Fluorescence Characteristics of Coalbed Methane Produced Water and Its Influence on Freshwater Bacteria in the South Qinshui Basin, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Geological Setting of the Study Area
2.2. Sample Collection and Water Quality Analysis Method
2.3. Fluorescence Features Test
- —Integral standard volume of the fluorescence region;
- —Multiplication factor, which represents the reciprocal of the ratio of the area of a certain fluorescence integration area to the area of the total fluorescence integration area;
- —Volume integration of the fluorescence, au·nm2;
- —Excitation wavelength, nm;
- —Emission wavelength, nm;
- —Volume integration of fluorescence, au·nm2;
- —Total fluorescence area integration standard volume;
- —percentage of integral standard volum of the total fluorescence integration region;
- —Fluorescence intensity corresponding to excitation wavelength or emission wavelength, au.
2.4. Bacterial Growth Experiment and Drainage Simulation Experiment
2.4.1. Freshwater Bacterial Growth Experiment
2.4.2. Drainage Simulation Experiment of CBM Produced Water
3. Results and Discussion
3.1. Water Quality Analysis of CBM Produced Water
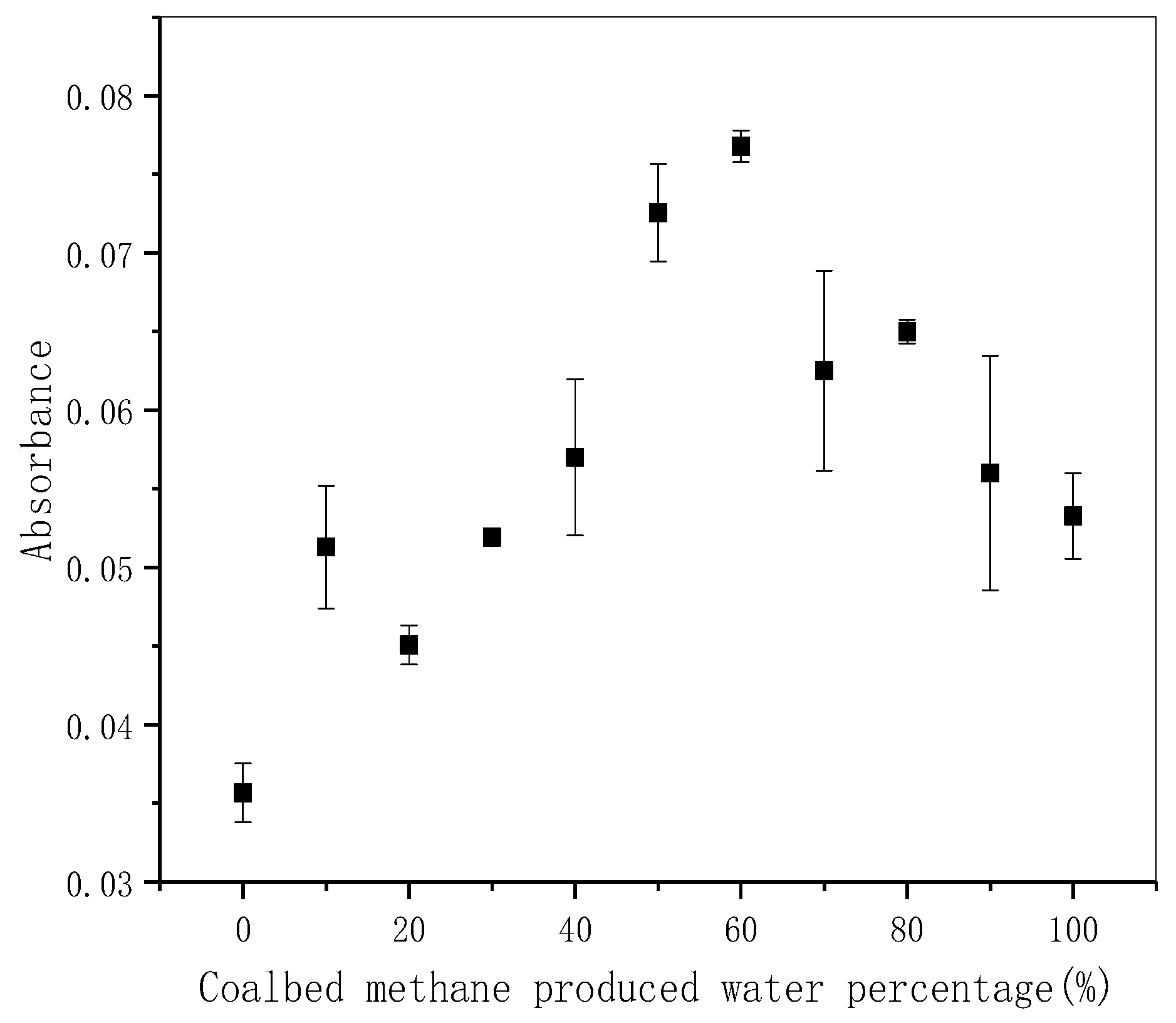
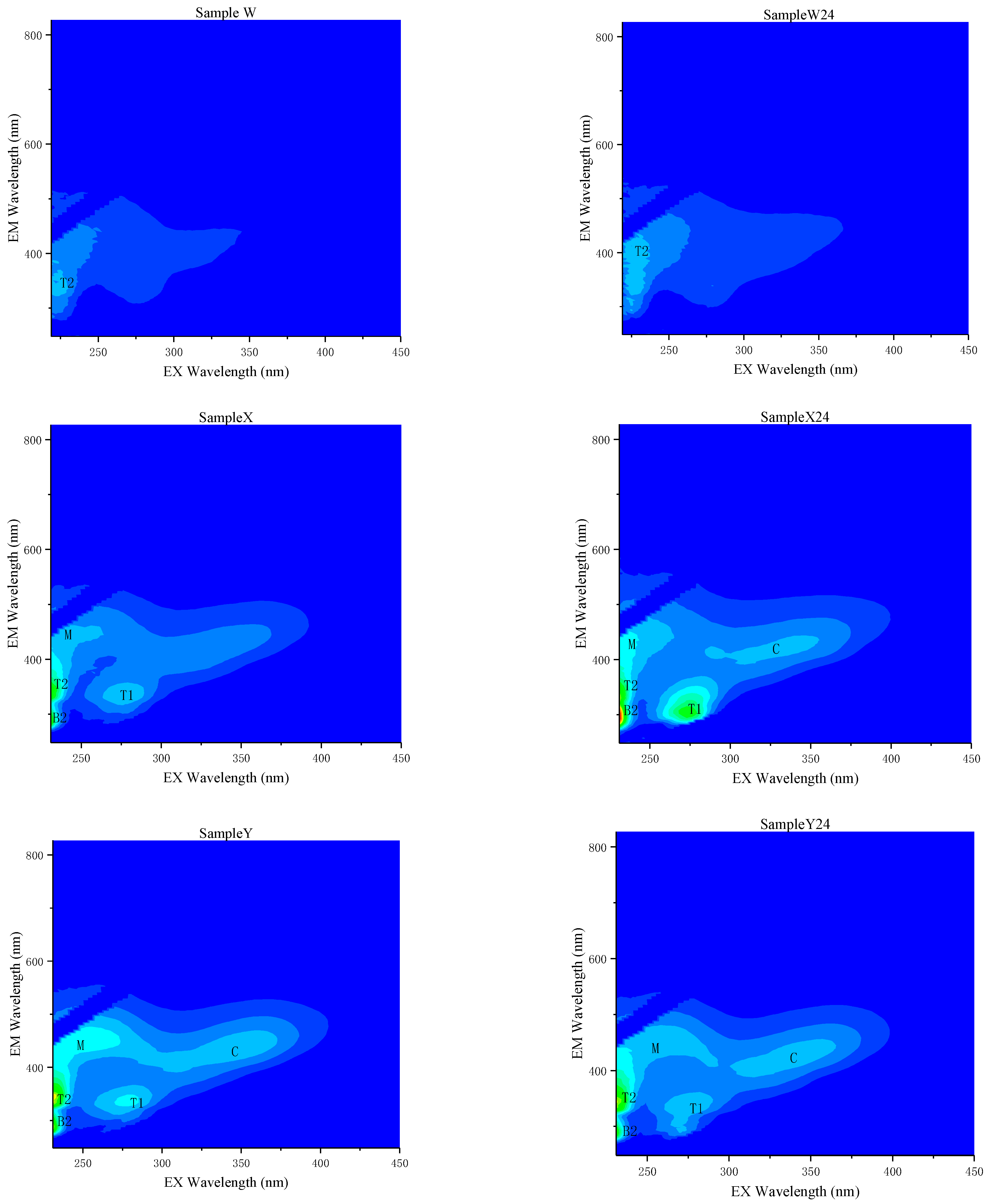
3.2. Fluorescence Characteristics of CBM Produced Water
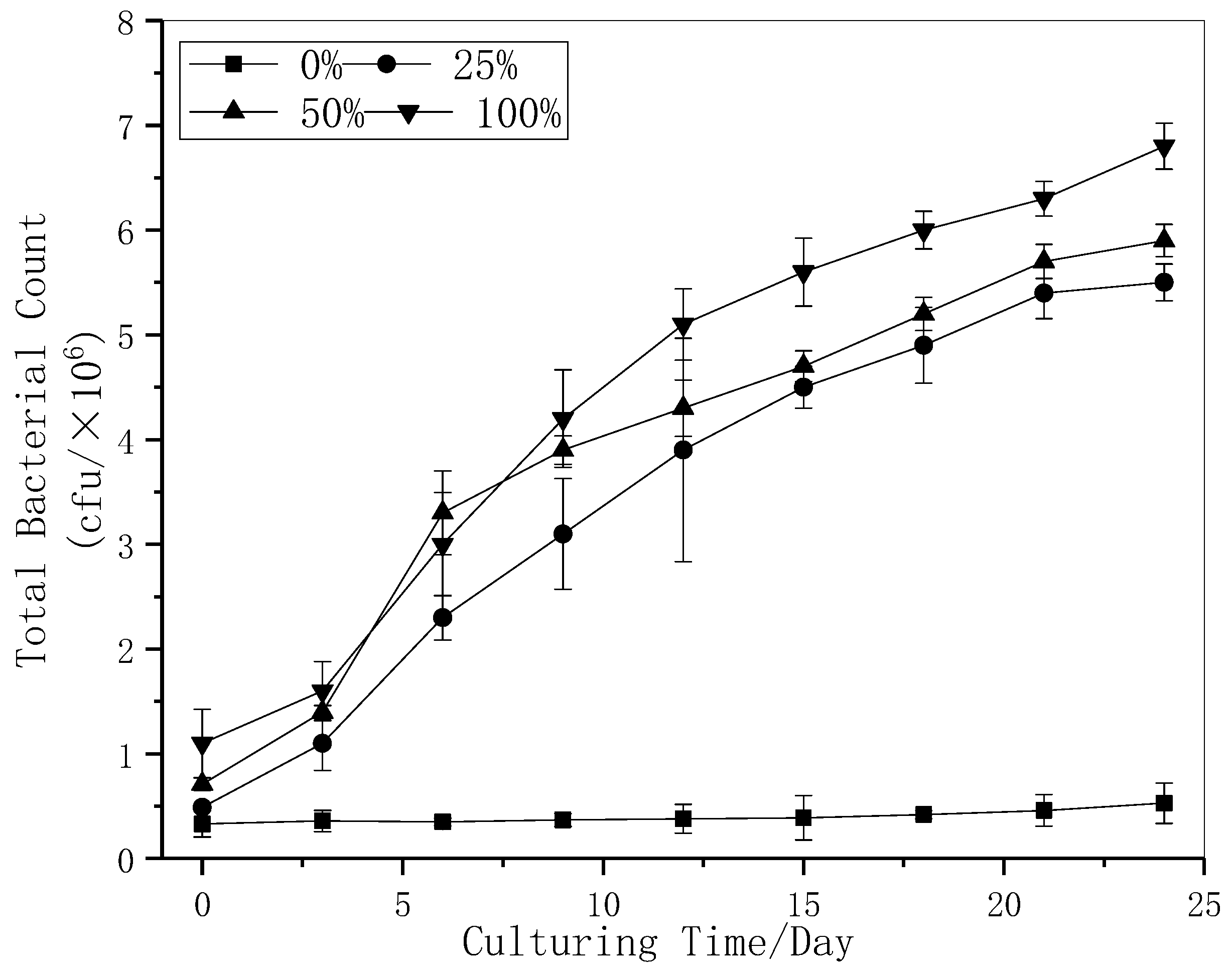
3.3. Bacterial Culture Experiment Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teng, J.-W.; Wang, Y.-C.; Si, X.; Liu, S.-H.; Wang, Y.-R. Diversified Transformation of Coal and Coalbed MethaneChina’s Fossil Energy Exploration, Development, Supply and Demand. Sci. Technol. Eng. 2021, 21, 9169–9193. [Google Scholar]
- Yaofeng, W. Current Situation and Prospect of Gas Extraction Technology and Equipment for Coal Mines in China. Saf. Coal Mines 2020, 51, 67–77. [Google Scholar]
- Bryner, G. Coalbed Methane Development in the Intermountain West: Producing Energy and Protecting Water. Wyo. Law Rev. 2017, 4, 541. [Google Scholar]
- Orem, W.; Tatu, C.; Varonka, M.; Lerch, H.; Bates, A.; Engle, M.; Crosby, L.; McIntosh, J. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale. Int. J. Coal Geol. 2014, 126, 20–31. [Google Scholar] [CrossRef]
