Towards an Efficient Generalization of the Online Dosage of Hydrogen Peroxide in Photo-Fenton Process to Treat Industrial Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pilot Plant
2.3. Analytical Methods
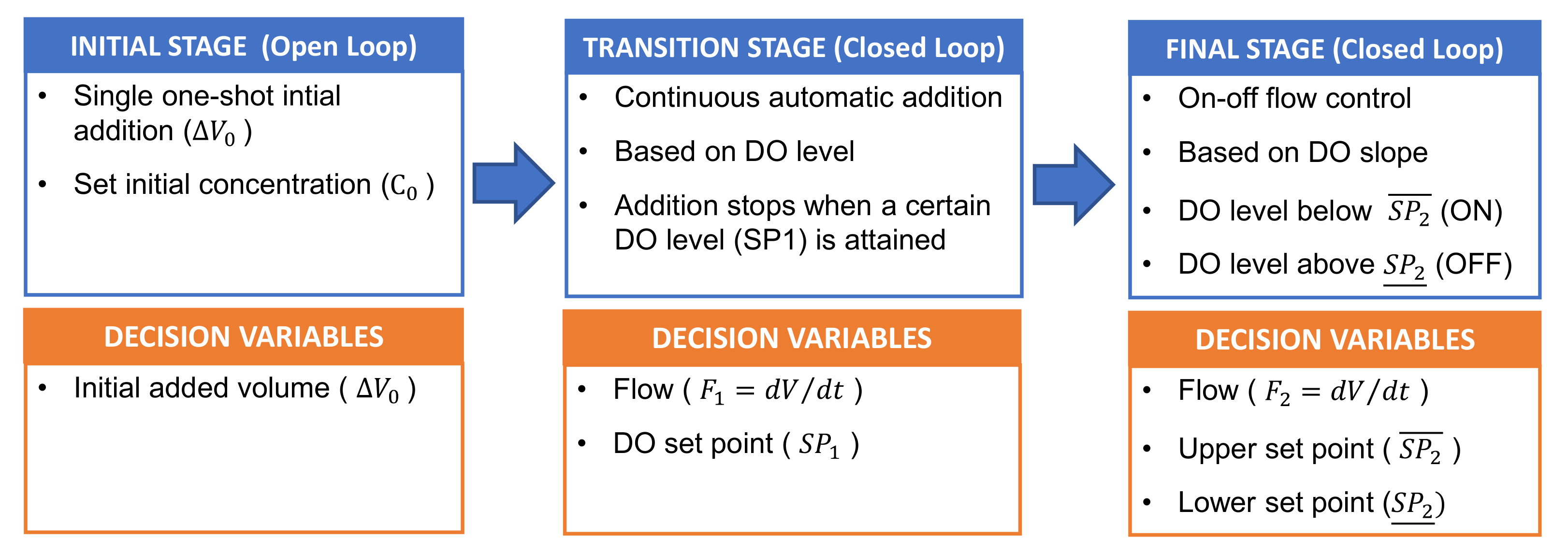
2.4. Experimental Procedure
- I.
- Initial stage (open-loop): Single initial addition at the beginning of the assay. The initial H2O2 concentrations are selected as a percentage of the H2O2 stoichiometric concentration (i.e., the stoichiometric concentration for 100 mg L−1 of PCT is 472 mg L−1; for 123 mg L−1 of SMT it is 631 mg L−1),
- II.
- Transition stage (close-loop): Continuous automatic addition of H2O2 from the beginning of the assays at a pre-fixed flow rate of 0.287 mL min−1. This addition is maintained until attaining the set DO level. The previously proposed DO set point is 4 mg L−1.
- III.
- Final stage (closed-loop): after the transition stage, automatic H2O2 addition (0.287 mL min−1 prefixed flow rate) is based on an on-off controller depending on the DO slope value. In general, the H2O2 addition is turned off when the DO slope reaches a maximum threshold of 0.2 mg L−1 min−1, while it is turned on when the DO slope falls below a minimum threshold of 0.1 mg L−1 min−1.
2.5. Experiments Codification
- I.
- Pollutant: codification starts with the information about the pollutant or pollutants mixture present in the wastewater by including the abbreviation of the compound preceded by a number that refers to its initial concentration. By way of illustration, PCT at 100 mg L−1 corresponds to 1PCT (63 mg L−1 of TOC), SMT at 123 mg L−1 corresponds to 1SMT (63 mg L−1 of TOC) and a mixture of SMT and PCT at 50% each corresponds to 0.5SMT+0.5PCT (also 63 mg L−1 of TOC).
- II.
- Initial stage: since initial additions were based on theoretical H2O2 stoichiometric amounts (coded as S), this information was coded accordingly. By way of illustration, an addition corresponding to 40% of the stoichiometric amount of the pollutant concentration was named 0.4S, which was added after the codification related to the nature and concentration of the pollutant.
- III.
- Transition stage: the DO set point that marks the stop of the H2O2 continuous addition is then added to the codification. By way of illustration, an initial addition corresponding to 40% stoichiometric amount of PCT at 100 mg L−1 using 4 mg L−1 of DO as set point to stop the continuous addition was codified as 1PCT_0.4S_DO4. For special assays in which continuous addition during transition stage was never stopped the codification was CA instead of DO4. If the transition stage was not carried out, no codification was added.
- IV.
- Final stage: the selected codification consisted in the addition of the minimum and maximum bounds of the DO slope used for the automatic start and stop of the H2O2 dosage. By way of illustration, an initial addition corresponding to 40% stoichiometric amount of PCT at 100 mg L−1 using 4 mg L−1 of DO as set point to stop the continuous addition and 0.2 mg L−1 min−1 and 0.1 mg L−1 min−1 as high and low DO slope thresholds of the on-off control system, respectively, was codified as 1PCT_0.4S_DO4_L0.1_H0.2.
- V.
