Prevalence of Anaemia, Iron Deficiency, and Iron Deficiency Anaemia in Women of Reproductive Age and Children under 5 Years of Age in South Africa (1997–2021): A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Source
2.2. Operationalization of Variables
2.3. Eligibility Criteria
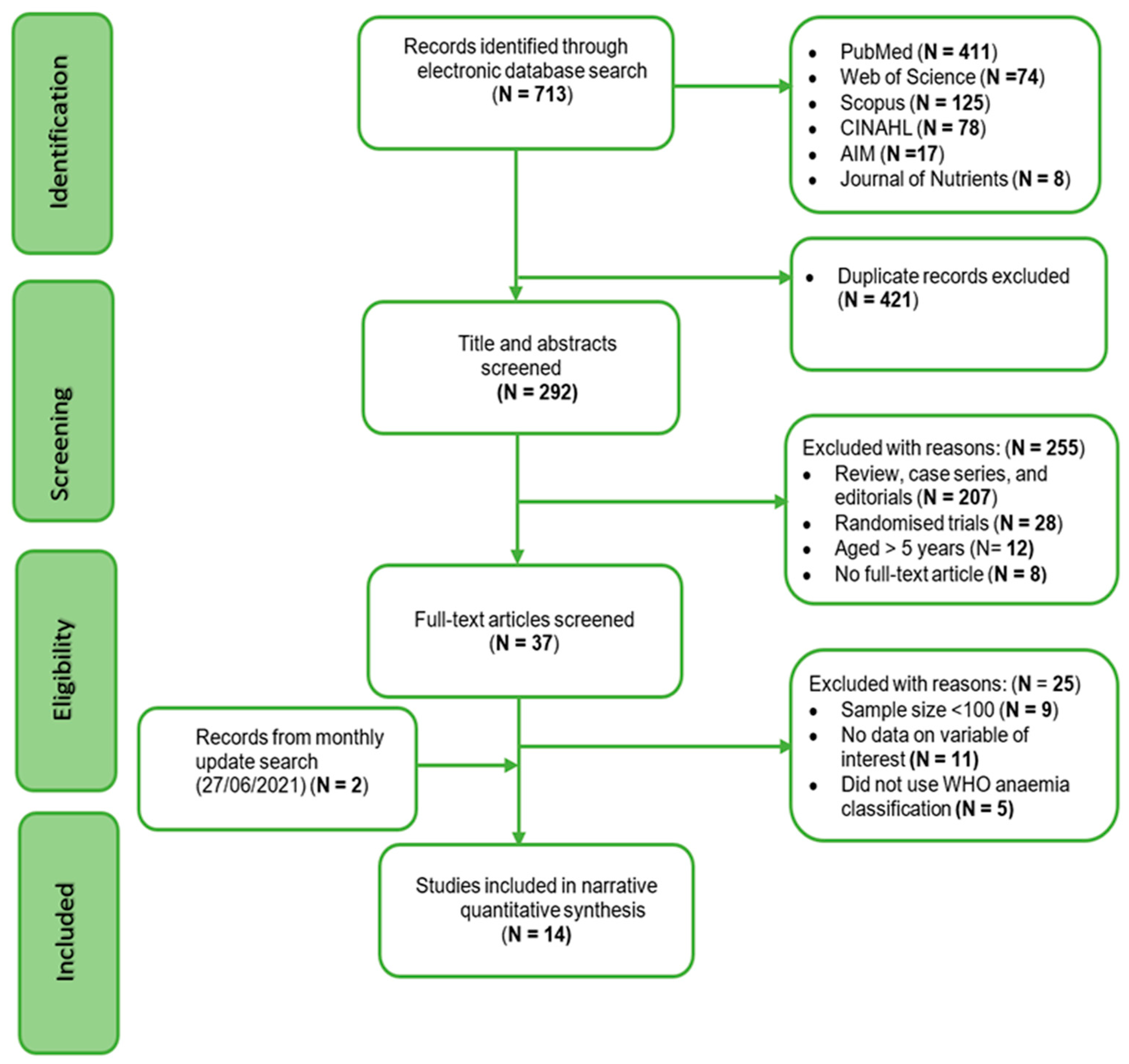
2.4. Study Selection
2.5. Quality Assessment and Data Extraction
2.6. Data Synthesis and Analyses
2.7. Patient and Public Involvement
3. Results
3.1. Study Results
3.2. Characteristics of Included Studies Are Summarised in Tables 1 and 2
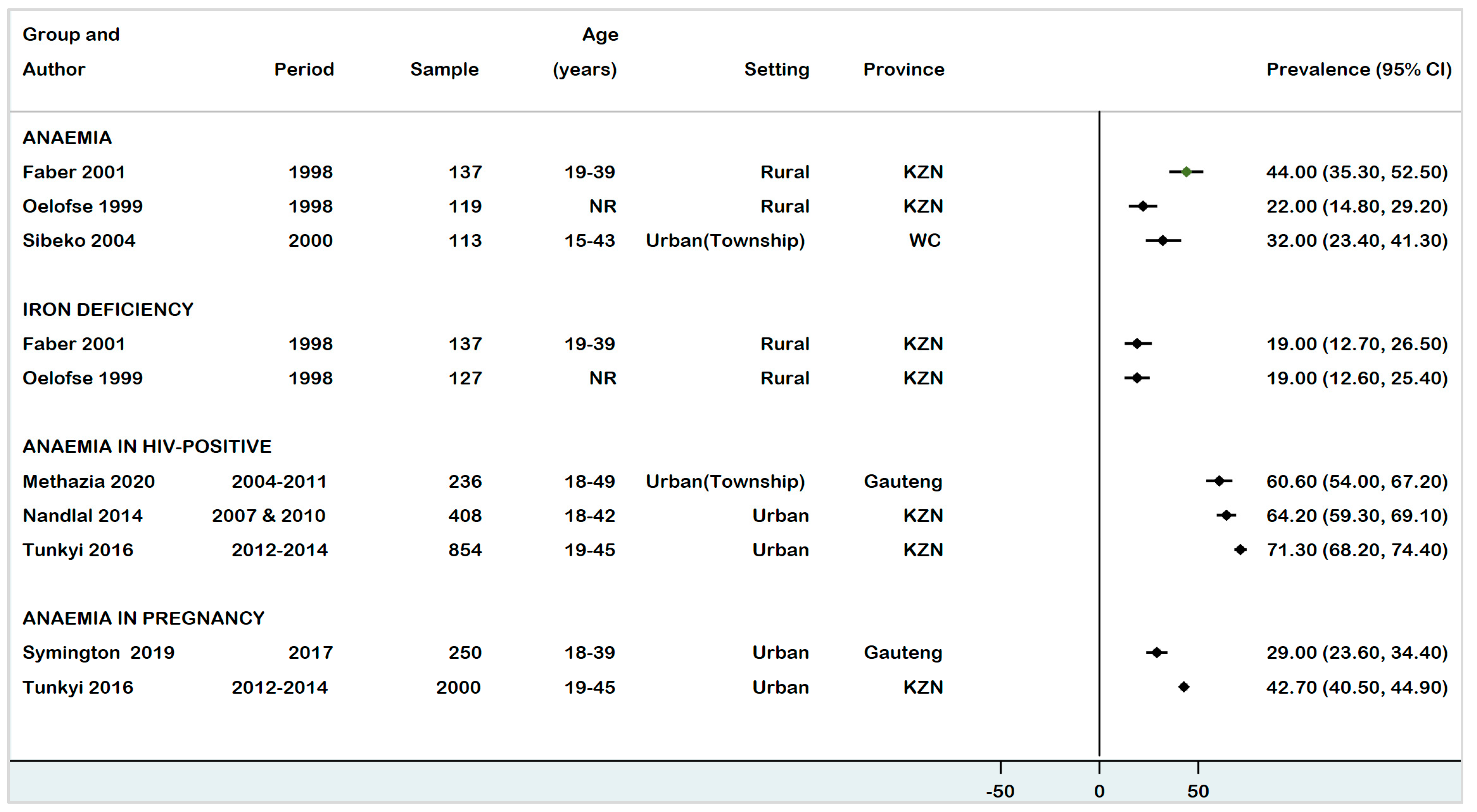
3.3. Prevalence of Anaemia, ID, and IDA in WRA (15–49 Years)
3.3.1. Anaemia
3.3.2. Iron Deficiency
3.3.3. Iron Deficiency Anaemia