- Jabbari, N.; Aminzadeh, F.; Barros, F. Hydraulic fracturing and the environment: Risk assessment for groundwater contamination from well casing failure. Stoch. Environ. Res. Risk Assess. 2016, 31, 1527–1542. [Google Scholar] [CrossRef]
- Yang, X.; Wen, G.; Lu, T.; Wang, B.; Yuan, G. Optimization and Field Application of CO2 Gas Fracturing Technique for Enhancing CBM Extraction. Nat. Resour. Res. 2019, 29, 1875–1896. [Google Scholar] [CrossRef]
- Stearman, W.; Taulis, M.; Smith, J.; Corkeron, M. Assessment of Geogenic Contaminants in Water Co-Produced with Coal Seam Gas Extraction in Queensland, Australia: Implications for Human Health Risk. Geosciences 2014, 4, 219–239. [Google Scholar] [CrossRef]
- Botner, E.C.; Townsend-Small, A.; Nash, D.B.; Xu, X.; Miller, J.H. Monitoring concentration and isotopic composition of methane in groundwater in the Utica Shale hydraulic fracturing region of Ohio. Environ. Monit. Assess. 2018, 190, 322. [Google Scholar] [CrossRef] [PubMed]
- Digiulio, D.C.; Jackson, R.B. Impact to Underground Sources of Drinking Water and Domestic Wells from Production Well Stimulation and Completion Practices in the Pavillion, Wyoming, Field. Environ. Sci. Technol. 2016, 50, 4524–4536. [Google Scholar] [CrossRef]
- Ganjegunte, G.K.; Vance, G.F.; King, L.A. Soil Chemical Changes Resulting from Irrigation with Water Co-Produced with Coalbed Natural Gas. J. Environ. Qual. 2005, 34, 2217–2227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vance, G.F.; King, L.A.; Ganjegunte, G.K. Soil and plant responses from land application of saline-sodic waters: Implications of management. J. Environ. Qual. 2008, 37, S139–S148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, Q.; Qian, B.; Meng, Q.; Li, X. Acute Toxicity and Ilnfluence of Antioxidant System of Coal Bed Methane Water on Carassius auratus. Environ. Sci. Technol. 2016, 39, 32–36. [Google Scholar]
- Li, X.; Bo, F.; Feng, Q.; Lai, Z.; Yue, S. Effects of Coalbed Methane Co-produced Water for Irrigation in China’s Qinshui Basin: An Experimental Field Study. Mine Water Environ. 2018, 37, 263–271. [Google Scholar] [CrossRef]
- Li, Y.; Tang, S.; Zhang, S.; Xi, Z.; Wang, P. Biogeochemistry and the associated redox signature of co-produced water from coalbed methane wells in the Shizhuangnan block in the southern Qinshui Basin, China. Energy Explor. Exploit. 2020, 38, 1034–1053. [Google Scholar] [CrossRef]