- Iron concentration: 20 mg L−1 of iron were used for most of the experiments; however, the catalyst concentration was doubled in a few assays. However, in this last case codification about iron concentration was added at the end (2Fe).
3. Results
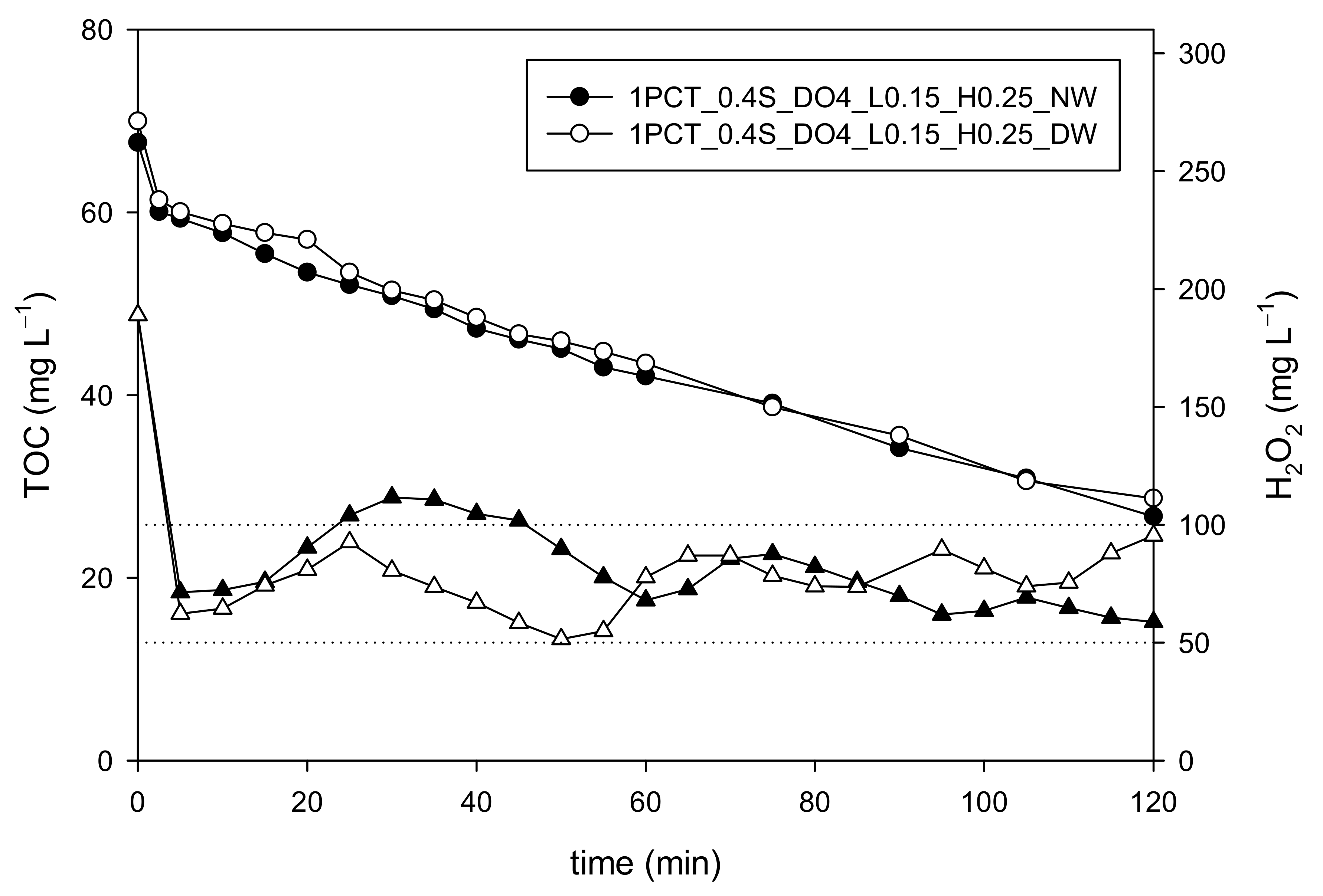
3.1. Water Matrix Effect on Dosage Strategy: Distilled Water vs. Natural Water
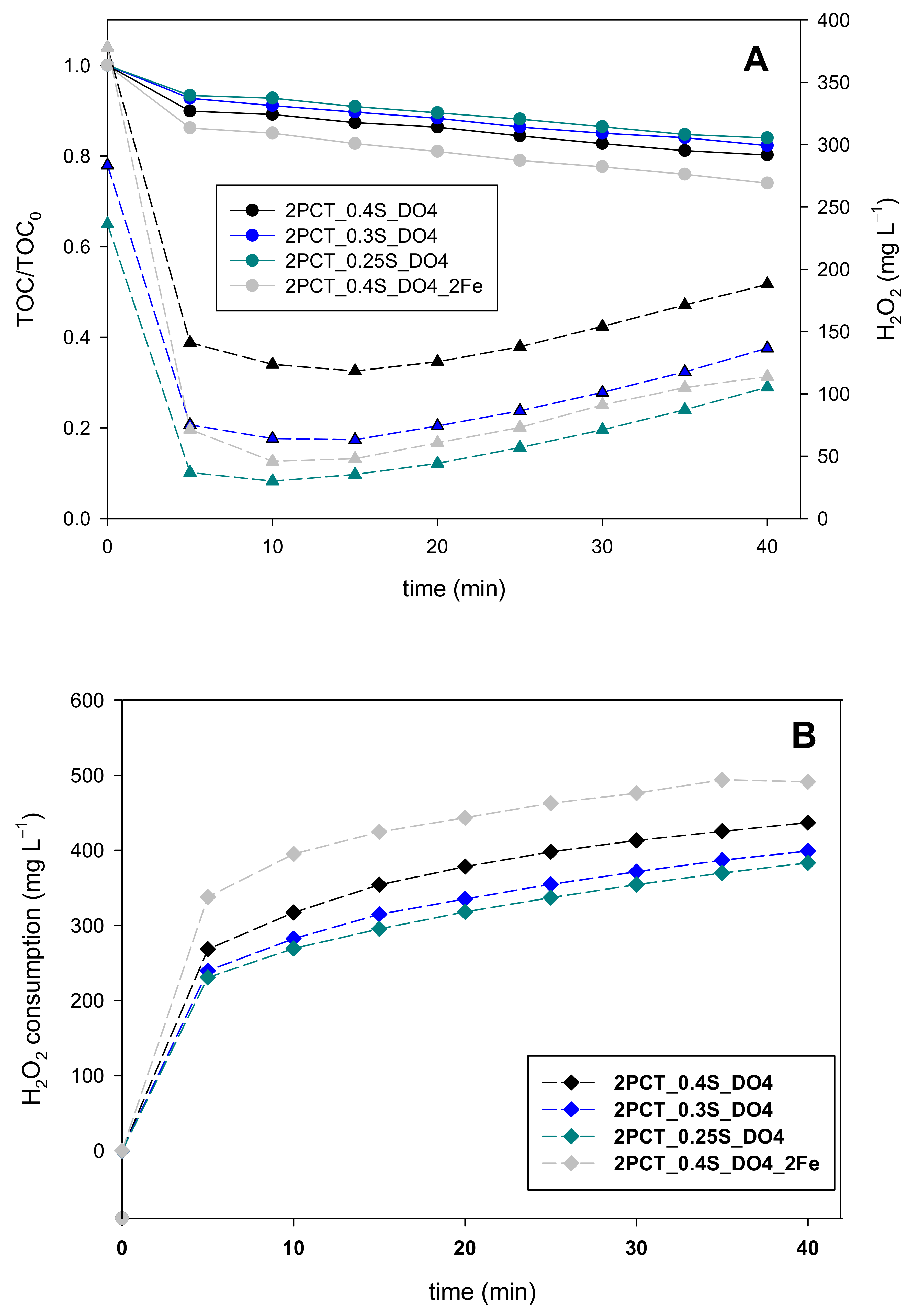
3.2. Validating the Dosage Strategy with Different Pollutant Concentrations
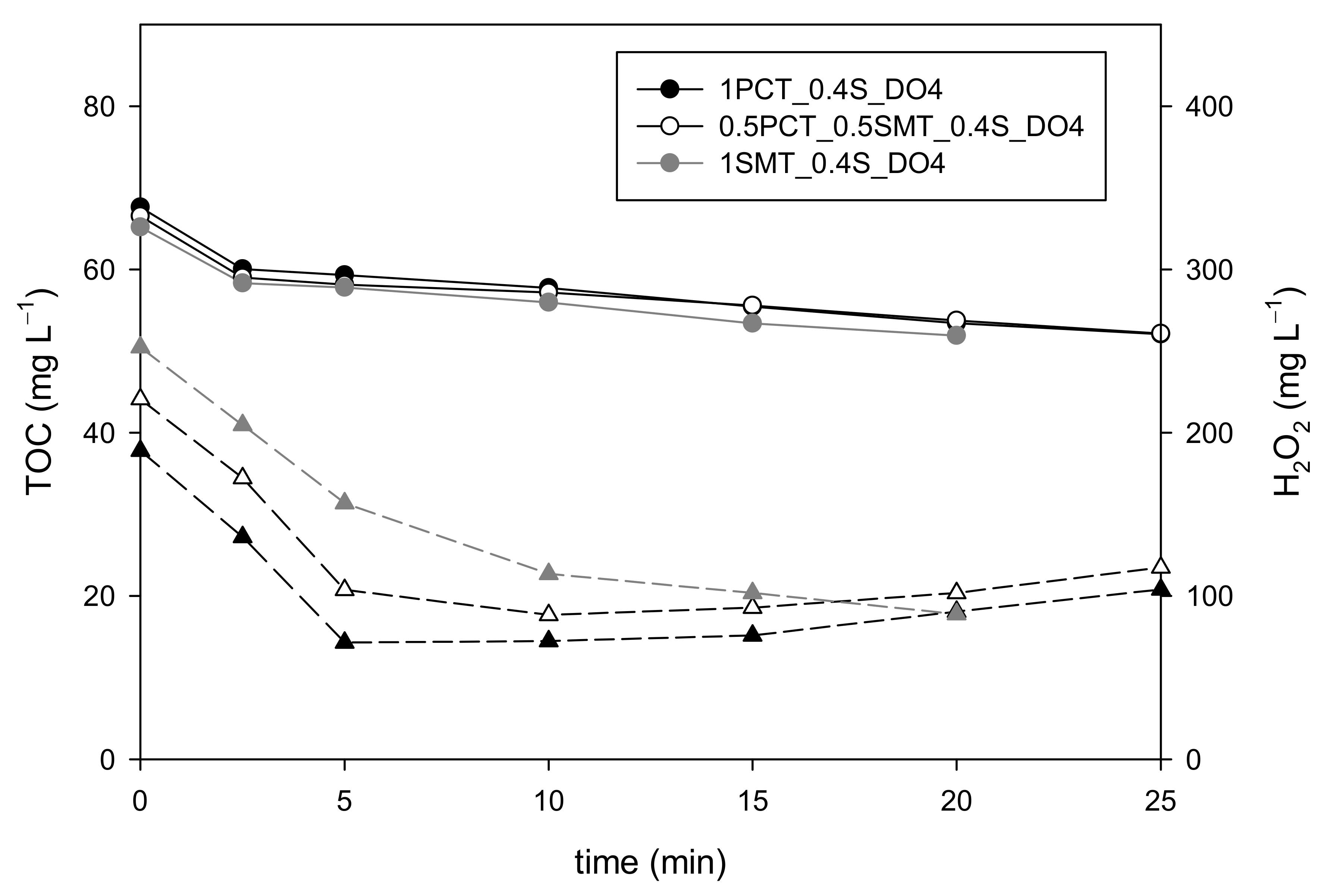
3.3. Modifying the Wastewater Organic Matter Nature
3.3.1. Paracetamol and Sulfamethoxazole Mixtures
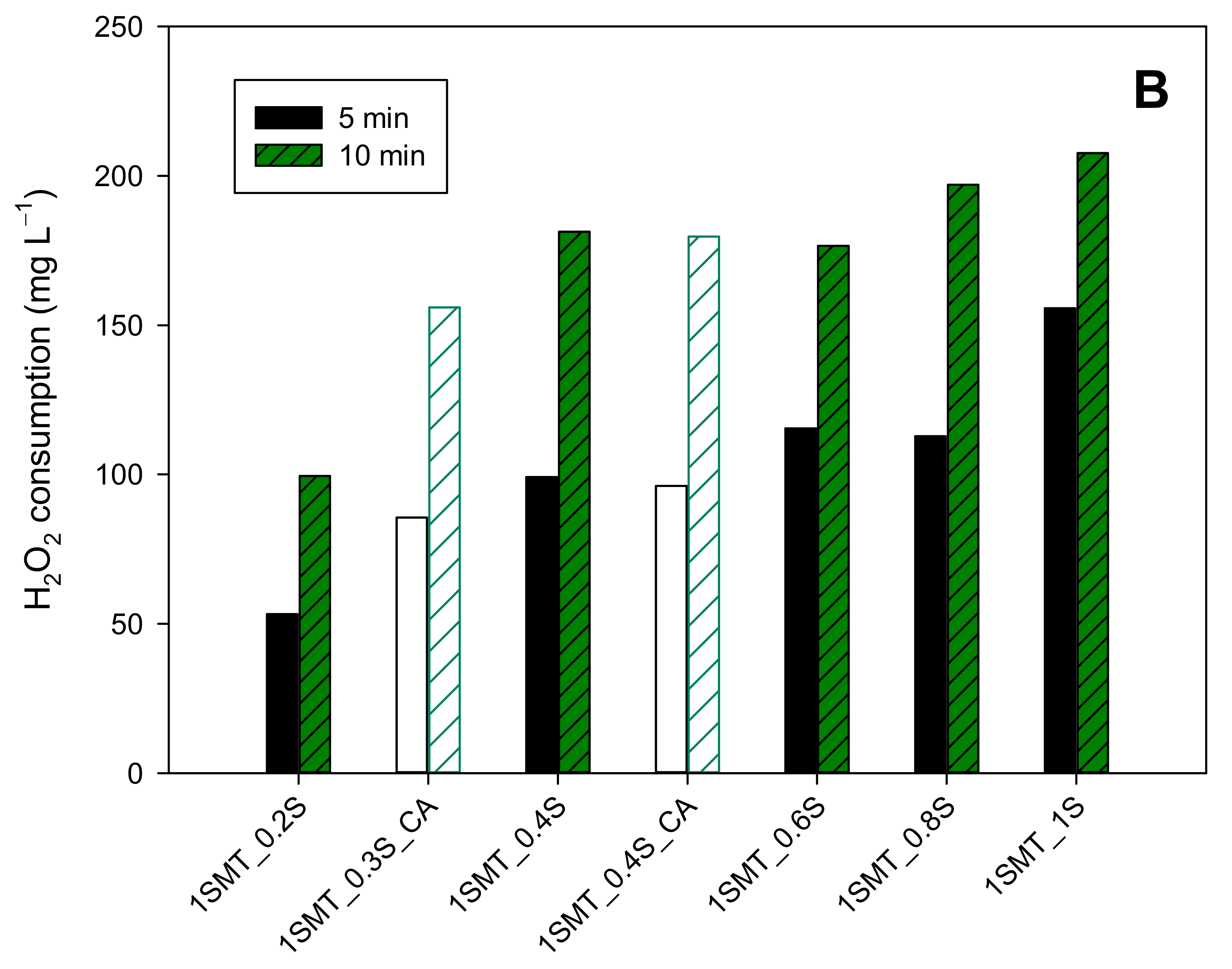
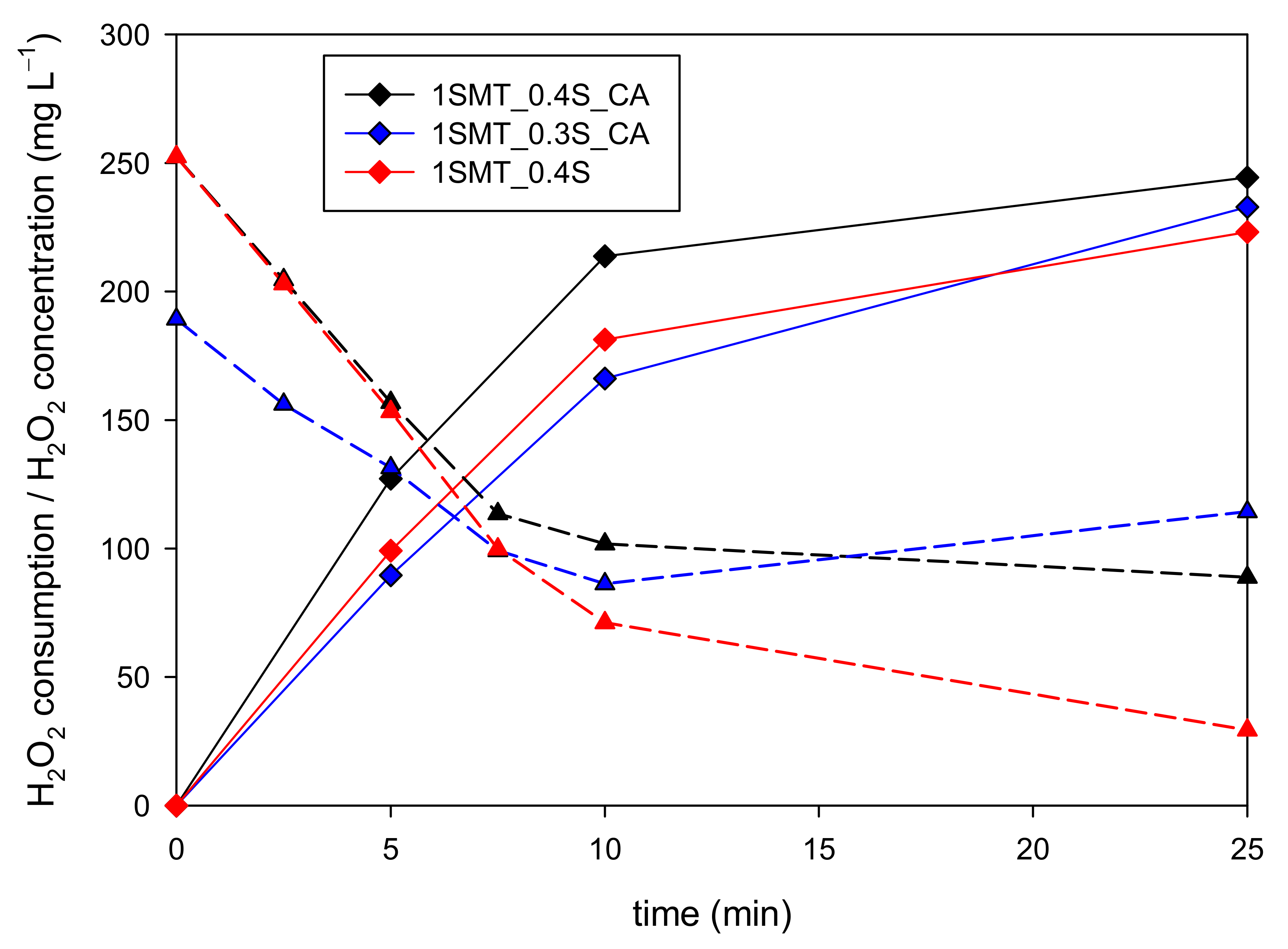
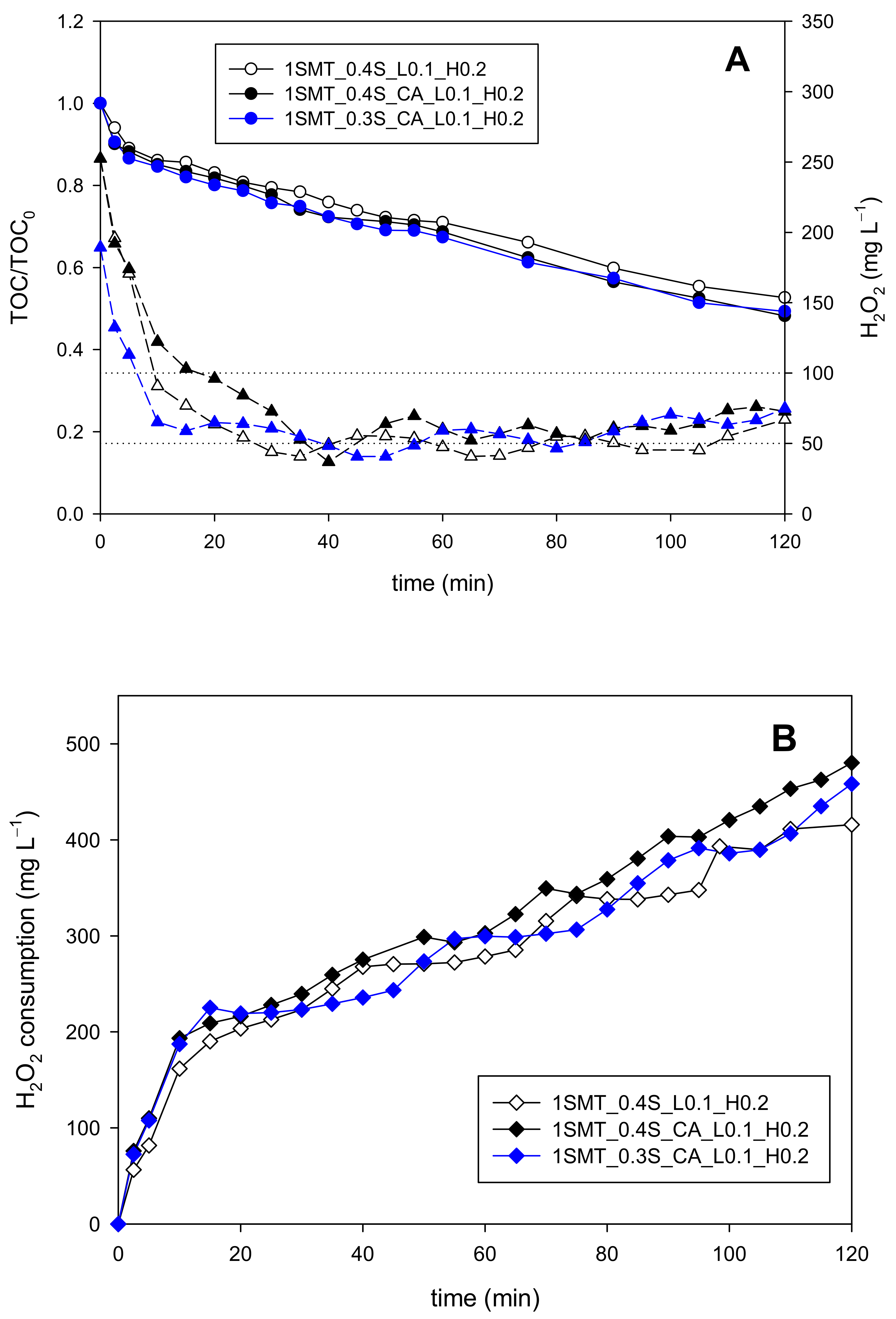
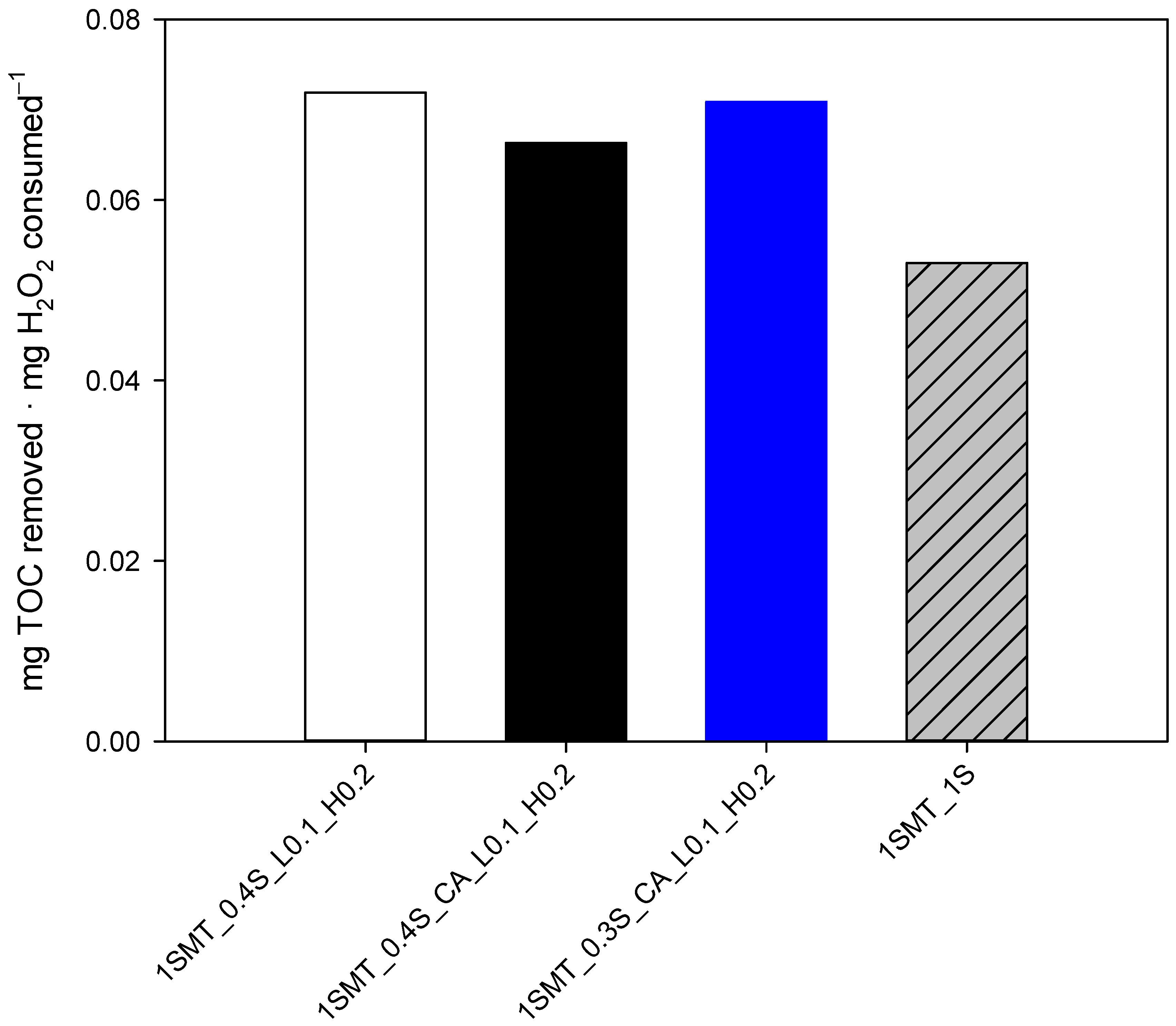
3.3.2. Sulfamethazine Contaminated Wastewater
4. Discussion
4.1. Water Matrix Effect on Dosage Strategy: Distilled Water vs. Natural Water
4.2. Validating the Dosage Strategy with Different Pollutant Concentrations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Tokumura, M.; Morito, R.; Hatayama, R.; Kawase, Y. Iron Redox Cycling in Hydroxyl Radical Generation during the Photo-Fenton Oxidative Degradation: Dynamic Change of Hydroxyl Radical Concentration. Appl. Catal. B Environ. 2011, 106, 565–576. [Google Scholar] [CrossRef]
- Yamal-Turbay, E.; Graells, M.; Pérez-Moya, M. Systematic Assessment of the Influence of Hydrogen Peroxide Dosage on Caffeine Degradation by the Photo-Fenton Process. Ind. Eng. Chem. Res. 2012, 51, 4770–4778. [Google Scholar] [CrossRef]
