Incidence of Anxiety in Latest Life and Risk Factors. Results of the AgeCoDe/AgeQualiDe Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Consideration
2.3. Measures
2.3.1. Geriatric Anxiety Symptoms
2.3.2. Predictor Variables
2.4. Statistical Analyses
3. Results
3.1. Study Population
3.2. Age- and Gender-Specific Incidence Rates of Anxiety Symptoms
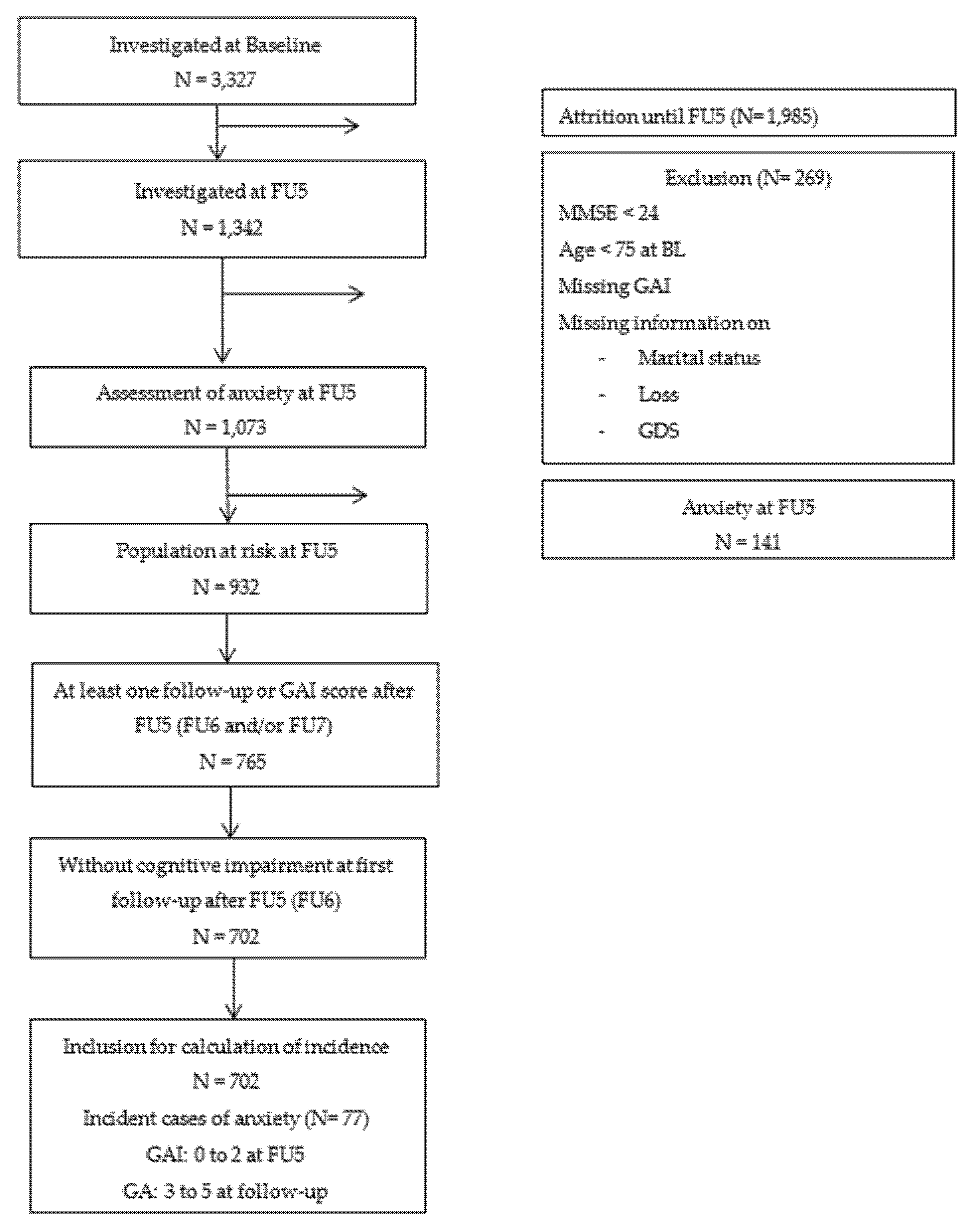
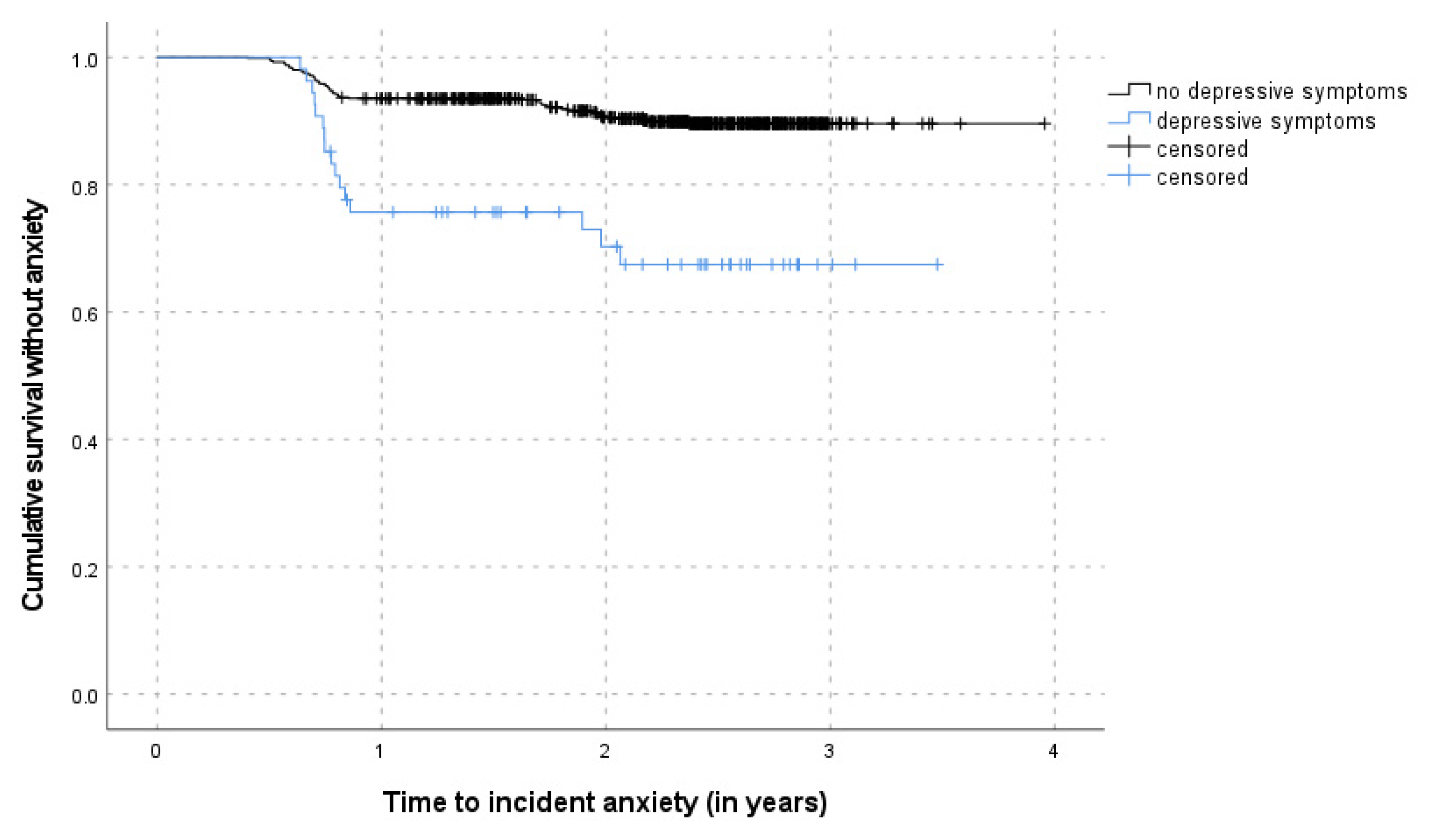
3.3. Risk Factors of Incident Anxiety Symptoms
3.4. Course of Anxiety Symptoms
4. Discussion
4.1. Incident Anxiety Symptoms in Late Life
4.2. Risk Factors for Incident Anxiety in Late Life
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welzel, F.D.; Stein, J.; Roehr, S.; Fuchs, A.; Pentzek, M.; Mösch, E.; Bickel, H.; Weyerer, S.; Werle, J.; Wiese, B.; et al. Prevalence of Anxiety Symptoms and Their Association With Loss Experience in a Large Cohort Sample of the Oldest-Old. Results of the AgeCoDe/AgeQualiDe Study. Front. Psychiatry 2019, 10, 285. [Google Scholar] [CrossRef]
- Canuto, A.; Weber, K.; Baertschi, M.; Andreas, S.; Volkert, J.; Dehoust, M.C.; Sehner, S.; Suling, A.; Wegscheider, K.; Ausín, B.; et al. Anxiety Disorders in Old Age: Psychiatric Comorbidities, Quality of Life, and Prevalence According to Age, Gender, and Country. Am. J. Geriatr. Psychiatry 2018, 26, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.; Ancelin, M.L.; Stewart, R.; Berr, C.; Ritchie, K.; Carrière, I. Anxiety symptoms and disorder predict activity limitations in the elderly. J. Affect. Disord. 2012, 141, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Miloyan, B.; Pachana, N.A. Clinical significance of worry and physical symptoms in late-life generalized anxiety disorder. Int. J. Geriatr. Psychiatry 2015, 30, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Kirmizioglu, Y.; Doğan, O.; Kuğu, N.; Akyüz, G. Prevalence of anxiety disorders among elderly people. Int. J. Geriatr. Psychiatry 2009, 24, 1026–1033. [Google Scholar] [CrossRef]