- Paerl, H.W.; Julianne, D.; Moisander, P.H.; Noble, R.T.; Piehler, M.F.; Pinckney, J.L.; Steppe, T.F.; Luke, T.; Valdes, L.M. Microbial indicators of aquatic ecosystem change: Current applications to eutrophication studies. Fems Microbiol. Ecol. 2010, 46, 233–246. [Google Scholar] [CrossRef]
- Saha, N.C.; Bhunia, F.; Kaviraj, A. Comparative Toxicity of Three Organic Acids to Freshwater Organisms and Their Impact on Aquatic Ecosystems. Hum. Ecol. Risk Assess. Int. J. 2006, 12, 192–202. [Google Scholar] [CrossRef]
- Marce, R.; Verdura, L.; Leung, N. Dissolved organic matter spectroscopy reveals a hot spot of organic matter changes at the river-reservoir boundary. Aquat. Sci. 2021, 83. [Google Scholar] [CrossRef]
- Mostofa, K.M.G.; Yoshioka, T.; Konohira, E.; Tanoue, E. Dynamics and characteristics of fluorescent dissolved organic matter in the groundwater, river and lake water. Water Air Soil Pollut. 2007, 184, 157–176. [Google Scholar] [CrossRef]
- Massicotte, P.; Frenette, J.J. Spatial connectivity in a large river system: Resolving the sources and fate of dissolved organic matter. Ecol. Appl. 2011, 21, 2600–2617. [Google Scholar] [CrossRef]
- Guo, L.; Lu, M.M.; Li, Q.Q.; Zhang, J.W.; Zong, Y.; She, Z.L. Three-dimensional fluorescence excitation-emission matrix (EEM) spectroscopy with regional integration analysis for assessing waste sludge hydrolysis treated with multi-enzyme and thermophilic bacteria. Bioresour. Technol. 2014, 171, 22–28. [Google Scholar] [CrossRef]
- Sgroi, M.; Roccaro, P.; Korshin, G.V.; Greco, V.; Sciuto, S.; Anumol, T.; Snyder, S.A.; Vagliasindi, F.G.A. Use of fluorescence EEM to monitor the removal of emerging contaminants in full scale wastewater treatment plants. J. Hazard. Mater. 2017, 323, 367–376. [Google Scholar] [CrossRef]
- Rodríguez-Vidal, F.J.; García-Valverde, M.; Ortega-Azabache, B.; González-Martínez, Á.; Bellido-Fernández, A. Characterization of urban and industrial wastewaters using excitation-emission matrix (EEM) fluorescence: Searching for specific fingerprints. J. Environ. Manag. 2020, 263, 110396. [Google Scholar] [CrossRef]
- Su, X.B.; Lin, X.Y.; Liu, S.B.; Zhao, M.J.; Song, Y. Geology of coalbed methane reservoirs in the Southeast Qinshui basin of China. Int. J. Coal Geol. 2005, 62, 197–210. [Google Scholar] [CrossRef]