- Yu, X.; Somoza-Tornos, A.; Graells, M.; Pérez-Moya, M. An Experimental Approach to the Optimization of the Dosage of Hydrogen Peroxide for Fenton and Photo-Fenton Processes. Sci. Total Environ. 2020, 743, 140402. [Google Scholar] [CrossRef] [PubMed]
- Ince, N.H. “Critical” Effect of Hydrogen Peroxide in Photochemical Dye Degradation. Water Res. 1999, 33, 1080–1084. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of Pharmaceuticals from Water by Homo/Heterogonous Fenton-Type Processes—A Review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Gulkaya, I.; Surucu, G.A.; Dilek, F.B. Importance of H2O2/Fe2+ Ratio in Fenton’s Treatment of a Carpet Dyeing Wastewater. J. Hazard. Mater. 2006, 136, 763–769. [Google Scholar] [CrossRef]
- Ortega-Gómez, E.; Moreno Úbeda, J.C.; Álvarez Hervás, J.D.; Casas López, J.L.; Santos-Juanes Jordá, L.; Sánchez Pérez, J.A. Automatic Dosage of Hydrogen Peroxide in Solar Photo-Fenton Plants: Development of a Control Strategy for Efficiency Enhancement. J. Hazard. Mater. 2012, 237–238, 223–230. [Google Scholar] [CrossRef]
- Audino, F.; Campanyà, G.; Pérez-Moya, M.; Espuña, A.; Graells, M. Systematic Optimization Approach for the Efficient Management of the Photo-Fenton Treatment Process. Sci. Total Environ. 2019, 646, 902–913. [Google Scholar] [CrossRef] [Green Version]
- Chu, W.; Chan, K.H.; Kwan, C.Y.; Choi, K.Y. Degradation of Atrazine by Modified Stepwise-Fenton’s Processes. Chemosphere 2007, 67, 755–761. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; San Martín, I.; Aguirre, M. Effect of Continuous Addition of H2O2 and Air Injection on Ferrioxalate-Assisted Solar Photo-Fenton Degradation of Orange II. Appl. Catal. B Environ. 