- Porensky, E.K.; Dew, M.A.; Karp, J.F.; Skidmore, E.; Rollman, B.L.; Shear, M.K.; Lenze, E.J. The Burden of Late-Life Generalized Anxiety Disorder: Effects on Disability, Health-Related Quality of Life, and Healthcare Utilization. Am. J. Geriatr. Psychiatry 2009, 17, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Santabárbara, J.; Lopez-Anton, R.; De La Cámara, C.; Lobo, E.; Gracia-García, P.; Villagrasa, B.; Bueno-Notivol, J.; Marcos, G.; Lobo, A. Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatr. Scand. 2019, 139, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Vasiliadis, H.-M.; Dionne, P.-A.; Préville, M.; Gentil, L.; Berbiche, D.; Latimer, E. The Excess Healthcare Costs Associated With Depression and Anxiety in Elderly Living in the Community. Am. J. Geriatr. Psychiatry 2013, 21, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Carrière, I.; Ryan, J.; Norton, J.; Scali, J.; Stewart, R.; Ritchie, K.; Ancelin, M.L. Anxiety and mortality risk in community-dwelling elderly people. Br. J. Psychiatry 2013, 203, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Holwerda, T.J.; Schoevers, R.A.; Dekker, J.; Deeg, D.J.H.; Jonker, C.; Beekman, A.T.F. The relationship between generalized anxiety disorder, depression and mortality in old age. Int. J. Geriatr. Psychiatry 2007, 22, 241–249. [Google Scholar] [CrossRef]
- Van Hout, H.P.J.; Beekman, A.T.F.; De Beurs, E.; Comijs, H.; Van Marwijk, H.; De Haan, M.; Van Tilburg, W.; Deeg, R.J.H. Anxiety and the risk of death in older men and women. Br. J. Psychiatry 2004, 185, 399–404. [Google Scholar] [CrossRef]
- Kang, H.-J.; Bae, K.-Y.; Kim, S.-W.; Shin, I.-S.; Yoon, J.-S.; Kim, J.-M. Anxiety symptoms in Korean elderly individuals: A two-year longitudinal community study. Int. Psychogeriatr. 2016, 28, 423–433. [Google Scholar] [CrossRef]
- Mehta, K.M.; Yaffe, K.; Brenes, G.A.; Newman, A.B.; Shorr, R.I.; Simonsick, E.M.; Ayonayon, H.N.; Rubin, S.M.; Covinsky, K.E. Anxiety Symptoms and Decline in Physical Function over 5 Years in the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2007, 55, 265–270. [Google Scholar] [CrossRef]
- Andreescu, C.; Varon, D. New Research on Anxiety Disorders in the Elderly and an Update on Evidence-Based Treatments. Curr. Psychiatry Rep. 2015, 17, 53. [Google Scholar] [CrossRef]