- Chen, S.; Tao, S.; Tian, W.; Tang, D.; Zhang, B.; Liu, P. Hydrogeological control on the accumulation and production of coalbed methane in the Anze Block, southern Qinshui Basin, China. J. Pet. Sci. Eng. 2021, 198, 108138. [Google Scholar] [CrossRef]
- Mitra, B.K.; Sasaki, C.; Enari, K.; Matsuyama, N.; Pongpattanasiri, S. Suitability Assessment of Shallow Groundwater for Irrigation in Sand Dune Area of Northwest Honshu Island, Japan. Int. J. Agric. Res. 2007, 2, 518–527. [Google Scholar]
- Gran, G. Determination of the equivalence point in potentiometric titrations. Part II. Analyst 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Kanadhia, K.C.; Ramavataram, D.V.S.S.; Nilakhe, S.P.D.; Patel, S. A study of water hardness and the prevalence of hypomagnesaemia and hypocalcaemia in healthy subjects of Surat district (Gujarat). Magnes. Res. 2014, 27, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation—Emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Poojamnong, K.; Tungsudjawong, K.; Khongnakorn, W.; Jutaporn, P. Characterization of reversible and irreversible foulants in membrane bioreactor (MBR) for eucalyptus pulp and paper mill wastewater treatment using fluorescence regional integration. J. Environ. Chem. Eng. 2020, 8, 10. [Google Scholar] [CrossRef]
- Cy, A.; Hl, A.; Ys, A.; Xian, H.A.; Mn, A.; Qi, H.A.; Mb, B. Fluorescence characterization of fractionated dissolved organic matter in the five tributaries of Poyang Lake, China. Sci. Total Environ. 2018, S637–638, 1311–1320. [Google Scholar]
- Miller, M.P.; McKnight, D.M. Comparison of seasonal changes in fluorescent dissolved organic matter among aquatic lake and stream sites in the Green Lakes Valley. J. Geophys. Res.-Biogeosci. 2010, 115, 14. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Lavonen, E.E.; Kothawala, D.N.; Tranvik, L.J.; Gonsior, M.; Schmitt-Kopplin, P.; Köhler, S.J. Tracking changes in the optical properties and molecular composition of dissolved organic matter during drinking water production. Water Res. 2015, 85, 286–294. [Google Scholar] [CrossRef]
- Ohno, T.; Fernandez, I.J.; Hiradate, S.; Sherman, J.F. Effects of soil acidification and forest type on water soluble soil organic matter properties. Geoderma 2007, 140, 176–187. [Google Scholar] [CrossRef]