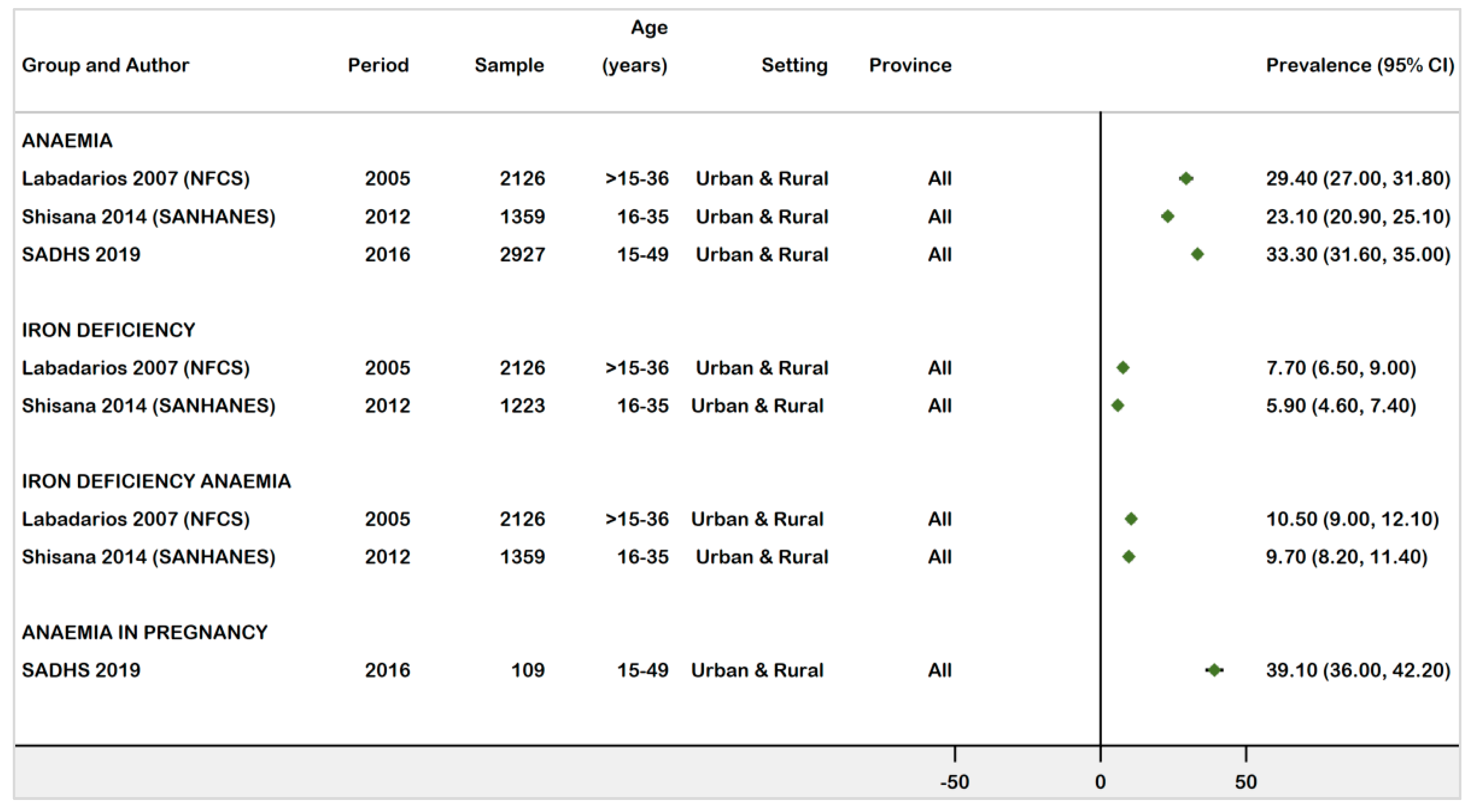
3.3.4. National Prevalence Estimates of Anaemia, ID, and IDA in WRA (1997−2021)
3.4. Prevalence Estimate in Children under 5 years
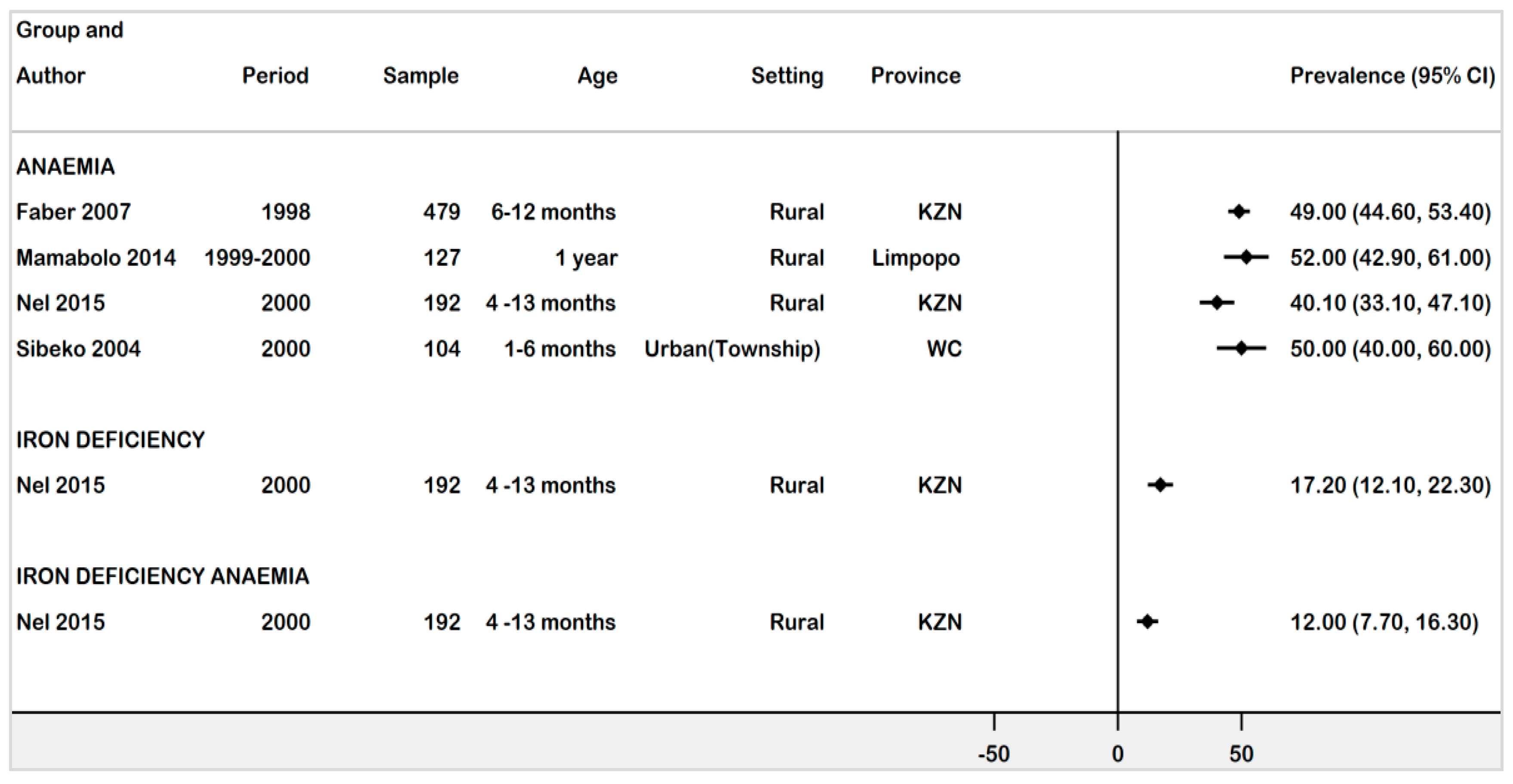
3.4.1. Prevalence of Anaemia, ID, and IDA in Infants Age 1 Month -13 Months
3.4.2. Prevalence of Anaemia, ID, and IDA in Children Age 24 -59 Months
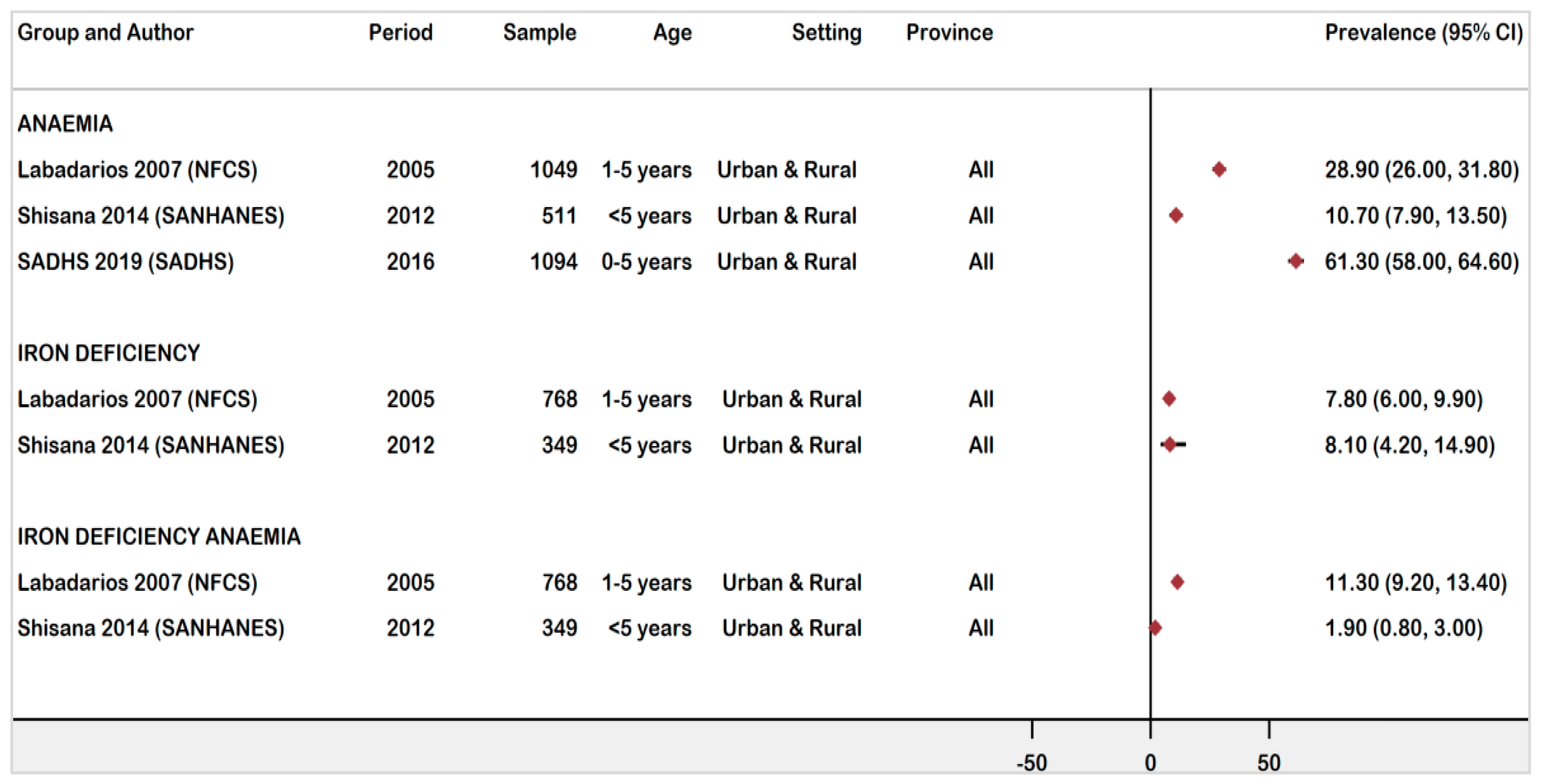
3.4.3. National Prevalence of Anaemia, ID, and IDA Children Ages 0 -5 Years Old
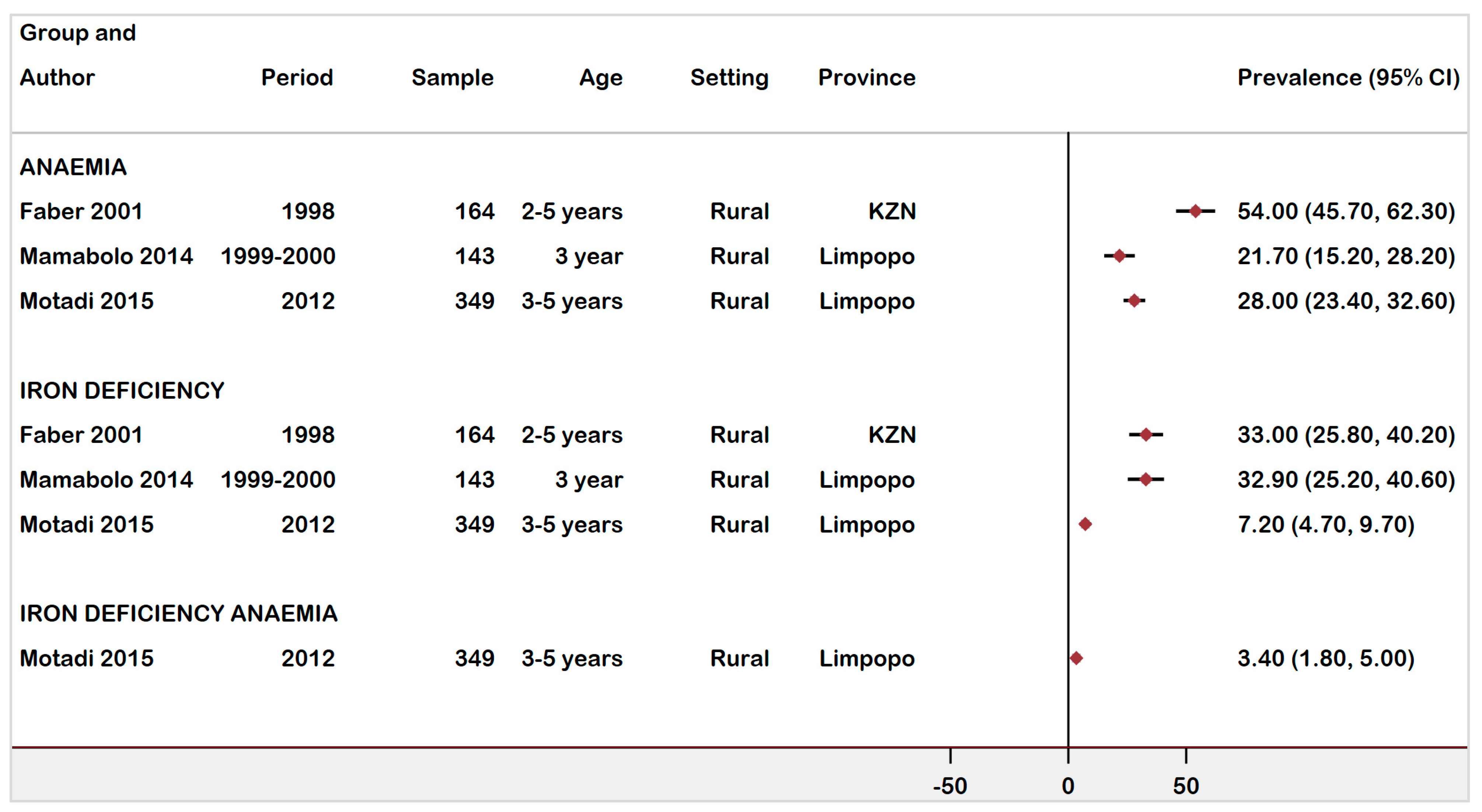
3.5. Anaemia Prevalence in Rural vs. Urban Settings in Children under 5 Years
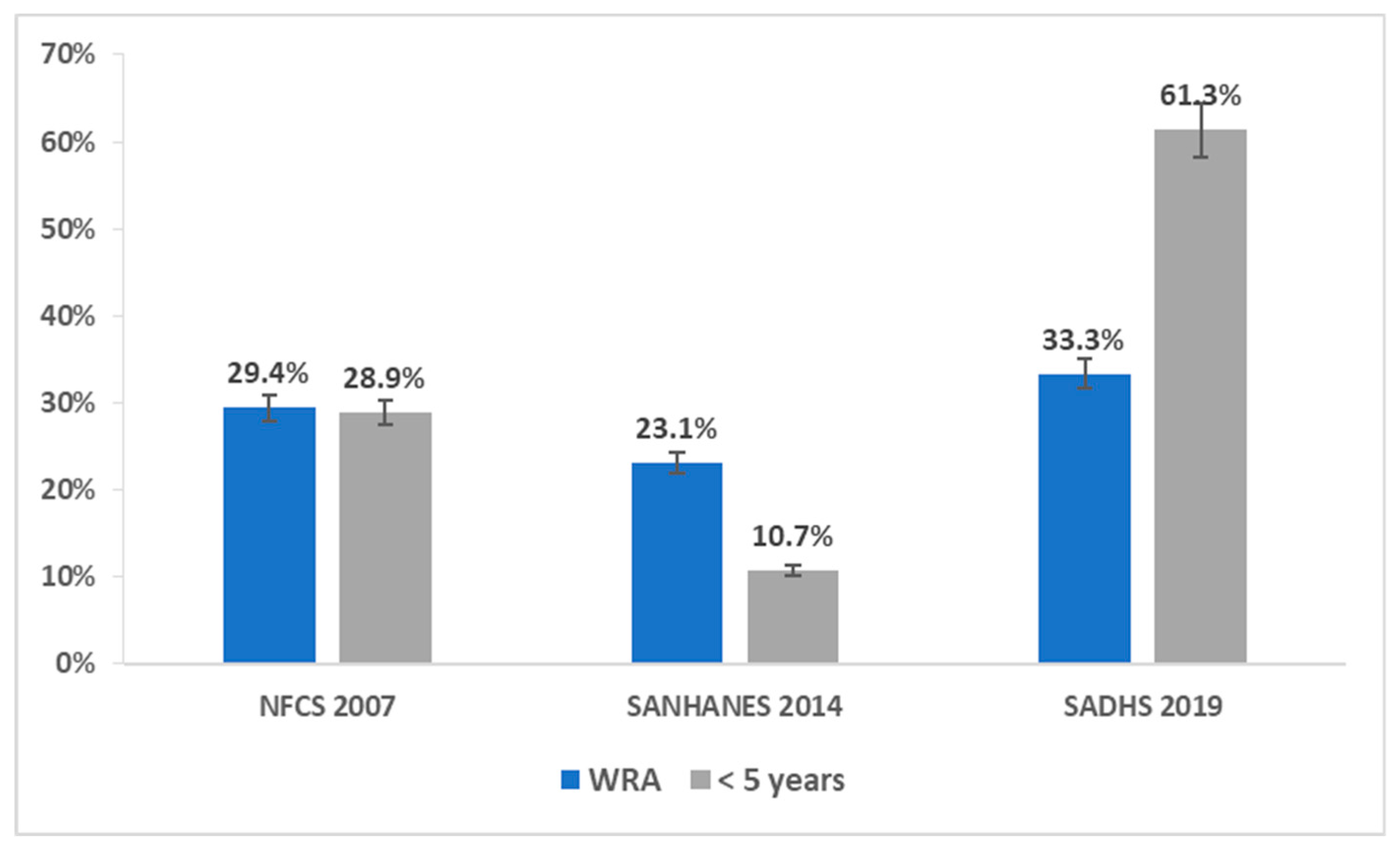
3.6. Comparison of Prevalence of Anaemia in WRA and Children under 5 Years of Age
4. Discussion