- Somers, J.M.; Goldner, E.M.; Waraich, P.; Hsu, L. Prevalence and Incidence Studies of Anxiety Disorders: A Systematic Review of the Literature. Can. J. Psychiatry 2006, 51, 100–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandanger, I.; Nygård, J.F.; Ingebrigtsen, G.; Sørensen, T.; Dalgard, O.S. Prevalence, incidence and age at onset of psychiatric disorders in Norway. Soc. Psychiatry Psychiatr. Epidemiol. 1999, 34, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Norton, J.; Mann, A.; Carriere, I.; Ancelin, M.-L. Late-Onset Agoraphobia: General Population Incidence and Evidence for a Clinical Subtype. Am. J. Psychiatry 2013, 170, 790–798. [Google Scholar] [CrossRef]
- Martín-Merino, E.; Ruigómez, A.; Wallander, M.-A.; Johansson, S.; García-Rodríguez, L.A. Prevalence, incidence, morbidity and treatment patterns in a cohort of patients diagnosed with anxiety in UK primary care. Fam. Pr. 2009, 27, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejtzén, N.; Sundquist, J.; Sundquist, K.; Li, X. Depression and anxiety in Swedish primary health care: Prevalence, incidence, and risk factors. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 264, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heesterbeek, T.J.; Van Der Aa, H.P.A.; Van Rens, G.H.M.B.; Twisk, J.W.R.; Van Nispen, R.M.A. The incidence and predictors of depressive and anxiety symptoms in older adults with vision impairment: A longitudinal prospective cohort study. Ophthalmic Physiol. Opt. 2017, 37, 385–398. [Google Scholar] [CrossRef]
- Joling, K.J.; van Marwijk, H.; Veldhuijzen, A.E.; van der Horst, H.E.; Scheltens, P.; Smit, F.; van Hout, H. The Two-Year Incidence of Depression and Anxiety Disorders in Spousal Caregivers of Persons with Dementia: Who is at the Greatest Risk? Am. J. Geriatr. Psychiatry 2015, 23, 293–303. [Google Scholar] [CrossRef]
- Joling, K.J.; van Hout, H.P.; Schellevis, F.G.; van der Horst, H.E.; Scheltens, P.; Knol, D.L.; van Marwijk, H.W. Incidence of Depression and Anxiety in the Spouses of Patients With Dementia: A Naturalistic Cohort Study of Recorded Morbidity With a 6-Year Follow-Up. Am. J. Geriatr. Psychiatry 2010, 18, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuelsson, G.; McCamish-Svensson, C.; Hagberg, B.; Sundström, G.; Dehlin, O. Incidence and risk factors for depression and anxiety disorders: Results from a 34-year longitudinal Swedish cohort study. Aging Ment. Heal. 2005, 9, 571–575. [Google Scholar] [CrossRef]