2009, 89, 510–518. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, L.; Oller, I.; Zapata, A.; Agüera, A.; Malato, S. Hydrogen Peroxide Automatic Dosing Based on Dissolved Oxygen Concentration during Solar Photo-Fenton. Catal. Today 2011, 161, 247–254. [Google Scholar] [CrossRef]
- Yu, X.; Graells, M.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Pérez-Moya, M. An Improved Hybrid Strategy for Online Dosage of Hydrogen Peroxide in Photo-Fenton Processes. J. Environ. Chem. Eng. 2021, 9, 105235. [Google Scholar] [CrossRef]
- Cabrera Reina, A.; Miralles-Cuevas, S.; Casas López, J.L.; Sánchez Pérez, J.A. Pyrimethanil Degradation by Photo-Fenton Process: Influence of Iron and Irradiance Level on Treatment Cost. Sci. Total Environ. 2017, 605–606, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E. Extremum-Seeking Control of Redox Processes in Wastewater Chemical Treatment Plants. Comput. Aided Chem. Eng. 2007, 24, 865–870. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; Sánchez, J.L.G.; López, J.L.C.; Oller, I.; Malato, S.; Sánchez Pérez, J.A. Dissolved Oxygen Concentration: A Key Parameter in Monitoring the Photo-Fenton Process. Appl. Catal. B Environ. 2011, 104, 316–323. [Google Scholar] [CrossRef]
- Dalgic, G.; Turkdogan, I.F.; Yetilmezsoy, K.; Kocak, E. Treatment of real paracetamol wastewater by Fenton process. Chem. Ind. Chem. Eng. Q. 2017, 23, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Roshanfekr Rad, L.; Irani, M.; Divsar, F.; Pourahmad, H.; Sadegh Sayyafan, M.; Haririan, I. Simultaneous degradation of phenol and paracetamol during photo-Fenton process: Design and optimization. J. Taiwan Inst. Chem. Eng. 2015, 47, 190–196. [Google Scholar]