4.1. Prevalence of Anaemia, ID, and IDA in WRA
4.2. Prevalence of Anaemia, ID, and IDA in Children under 5 Years
4.3. Strengths and Limitations of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stephen, G.; Mgongo, M.; Hashim, T.H.; Katanga, J.; Stray-Pedersen, B.; Msuya, S.E. Anaemia in Pregnancy: Prevalence, Risk Factors, and Adverse Perinatal Outcomes in Northern Tanzania. Anemia 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; De Benoist, B., McLean, E., Egli, I., Cogswell, M., Eds.; WHO Press: Geneva, Switzerland, 2008; Available online: https://apps.who.int/iris/handle/10665/43894 (accessed on 29 September 2021).
- World Health Organization. Anaemia in Women and Children: WHO Global Anaemia Estimates, 2021 Edition; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 29 September 2021).
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Black, R.e.; Victora, C.g.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- World Health Organization. The Global Prevalence of Anaemia in 2011; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/handle/10665/177094 (accessed on 29 September 2021).
- Gautam, S.; Min, H.; Kim, H.; Jeong, H.-S. Determining factors for the prevalence of anemia in women of reproductive age in Nepal: Evidence from recent national survey data. PLoS ONE 2019, 14, e0218288. [Google Scholar] [CrossRef]
- Simbauranga, R.H.; Kamugisha, E.; Hokororo, A.; Kidenya, B.R.; Makani, J. Prevalence and factors associated with severe anaemia amongst under-five children hospitalized at Bugando Medical Centre, Mwanza, Tanzania. BMC Hematol. 2015, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, R.J.; Mullany, M.L.; Black, R.E. Iron deficiency anaemia. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Lopez, D., Rodgers, A., Murray, C.J.L., Eds.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Southern African Development Community Annual Report 2020–2021. Available online: https://www.sadc.int/documents-publications/annual-reports/ (accessed on 22 November 2021).
- World Health Organization. Global Nutrition Targets 2025: Anaemia Policy Brief; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/148556 (accessed on 29 September 2021).