- BSI. Water Quality—Enumeration Of Culturable Microorganisms—Population Method For Inoculation In Nutrient Agar Medium; BS EN ISO 6222-1999, 8P; BSI: London, UK, 1999; 4p. [Google Scholar]
- Guo, X.P.; Zhang, M.M.; Gao, Y.; Li, W.J.; Lu, D. Determining survival fractions of Saccharomyces cerevisiae in response to ionizing radiation in liquid culture. J. Radiat. Res. 2018, 59, 760–764. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Li, Z.; Guo, Q.; Pan, Z. Stable isotope characteristics of CBM co-produced water and implications for CBM development: The example of the Shizhuangnan block in the southern Qinshui Basin, China. J. Nat. Gas Sci. Eng. 2015, 27, 1400–1411. [Google Scholar] [CrossRef]
- Institute of Environmental Standards; CEPA (Beijing); Hangzhou Institute of Environmental Protection; The General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Emission Standard for Pollutants from Coal Industry; GB 20426-2006; China Environmental Science Publishing House: Beijing, China, 2006; 11p. [Google Scholar]
- Voast, V.; Wayne, A. Geochemical signature of formation waters associated with coalbed methane. AAPG Bull. 2003, 87, 667–676. [Google Scholar] [CrossRef]
- Cheung, K.; Sanei, H.; Klassen, P.; Mayer, B.; Goodarzi, F. Produced fluids and shallow groundwater in coalbed methane (CBM) producing regions of Alberta, Canada: Trace element and rare earth element geochemistry. Int. J. Coal Geol. 2009, 77, 338–349. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Li, Z.; Pan, Z.; Shi, W. Study of hydrochemical characteristics of CBM co-produced water of the Shizhuangnan Block in the southern Qinshui Basin, China, on its implication of CBM development. Int. J. Coal Geol. 2016, 159, 169–182. [Google Scholar] [CrossRef]
- Wang, H.; Fu, X.; Zhang, X.; Niu, Q.; Ge, Y.; Tian, J.; Cheng, X.; Chen, N.; Hou, X.; Du, H. Source, Age, and Evolution of Coal Measures Water in Central-South Qinshui Basin, China. Energy Fuels 2018, 32, 7358–7373. [Google Scholar] [CrossRef]
- Houatmia, F.; Azouzi, R.; Charef, A.; Bedir, M. Assessment of groundwater quality for irrigation and drinking purposes and identification of hydrogeochemical mechanisms evolution in Northeastern, Tunisia. Environ. Earth Sci. 2016, 75, 746. [Google Scholar] [CrossRef]