- Nogueira, R.F.P.; Oliveira, M.C.; Paterlini, W.C. Simple and Fast Spectrophotometric Determination of H2O2 in Photo-Fenton Reactions Using Metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef]
- Baffi, R.; Dolch, G.; Garnick, R.; Huang, Y.F.; Mar, B.; Matsuhiro, D.; Niepelt, B.; Parra, C.; Stephan, M. A total organic carbon analysis method for validating cleaning between products in biopharmaceutical manufacturing. J. Parenter. Sci. Technol. 1991, 45, 13–19. [Google Scholar]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of Water Matrix on the Removal of Micropollutants by Advanced Oxidation Technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and Disinfection of Water by Solar Photocatalysis: Recent Overview and Trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Zapata, A.; Oller, I.; Bizani, E.; Sánchez-Pérez, J.A.; Maldonado, M.I.; Malato, S. Evaluation of Operational Parameters Involved in Solar Photo-Fenton Degradation of a Commercial Pesticide Mixture. Catal. Today 2009, 144, 94–99. [Google Scholar] [CrossRef]
- Ballesteros Martín, M.M.; Sánchez Pérez, J.A.; Casas López, J.L.; Oller, I.; Malato Rodríguez, S. Degradation of a Four-Pesticide Mixture by Combined Photo-Fenton and Biological Oxidation. Water Res. 2009, 43, 653–660. [Google Scholar] [CrossRef]