- Sunguya, B.F.; Zhu, S.; Paulo, L.S.; Ntoga, B.; Abdallah, F.; Assey, V.; Mpembeni, R.; Huang, J. Regional Disparities in the Decline of Anemia and Remaining Challenges among Children in Tanzania: Analyses of the Tanzania Demographic and Health Survey 2004–2015. Int. J. Environ. Res. Public Health 2020, 17, 3492. [Google Scholar] [CrossRef] [PubMed]
- Kozuki, N.; Lee, A.C.; Katz, J. Child Health Epidemiology Reference Group. Moderate to Severe, but Not Mild, Maternal Anemia Is Associated with Increased Risk of Small-for-Gestational-Age Outcomes. J. Nutrition. 2011, 142, 358–362. [Google Scholar]
- Department of Health, Republic of South Africa. Regulations Relating to the Fortification of Certain Foodstuffs (No. R. 504 of 2003); Department of Health, Republic of South Africa: Pretoria, South Africa, 2003; pp. 3–26. [Google Scholar]
- Hoque, M.; Kader, S.B.; Hoque, E. Prevalence of Anaemia in Pregnancy at Uthungulu Health district of KwaZulu-Natal, South Africa-2003. S. Afr. Fam. Pract. 2007, 14, 16. [Google Scholar] [CrossRef]
- Phatlhane, D.V.; Zemlin, A.E.; Matsha, T.E.; Hoffmann, M.; Naidoo, N.; Ichihara, K.; Smit, F.; Erasmus, R.T. The iron status of a healthy South African adult population. Clin. Chim. Acta 2016, 460, 240–245. [Google Scholar] [CrossRef]
- National Department of Health (NDoH); Statistics South Africa (Stats SA); South African Medical Research Council (SAMRC); ICF. South Africa Demographic and Health Survey 2016; NDOH: Pretoria, South Africa; Rockville, MD, USA, 2019. [Google Scholar]
- Labadarios, D.; Swart, R.; Maunder, E.; Kruger, H.; Gericke, G.; Kuzwayo, P.; Ntsie, P.; Steyn, N.; Schloss, I.; Dhansay, M.; et al. The National Food Consumption Survey Fortification Baseline (NFCS-FB). S. Afr. J.Clin. Nutr. 2008, 21, 246–271. [Google Scholar]
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A. The South African National Health and Nutrition Examination Survey 2014 SANHANES-1: The Health and Nutritional Status of the Nation; HSRC Press: Cape Town, South Africa, 2014. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed on 29 September 2021).
- World Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications-detail-redirect/9789240000124 (accessed on 29 September 2021).
- Pillay-van Wyk, V.; Roomaney, R.A.; Awotiwon, O.F.; Turawa, E.; Nglazi, M.D.; Ebrahim, A.H.; Glass, T.; Bradshaw, D. Burden of Disease Review Manager for Systematic Review of Observational Studies; Technical Report and User Guide; South African Medical Research Council: Cape Town, South Africa, 2018. [Google Scholar]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; Peterson, J.; Welch, V.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Health Research Institute: Canada, Ottawa, 2009; Available online: http://www.ohri.ca/programs/clinicalepidemiology/oxford.Asp (accessed on 29 September 2021).
- StataCorp. Stata Statistical Software College Station. TX: StataCorp LLC. Available online: https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/ (accessed on 29 September 2021).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Faber, M.; Benadé, A.S. Factors associated with low serum retinol levels in children aged 6–24 months in a rural South African community. Publ. Health Nutr. 2000, 3, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Jogessar, V.B.; Benadé, A.J.S. Nutritional status and dietary intakes of children aged 2–5 years and their caregivers in a rural South African community. Int. J. Food Sci. Nutr. 2001, 52, 401–411. [Google Scholar] [PubMed]
- Motadi, S.A.; Mbhenyane, X.G.; Mbhatsani, H.V.; Mabapa, N.S.; Mamabolo, R.L. Prevalence of iron and zinc deficiencies among preschool children ages 3 to 5 y in Vhembe district, Limpopo province, South Africa. Nutrition 2015, 31, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Nel, E.; Kruger, H.S.; Baumgartner, J.; Faber, M.; Smuts, C.M. Differential ferritin interpretation methods that adjust for inflammation yield discrepant iron deficiency prevalence. Matern. Child Nutr. 2015, 11, 221–228. [Google Scholar] [CrossRef]
- Oelofse, A.; Faber, M.; Benade, J.G.; Benade, A.J.; Kenoyer, D.G. The nutritional status of a rural community in KwaZulu-Natal, South Africa: The Ndunakazi project. Cent. Afr. J. Med. 1999, 45, 14–19. [Google Scholar] [CrossRef]
- Sibeko, L.; Dhansay, M.; Charlton, K.; Johns, T.; van Stuijvenberg, M.; Gray-Donald, K.; Charlton, K. Full-term, peri-urban South African infants under 6 months of age are at risk for early-onset anaemia. Publ. Health Nutr. 2004, 7, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Tunkyi, K.; Moodley, J. Prevalence of anaemia in pregnancy in a regional health facility in South Africa. S. Afr. Med. J. 2015, 106, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Mamabolo, R.L.; Alberts, M. Prevalence of anaemia and its associated factors in African children at one and three years residing in the Capricorn District of Limpopo Province, South Africa. Curationis 2014, 37, 1–9. [Google Scholar] [CrossRef][Green Version]
- Methazia, J.; Ngamasana, E.L.; Utembe, W.; Ogunrombi, M.; Nyasulu, P. An investigation of maternal anaemia among HIV infected pregnant women on antiretroviral treatment in Johannesburg, South Africa. Pan Afr. Med. J. 2020, 37, 93. [Google Scholar] [CrossRef]
- Nandlal, V.; Moodley, D.; Grobler, A.; Bagratee, J.; Maharaj, N.R.; Richardson, P. Anaemia in Pregnancy Is Associated with Advanced HIV Disease. PLoS ONE 2014, 9, e106103. [Google Scholar] [CrossRef] [PubMed]
- Symington, E.A.; Baumgartner, J.; Malan, L.; Wise, A.J.; Ricci, C.; Zandberg, L.; Smuts, C. Maternal iron-deficiency is associated with premature birth and higher birth weight despite routine antenatal iron supplementation in an urban South African setting: The NuPED prospective study. PLoS ONE 2019, 14, e0221299. [Google Scholar] [CrossRef]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, C.; Zeh, C.; Angira, F.; Opollo, V.; Akinyi, B.; Masaba, R.; Williamson, J.M.; Otieno, J.; Mills, L.A.; Lecher, S.L.; et al. Anaemia in HIV-infected pregnant women receiving triple antiretroviral combination therapy for prevention of mother-to-child transmission: A secondary analysis of the Kisumu breastfeeding study (KiBS). Trop. Med. Int. Health 2016, 21, 373–384. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory Data Repository: Prevalence of Anaemia in Women Estimates by Country; World Health Organization: Geneva, Switzerland, 2019; Available online: http://apps.who.int/gho/data/view.main.GSWCAH28v?lang=en (accessed on 29 September 2021).