- Dalmagro, H.J.; Lathuilliere, M.J.; Sallo, F.D.S.; Guerreiro, M.F.; Johnson, M.S. Streams with Riparian Forest Buffers versus Impoundments Differ in Discharge and DOM Characteristics for Pasture Catchments in Southern Amazonia. Water 2019, 11, 390. [Google Scholar] [CrossRef]
- Liu, C.; Du, Y.; Yin, H.; Fan, C.; Chen, K.; Zhong, J.; Gu, X. Exchanges of nitrogen and phosphorus across the sediment-water interface influenced by the external suspended particulate matter and the residual matter after dredging. Environ. Pollut. 2019, 246, 207–216. [Google Scholar] [CrossRef]
- Tomco, P.L.; Zulueta, R.C.; Miller, L.C.; Zito, P.A.; Welker, J. DOC export is exceeded by C fixation in May Creek: A late-successional watershed of the Copper River Basin, Alaska. PLoS ONE 2019, 14, e0225271. [Google Scholar] [CrossRef]
- Pitta, E.; Zeri, C. The impact of combining data sets of fluorescence excitation—Emission matrices of dissolved organic matter from various aquatic sources on the information retrieved by PARAFAC modeling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 5, 119800. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Kang, H.W.; Kim, S.H.; Kang, D.J. Tracing water mass fractions in the deep western Indian Ocean using fluorescent dissolved organic matter. Mar. Chem. 2020, 218, 103720. [Google Scholar] [CrossRef]
- Guo, W.; Jing, X.; Wang, J.; Wen, Y.; Zhuo, J.; Yan, Y. Characterization of dissolved organic matter in urban sewage using excitation emission matrix fluorescence spectroscopy and parallel factor analysis. J. Environ. Sci. 2010, 22, 1728–1734. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Yoshioka, T. Photobiogeochemistry of Organic Matter: Principles and Practices in Water Environments (Environmental Science and Engineering); Springer: New York City, NY, USA, 2013. [Google Scholar]
- Cabrera, J.M.; García, P.; Pedrozo, F.L.; Queimaliños, C. Dynamics of the dissolved organic matter in a stream-lake system within an extremely acid to neutral pH range: Agrio-Caviahue watershed. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118278. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, T.H.; Sang, R.P.; Lee, H.J.; Kim, J.K. Factors controlling the distributions of dissolved organic matter in the East China Sea during summer. Sci. Rep. 2020, 10, 11854. [Google Scholar] [CrossRef]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Beckmann, S.; Luk, A.; Gutierrez-Zamora, M.L.; Chong, N.; Thomas, T.; Lee, M.; Manefield, M. Long-term succession in a coal seam microbiome during in situ biostimulation of coalbed-methane generation. ISME J. 2018, 13, 632–650. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption Spectral Slopes and Slope Ratios as Indicators of Molecular Weight, Source, and Photobleaching of Chromophoric Dissolved Organic Matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Formolo, M.; Martini, A.; Petsch, S. Biodegradation of sedimentary organic matter associated with coalbed methane in the Powder River and San Juan Basins, U.S.A. Int. J. Coal Geol. 2008, 76, 86–97. [Google Scholar] [CrossRef]
- Strąpoć, D.; Mastalerz, M.; Dawson, K.; Macalady, J.; Callaghan, A.V.; Wawrik, B.; Turich, C.; Ashby, M. Biogeochemistry of Microbial Coal-Bed Methane. Annu. Rev. Earth Planet. Sci. 2011, 39, 617–656. [Google Scholar] [CrossRef]
- Huang, J.; Ling, L.; Zhang, H.; Ling, Q.; Liu, P.R. Fluorescence spectra of dissolved organic matter in food wastewater treatment by ASBR process. China Environ. Sci. 2016, 36, 1746–1751. [Google Scholar]
- Chen, M.; Li, C.; Spencer, R.G.M.; Maie, N.; Hur, J.; McKenna, A.M.; Yan, F. Climatic, land cover, and anthropogenic controls on dissolved organic matter quantity and quality from major alpine rivers across the Himalayan-Tibetan Plateau. Sci. Total Environ. 2021, 754, 142411. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, T.; Dong, H.; Wang, Y.; Yang, H.; Qiang, Z. Tracking spatio-temporal dynamics of fluorescence characteristics of Huangpu River, China by parallel factor analysis: Correlation with disinfection by-product precursor and pesticide level variations. Chemosphere 2021, 283, 131198. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.; Baker, A.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]

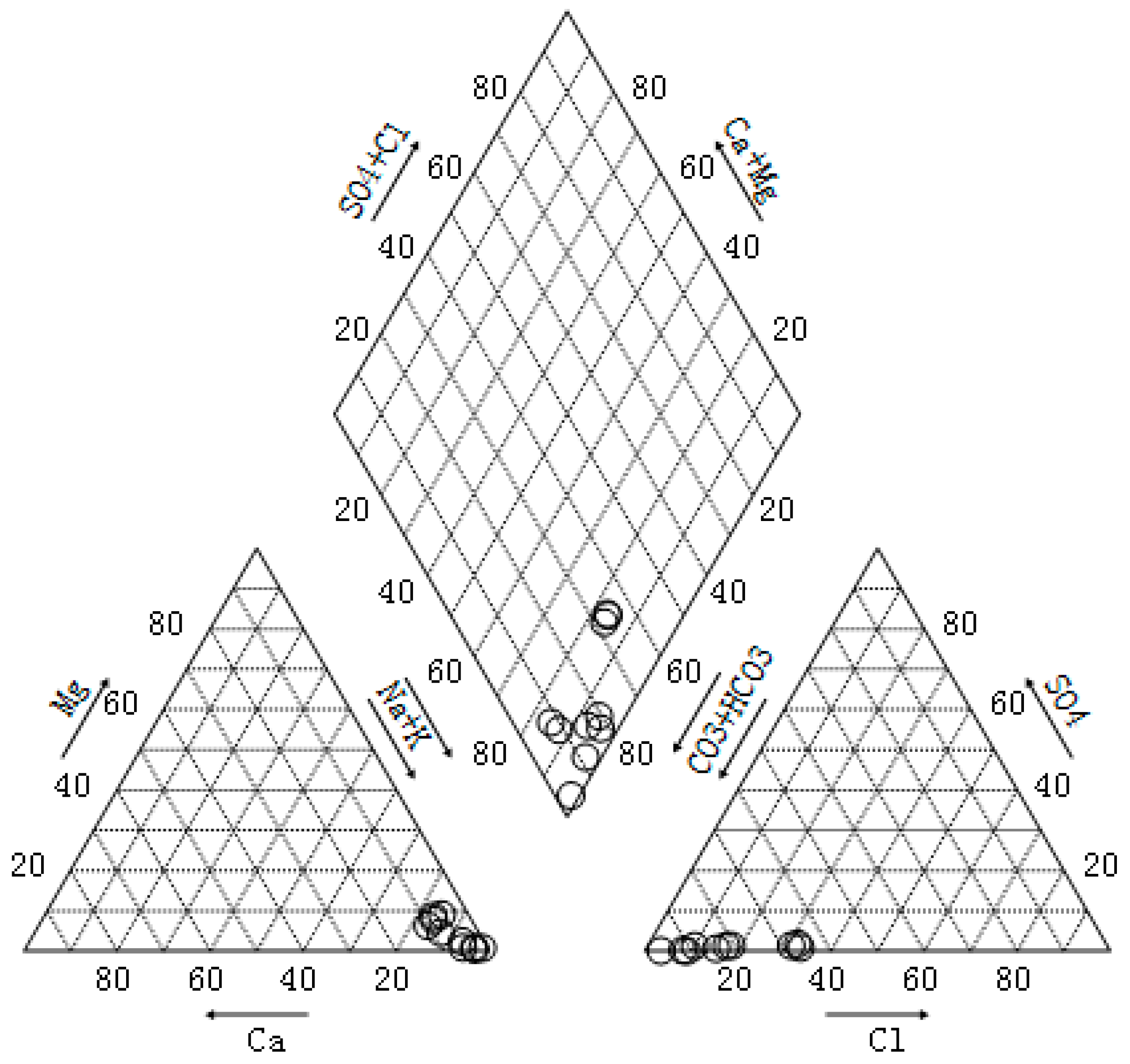
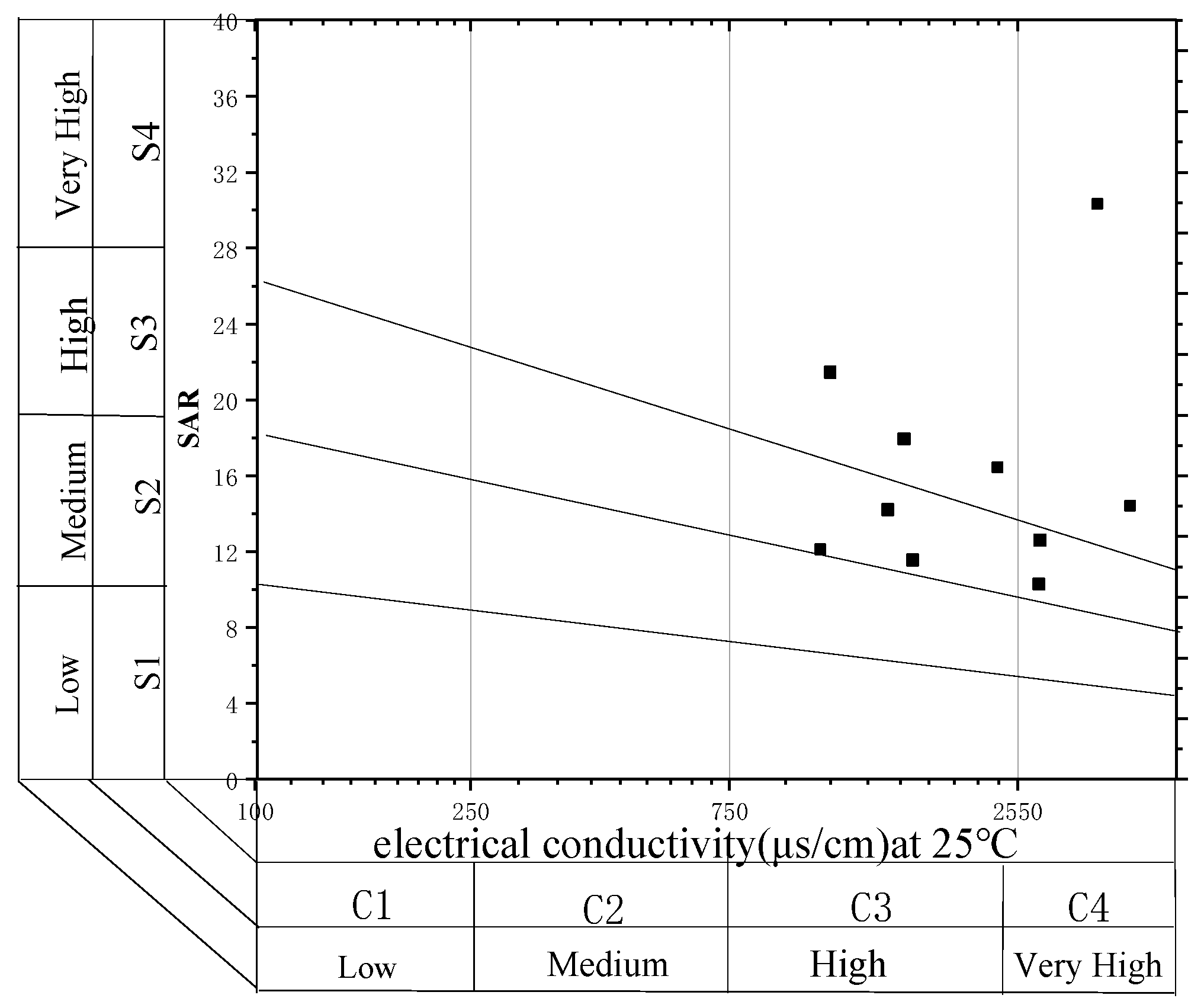

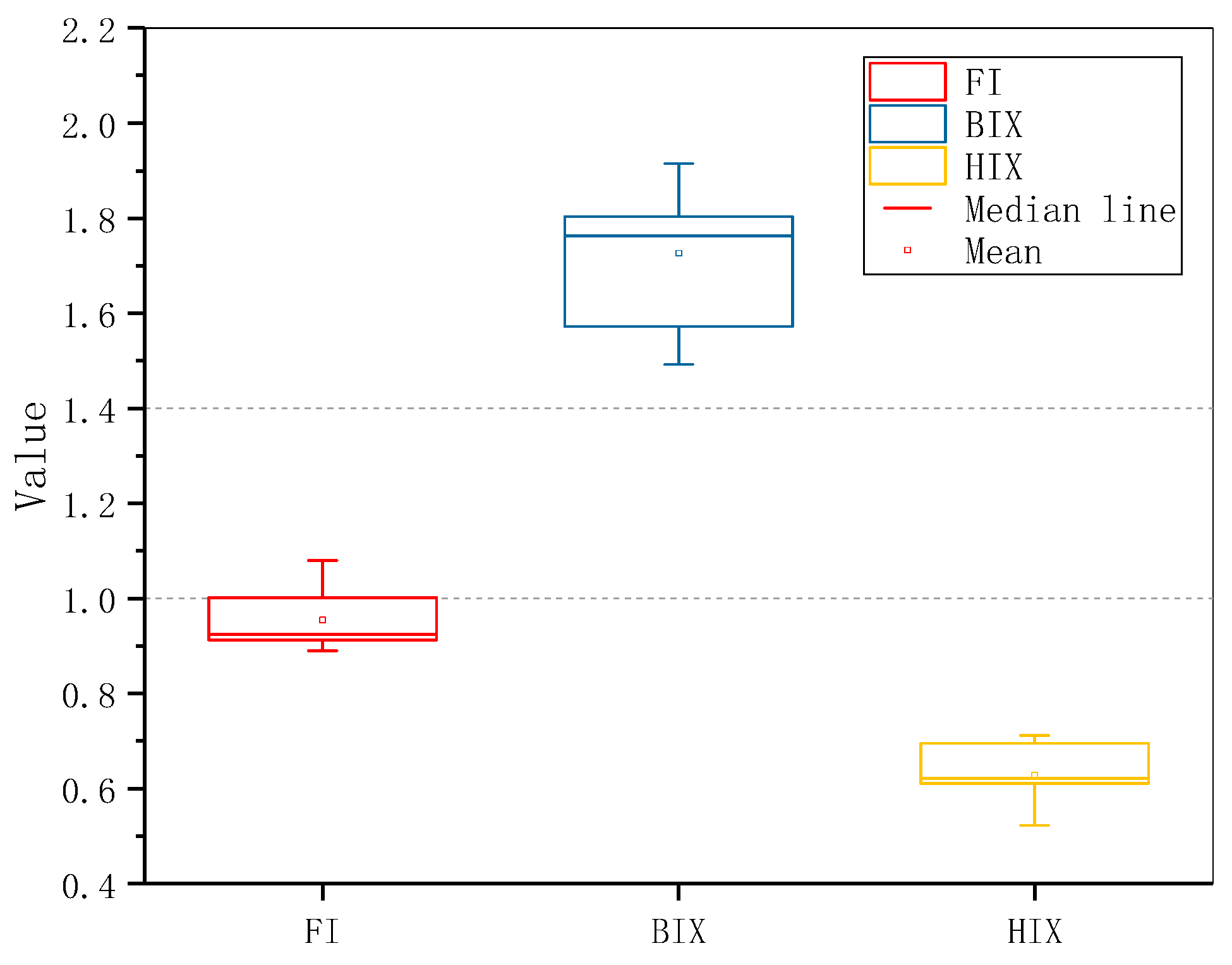


| Sample | Total Hardness (mg/L) | Total Alkalinity (mg/L) | Electrical Conductivity (μs/cm) | pH | TDS (mg/L) | SS (mg/L) | COD (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 23.45 ± 0.27 | 997.43 ± 4.00 | 4107 ± 6 | 8.34 ± 0.01 | 3880 ± 17 | 246.3 ± 4.8 | 2680 ± 4 |