- Gernjak, W.; Fuerhacker, M.; Fernández-Ibañez, P.; Blanco, J.; Malato, S. Solar Photo-Fenton Treatment—Process Parameters and Process Control. Appl. Catal. B Environ. 2006, 64, 121–130. [Google Scholar] [CrossRef]
- Audino, F.; Conte, L.O.; Schenone, A.V.; Pérez-Moya, M.; Graells, M.; Alfano, O.M. A Kinetic Study for the Fenton and Photo-Fenton Paracetamol Degradation in an Annular Photoreactor. Environ. Sci. Pollut. Res. 2019, 26, 4312–4323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera Reina, A.; Miralles-Cuevas, S.; Cornejo, L.; Pomares, L.; Polo, J.; Oller, I.; Malato, S. The influence of location on solar photo-Fenton: Process performance, photoreactor scaling-up and treatment cost. Renew. Energy 2020, 145, 1890–1900. [Google Scholar] [CrossRef]



| Irradiated volume, L | 1.5 | |
| Annular irradiated height, mm | 130 | |
| Outer cylinder | Outer diameter, mm | 150 |
| Inner diameter, mm | 140 | |
| Inner cylinder | Outer diameter, mm | 70 |
| Inner diameter, mm | 63.6 |
| Assay Code | PCT | SMT | TOC | Iron | Initial H2O2 | Dosage Stage | ||
|---|---|---|---|---|---|---|---|---|
| mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | Initial | Transition | Final | |
| 1PCT_0.4S_DO4_L0.15_H0.25_DW | 100 | 0 | 63 | 20 | 189 | YES | YES | YES |
| 1PCT_0.4S_DO4_L0.15_H0.25_NW | 100 | 0 | 63 | 20 | 189 | YES | YES | YES |
| 2PCT_0.25S_DO4 | 200 | 0 | 126 | 20 | 236 | YES | YES | NO |
| 2PCT_0.3S_DO4 | 200 | 0 | 126 | 20 | 283 | YES | YES | NO |
| 2PCT_0.4S_DO4 | 200 | 0 | 126 | 20 | 378 | YES | YES | NO |
| 2PCT_0.4S_DO4_2Fe | 200 | 0 | 126 | 40 | 378 | YES | YES | NO |
| 2PCT_0.3S_DO4_L0.1_H0.2 | 200 | 0 | 126 | 20 | 283 | YES | YES | YES |