- Yang, F.; Liu, X.; Zha, P. Trends in Socioeconomic Inequalities and Prevalence of Anemia Among Children and Nonpregnant Women in Low- and Middle-Income Countries. JAMA Netw. Open 2018, 1, e182899. [Google Scholar] [CrossRef]
- Sunuwar, D.R.; Singh, D.R.; Chaudhary, N.K.; Pradhan, P.M.S.; Rai, P.; Tiwari, K. Prevalence and factors associated with anemia among women of reproductive age in seven South and Southeast Asian countries: Evidence from nationally representative surveys. PLoS ONE 2020, 15, e0236449. [Google Scholar] [CrossRef] [PubMed]
- National Department of Health. Saving Mothers 2010–2013: Sixth Report of Confidential Enquiries into Maternal Deaths in South Africa; NDOH: Pretoria, South Africa, 2013. [Google Scholar]
- Mocroft, A.; Kirk, O.; Barton, S.E.; Dietrich, M.; Proenca, R.; Colebunders, R.; Pradier, C.; Monforte, A.D.; Ledergerber, B.; Lundgren, J. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS 1999, 13, 943–950. [Google Scholar] [CrossRef]
- Moyle, G. Anaemia in persons with HIV infection: Prognostic marker and contributor to morbidity. Aids Rev. 2002, 4, 13–20. [Google Scholar]
- World Health Organization. WHO Methods and Data Sources for Mean Haemoglobin and Anaemia Estimates in Women of Reproductive Age and Pre-School Age Children 2000–2019; World Health Organization: Geneva, Switzerland, 2021; Available online: https://cdn.who.int/media/docs/default-source/anaemia-in-women-and-children/hb-methods-for-gather.pdf?sfvrsn=da0fbb5f_11 (accessed on 29 September 2021).
- Hruschka, D.J.; Williams, A.M.; Mei, Z.; Leidman, E.; Suchdev, P.S.; Young, M.F.; Namaste, S. Comparing hemoglobin distributions between population-based surveys matched by country and time. BMC Public Health 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shimanda, P.P.; Amukugo, H.J.; Norström, F. Socioeconomic factors associated with anemia among children aged 6–59 months in Namibia. J. Publ. Health Afr. 2020, 11, 1131. [Google Scholar] [CrossRef]
- Keats, E.C.; Neufeld, L.M.; Garrett, G.S.; Mbuya, M.N.; Bhutta, Z.A. Improved micronutrient status and health outcomes in low- and middle-income countries following large-scale fortification: Evidence from a systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1696–1708. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC); Food Fortification Initiative (FFI); Global Alliance for Improved Nutrition (GAIN); University of the Western Cape. Fortification Assessment Coverage Tool (FACT) Survey in Two South African Provinces: Gauteng and Eastern Cape, 2015; GAIN: Geneva, Switzerland, 2017. [Google Scholar]
- Park, K.; Kersey, M.; Geppert, J.; Story, M.; Cutts, D.; Himes, J.H. Household food insecurity is a risk factor for iron-deficiency anaemia in a multi-ethnic, low-income sample of infants and toddlers. Public Health Nutr. 2009, 12, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Kraef, C.; Wood, B.; Von Philipsborn, P.; Singh, S.; Peterson, S.S.; Kallestrup, P. Primary health care and nutrition. Bull. World Health Organ. 2020, 98, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Shankar, A.H.; Wu, L.S.-F.; Aboud, S.; Adu-Afarwuah, S.; Ali, H.; Agustina, R.; Arifeen, S.; Ashorn, P.; Bhutta, Z.A.; et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: A meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob. Health 2017, 5, e1090–e1100. [Google Scholar] [CrossRef]
- Black, R.E.; Dewey, K.G. Benefits of supplementation with multiple micronutrients in pregnancy. Ann. N. Y. Acad. Sci. 2019, 1444, 3–5. [Google Scholar] [CrossRef] [PubMed]
| Study ID | Study Design | Study Period | Setting | Population | Age (Year) | Condition | Case Definition | Sample (N) | Sample (n) | Prevalence | Risk of Bias Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | |||||||||||
| Faber (2001) [30] | Cross-sectional | 1998 | Rural | Women | 19–39 | Anaemia | Hb < 12 g/dL | 137 | 60 | 44 | 35.3–52.5 | Low |
| ID | SF < 15μg/L | 26 | 19 | 12.7–26.5 | ||||||||
| Labadarios (2007) [19] (NFCS) | Population-based survey | 2005 | National | Women | 16–36 | Anaemia | Hb < 12 g/dL | 2126 | 625 | 29.4 | 27.0–31.8 | Low |
| IDA | Hb < 12 g/dL & SF < 15μg/L | 223 | 10.5 | 9.0–12.1 | ||||||||
| ID | Hb ≥ 12 g/dL & SF < 15μg/L | 164 | 7.7 | 6.5–9.0 | ||||||||
| Methazia (2020) [37] | Cohort | 2004–2011 | Urban | HIV-infected pregnant | 18–49 | Anaemia | Hb < 10.0–10.9 g/dL | 236 | 142 | 60.6 | 54.0–67.2 | Low |
| Nandlal (2014) [38] | Cohort | 2007 & 2010 | Urban | HIV-infected pregnant | 18–42 | Anaemia | Hb < 11 g/dL | 408 | 262 | 64.2 | 59.3–69.1 | Moderate |
| Oelofse (1999) [33] | Cross-sectional | 1998 | Rural | Black African | NR | Anaemia | Hb < 12 g/dL | 119 | 26 | 22 | 14.8–29.2 | Moderate |
| ID | Hb > 12 g/dL & SF < 15μg/L | 127 | 24 | 19 | 12.6–25.4 | |||||||
| Shisana (2014) [20] (SANHANES) | Population-based survey | 2012 | National | Women | 16–35 | Anaemia | Hb < 12 g/dL | 1359 | 313 | 23.1 | 20.9–25.1 | Low |