| 2 | 9.57 ± 0.43 | 735.23 ± 7.05 | 2340 ± 3 | 8.98 ± 0.02 | 2690 ± 10 | 326.8 ± 3.0 | 2458 ± 10 |
| 3 | 65.32 ± 0.92 | 543.23 ± 3.41 | 1573 ± 6 | 8.63 ± 0.01 | 1200 ± 10 | 349.4 ± 1.2 | 2430 ± 6 |
| 4 | 32.45 ± 1.44 | 456.77 ± 4.67 | 1150 ± 10 | 8.84 ± 0.02 | 1400 ± 12 | 308.6 ± 3.5 | 2558 ± 3 |
| 5 | 46.34 ± 1.10 | 677.96 ± 7.00 | 3570 ± 6 | 8.70 ± 0.02 | 2700 ± 44 | 338.4 ± 0.4 | 2460 ± 3 |
| 6 | 37.98 ± 1.66 | 934.22 ± 5.27 | 2789 ± 5 | 8.55 ± 0.03 | 2560 ± 17 | 230.3 ± 1.4 | 2498 ± 5 |
| 7 | 25.32 ± 2.36 | 718.97 ± 3.91 | 1630 ± 7 | 8.73 ± 0.03 | 1898 ± 17 | 328.5 ± 7.3 | 2520 ± 4 |
| 8 | 97.33 ± 2.28 | 567.89 ± 6.16 | 1103 ± 8.6 | 8.65 ± 0.01 | 890 ± 26 | 320.4 ± 4.4 | 2570 ± 4.7 |
| 9 | 46.34 ± 1.23 | 489.34 ± 1.91 | 1468 ± 6.2 | 8.83 ± 0.02 | 1180 ± 26 | 306.4 ± 2.7 | 2376 ± 2.0 |
| 10 | 48.22 ± 1.30 | 712.65 ± 4.31 | 2800 ± 8.5 | 8.96 ± 0.01 | 2350 ± 44 | 349.8 ± 3.4 | 2550 ± 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, T.; Meng, Q.; Li, X.; Zhou, L. Fluorescence Characteristics of Coalbed Methane Produced Water and Its Influence on Freshwater Bacteria in the South Qinshui Basin, China. Int. J. Environ. Res. Public Health 2021, 18, 12921. https://doi.org/10.3390/ijerph182412921
Jin T, Meng Q, Li X, Zhou L. Fluorescence Characteristics of Coalbed Methane Produced Water and Its Influence on Freshwater Bacteria in the South Qinshui Basin, China. International Journal of Environmental Research and Public Health. 2021; 18(24):12921. https://doi.org/10.3390/ijerph182412921
Chicago/Turabian StyleJin, Tao, Qingjun Meng, Xiangdong Li, and Lai Zhou. 2021. "Fluorescence Characteristics of Coalbed Methane Produced Water and Its Influence on Freshwater Bacteria in the South Qinshui Basin, China" International Journal of Environmental Research and Public Health 18, no. 24: 12921. https://doi.org/10.3390/ijerph182412921
APA StyleJin, T., Meng, Q., Li, X., & Zhou, L. (2021). Fluorescence Characteristics of Coalbed Methane Produced Water and Its Influence on Freshwater Bacteria in the South Qinshui Basin, China. International Journal of Environmental Research and Public Health, 18(24), 12921. https://doi.org/10.3390/ijerph182412921