| 2PCT_0.4S_DO4_L0.1_H0.2 | 200 | 0 | 126 | 20 | 378 | YES | YES | YES |
| 2PCT_0.4S_DO4_L0.1_H0.2_2Fe | 200 | 0 | 126 | 40 | 378 | YES | YES | YES |
| 1PCT_0.4S_DO4 | 100 | 0 | 63 | 20 | 189 | YES | YES | NO |
| 1SMT_0.4S_DO4 | 0 | 123 | 63 | 20 | 252 | YES | YES | NO |
| 0.5PCT_0.5SMT_0.4S_DO4 | 50 | 61.5 | 63 | 20 | 221 | YES | YES | NO |
| 1SMT_0.2S | 0 | 123 | 63 | 20 | 126 | YES | NO | NO |
| 1SMT_0.3S_CA | 0 | 123 | 63 | 20 | 189 | YES | YES | NO |
| 1SMT_0.4S | 0 | 123 | 63 | 20 | 252 | YES | NO | NO |
| 1SMT_0.4S_CA | 0 | 123 | 63 | 20 | 252 | YES | YES | NO |
| 1SMT_0.6S | 0 | 123 | 63 | 20 | 379 | YES | NO | NO |
| 1SMT_0.8S | 0 | 123 | 63 | 20 | 505 | YES | NO | NO |
| 1SMT_1.0S | 0 | 123 | 63 | 20 | 631 | YES | NO | NO |
| 1SMT_0.4S_CA_L0.1_H0.2 | 0 | 123 | 63 | 20 | 252 | YES | NO | YES |
| 1SMT_0.3S_CA_L0.1_H0.2 | 0 | 123 | 63 | 20 | 189 | YES | NO | YES |
| 1SMT_0.4S_L0.1_H0.2 | 0 | 123 | 63 | 20 | 252 | YES | NO | YES |
| Dosage Strategy | |||||
|---|---|---|---|---|---|
| 2PCT_0.25S_DO4 | 2PCT_0.3S_DO4 | 2PCT_0.4S_DO4 | 2PCT_0.4S_DO4_2Fe | ||
| Initial H2O2 concentration (mg L−1) | 236 | 283 | 377 | 377 | |
| Minimum H2O2 concentration in reaction bulk (mg L−1) | 30 | 63 | 118 | 46 | |
| DO set point (4 mg L−1) | time (min) | 55 | 47 | 39 | 36 |
| Equivalent* H2O2 concentration (mg L−1) | 583 | 580 | 623 | 604 | |
| Actual H2O2 concentration (mg L−1) | 169 | 159 | 184 | 106 | |
| Efficiency (mg TOC mg H2O2−1) | 0.06 | 0.06 | 0.06 | 0.06 | |
| DO slope set point (0.2 mg L−1 min−1) | time (min) | 47 | 34 | 31 | 26 |
