd day | No | 42 (84%) | 45 (90%) | 0.552 |
| Yes | 8 (16%) | 5 (10%) | |||
| 7th day | No | 49 (98%) | 50 (100%) | 1.000 | |
| Yes | 1 (2%) | 0 (0%) | |||
| 14th day | No | 50 (100%) | 50 (100%) | 1.000 | |
| Yes | 0 (0%) | 0 (0%) | |||
| Skin warmth | 3rd day | No | 37 (74%) | 47 (94%) | 0.014 |
| Yes | 13 (26%) | 3 (6%) | |||
| 7th day | No | 50 (100%) | 50 (100%) | 1.000 | |
| Yes | 0 (0%) | 0 (0%) | |||
| 14th day | No | 50 (100%) | 50 (100%) | 1.000 | |
| Yes | 0 (0%) | 0 (0%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starzyńska, A.; Kaczoruk-Wieremczuk, M.; Lopez, M.A.; Passarelli, P.C.; Adamska, P. The Growth Factors in Advanced Platelet-Rich Fibrin (A-PRF) Reduce Postoperative Complications after Mandibular Third Molar Odontectomy. Int. J. Environ. Res. Public Health 2021, 18, 13343. https://doi.org/10.3390/ijerph182413343
Starzyńska A, Kaczoruk-Wieremczuk M, Lopez MA, Passarelli PC, Adamska P. The Growth Factors in Advanced Platelet-Rich Fibrin (A-PRF) Reduce Postoperative Complications after Mandibular Third Molar Odontectomy. International Journal of Environmental Research and Public Health. 2021; 18(24):13343. https://doi.org/10.3390/ijerph182413343
Chicago/Turabian StyleStarzyńska, Anna, Magdalena Kaczoruk-Wieremczuk, Michele Antonio Lopez, Pier Carmine Passarelli, and Paulina Adamska. 2021. "The Growth Factors in Advanced Platelet-Rich Fibrin (A-PRF) Reduce Postoperative Complications after Mandibular Third Molar Odontectomy" International Journal of Environmental Research and Public Health 18, no. 24: 13343. https://doi.org/10.3390/ijerph182413343
APA StyleStarzyńska, A., Kaczoruk-Wieremczuk, M., Lopez, M. A., Passarelli, P. C., & Adamska, P. (2021). The Growth Factors in Advanced Platelet-Rich Fibrin (A-PRF) Reduce Postoperative Complications after Mandibular Third Molar Odontectomy. International Journal of Environmental Research and Public Health, 18(24), 13343. https://doi.org/10.3390/ijerph182413343