| ID | Hb > 12 g/dL & SF < 15μg/L | 1223 | 72 | 5.9 | 4.6–7.4 | |||||||
| IDA | Hb ≤ 12 g/dL & SF ≤ 15μg/L | 1359 | 132 | 9.7 | 8.2–11.4 | |||||||
| Sibeko (2004) [34] | Cross-sectional | 2000 | Urban | Black African | 15–43 | Anaemia | Hb < 12 g/dL | 113 | 36 | 32 | 23.4–41.3 | Low |
| South Africa Demographic & Health Survey (2019) [18] (SADHS) | Population- based survey | 2016 | National | women | 15–49 | Anaemia | Hb < 12 g/dL | 2927 | 975 | 33.3 | 31.6–35.0 | Low |
| Pregnant women | Hb < 11 g/dL | 109 | 42 | 39.1 | 36–42.2 | |||||||
| Symington (2019) [39] | Cohort | 2017 | Urban | Pregnant women | 18–39 | Anaemia | Hb < 11 g/dL | 250 | 73 | 29 | 23.6–34.4 | Moderate |
| Tunkyi (2016) [35] | Cross-sectional | 2012–2014 | Urban | Pregnant women | 19–45 | Anaemia | Hb < 11 g/dL | 2000 | 854 | 42.7 | 40.5–44.9 | Moderate |
| HIV-infected pregnant | 854 | 609 | 71.3 | 68.2–74.4 | ||||||||
| Study ID | Study Design | Study Period | Setting | Population | Condition | Age | Case Definition | Sample | Sample | Prevalence | Risk of Bias Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N) | (n) | % | 95% CI | |||||||||
| Faber (2001) [30] | Cross-sectional | 1998 | Rural | Anaemia | 2–5 years | Hb < 11 g/dL | 88 | 54 | 45.7–61.5 | Low | ||
| African children | ID | SF < 12μg/L | 164 | 54 | 33 | 25.8–40.7 | ||||||
| Faber (2007) [29] | Cross-sectional | 1998 | Rural | Children | Anaemia | 6–12 months | Hb < 11 g/dL | 498 | 246 | 49 | 44.6–53.4 | Moderate |
| Labadarios (2007) [19] (NFCS-FB-1) | Population-based survey | 2005 | National | Anaemia | Hb < 11 g/dL | 1049 | 303 | 28.9 | 26.0–31.8 | Low | ||
| Children | ID | 1–5 years | Hb ≥ 11 g/dL & SF 12μg/L | 821 | 151 | 7.8 | 6.0–9.9 | |||||
| IDA | Hb < 11 g/dL& SF < 12μg/L | 768 | 87 | 11.3 | 9.2–13.4 | |||||||
| Mamabolo (2014) [36] | Cohort | 1999–2000 | Rural | Anaemia | 1 year | Hb < 11 g/dL & SF < 12μg/L | 127 | 66 | 52 | 42.9–60.9 | Moderate | |
| Children | 3 years | 143 | 31 | 21.7 | 15.2–28.2 | |||||||
| ID | 3 years | SF < 12μg/L | 143 | 47 | 32.9 | 25.2–40.6 | ||||||
| Motadi (2015) [31] | Cross-sectional | 2012 | Rural | Children | Anaemia | 3–5 years | Hb < 11 g/dL | 349 | 98 | 28 | 23.4–32.6 | Moderate |
| ID | SF < 12μg/L or TSAT < 15% | 25 | 7.2 | 4.7–9.7 | ||||||||
| IDA | Low Hb & TSAT or SF or both | 12 | 3.4 | 1.8–5.0 | ||||||||
| Nel (2015) [32] | Cross-sectional | Anaemia | 4–13 months | Hb < 11 g/dL | 77 | 40.1 | 33.1–47.1 | Low | ||||
| 2000 | Rural | African children | ID | SF < 12μg/L | 192 | 33 | 17.2 | 12.1–22.3 | ||||
| IDA | Hb < 11 g/dL & SF < 12μg/L | 23 | 12 | 7.7–16.3 | ||||||||
| Shisana (2014) [20] | Population-based survey | 2012 | National | Children | Anaemia | < 5 years | Hb < 11 g/dL | 511 | 56 | 10.7 | 7.9–13.5 | Low |
| (SANHANES) | ID | Hb ≥ 11 g/dL & SF < 12μg/mL | 349 | 28 | 8.1 | 5.4–11.4 | ||||||
| IDA | Hb < 11 g/dl & SF < 12μg/L | 7 | 1.9 | 0.8–4.0 | ||||||||
| Sibeko (2004) [34] | Cross-sectional | 2000 | Urban | African children | Anaemia | 1–6 months | Hb < 11 g/dL | 104 | 52 | 50 | 40.0–60 | Low |
| South Africa Demographic & Health Survey (2019) [18] (SADHS) | Population-based survey | 2016 | National | Children | Anaemia | 0–5 years | Hb < 11 g/dL | 1094 | 667 | 61.3 | 58.0–64.6 | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turawa, E.; Awotiwon, O.; Dhansay, M.A.; Cois, A.; Labadarios, D.; Bradshaw, D.; Pillay-van Wyk, V. Prevalence of Anaemia, Iron Deficiency, and Iron Deficiency Anaemia in Women of Reproductive Age and Children under 5 Years of Age in South Africa (1997–2021): A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12799. https://doi.org/10.3390/ijerph182312799
Turawa E, Awotiwon O, Dhansay MA, Cois A, Labadarios D, Bradshaw D, Pillay-van Wyk V. Prevalence of Anaemia, Iron Deficiency, and Iron Deficiency Anaemia in Women of Reproductive Age and Children under 5 Years of Age in South Africa (1997–2021): A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(23):12799. https://doi.org/10.3390/ijerph182312799
Chicago/Turabian StyleTurawa, Eunice, Oluwatoyin Awotiwon, Muhammad Ali Dhansay, Annibale Cois, Demetre Labadarios, Debbie Bradshaw, and Victoria Pillay-van Wyk. 2021. "Prevalence of Anaemia, Iron Deficiency, and Iron Deficiency Anaemia in Women of Reproductive Age and Children under 5 Years of Age in South Africa (1997–2021): A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 23: 12799. https://doi.org/10.3390/ijerph182312799
APA StyleTurawa, E., Awotiwon, O., Dhansay, M. A., Cois, A., Labadarios, D., Bradshaw, D., & Pillay-van Wyk, V. (2021). Prevalence of Anaemia, Iron Deficiency, and Iron Deficiency Anaemia in Women of Reproductive Age and Children under 5 Years of Age in South Africa (1997–2021): A Systematic Review. International Journal of Environmental Research and Public Health, 18(23), 12799. https://doi.org/10.3390/ijerph182312799