| Equivalent * H2O2 concentration (mg L−1) | 533 | 498 | 573 | 541 | |
| Actual H2O2 concentration (mg L−1) | 125 | 114 | 157 | 75 | |
| Efficiency (mg TOC mg H2O2−1) | 0.06 | 0.05 | 0.06 | 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Cabrera-Reina, A.; Graells, M.; Miralles-Cuevas, S.; Pérez-Moya, M. Towards an Efficient Generalization of the Online Dosage of Hydrogen Peroxide in Photo-Fenton Process to Treat Industrial Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 13313. https://doi.org/10.3390/ijerph182413313
Yu X, Cabrera-Reina A, Graells M, Miralles-Cuevas S, Pérez-Moya M. Towards an Efficient Generalization of the Online Dosage of Hydrogen Peroxide in Photo-Fenton Process to Treat Industrial Wastewater. International Journal of Environmental Research and Public Health. 2021; 18(24):13313. https://doi.org/10.3390/ijerph182413313
Chicago/Turabian StyleYu, Xiangwei, Alejandro Cabrera-Reina, Moisès Graells, Sara Miralles-Cuevas, and Montserrat Pérez-Moya. 2021. "Towards an Efficient Generalization of the Online Dosage of Hydrogen Peroxide in Photo-Fenton Process to Treat Industrial Wastewater" International Journal of Environmental Research and Public Health 18, no. 24: 13313. https://doi.org/10.3390/ijerph182413313
APA StyleYu, X., Cabrera-Reina, A., Graells, M., Miralles-Cuevas, S., & Pérez-Moya, M. (2021). Towards an Efficient Generalization of the Online Dosage of Hydrogen Peroxide in Photo-Fenton Process to Treat Industrial Wastewater. International Journal of Environmental Research and Public Health, 18(24), 13313. https://doi.org/10.3390/ijerph182413313