- Walters, K.; Rait, G.; Griffin, M.; Buszewicz, M.; Nazareth, I. Recent Trends in the Incidence of Anxiety Diagnoses and Symptoms in Primary Care. PLoS ONE 2012, 7, e41670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenze, E.J.; Wetherell, J.L. Bringing the bedside to the bench, and then to the community: A prospectus for intervention research in late-life anxiety disorders. Int. J. Geriatr. Psychiatry 2009, 24, 1–14. [Google Scholar] [CrossRef]
- Prigerson, H.G.; Shear, M.K.; Newsom, J.T.; Frank, E.; Reynolds, C.F., III; Maciejewski, P.K.; Houck, P.R.; Bierhals, A.J.; Kupfer, D.J. Anxiety among widowed elders: Is it distinct from depression and grief? Anxiety 1996, 2, 1–12. [Google Scholar] [CrossRef]
- Byrne, G.J.A.; Raphael, B. The Psychological Symptoms of Conjugal Bereavement in Elderly Men over the First 13 Months. Int. J. Geriatr. Psychiatry 1997, 12, 241–251. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bickel, H.; Eiffländer-Gorfer, S.; Fuchs, A.; Kaduszkiewicz, H.; Köhler, M.; Luck, T.; Mösch, E.; Pentzek, M.; et al. Prediction of Dementia in Primary Care Patients. PLoS ONE 2011, 6, e16852. [Google Scholar] [CrossRef]
- Hohls, J.K.; König, H.-H.; Eisele, M.; Mallon, T.; Mamone, S.; Wiese, B.; Weyerer, S.; Fuchs, A.; Pentzek, M.; Roehr, S.; et al. Help-seeking for psychological distress and its association with anxiety in the oldest old—Results from the AgeQualiDe cohort study. Aging Ment. Heal. 2021, 25, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J. Psychiatr. Res. 2009, 43, 411–431. [Google Scholar] [CrossRef]
- Byrne, G.J.; Pachana, N.A. Development and validation of a short form of the Geriatric Anxiety Inventory—The GAI-SF. Int. Psychogeriatr. 2010, 23, 125–131. [Google Scholar] [CrossRef]
- Johnco, C.; Knight, A.; Tadic, D.; Wuthrich, V.M. Psychometric properties of the Geriatric Anxiety Inventory (GAI) and its short-form (GAI-SF) in a clinical and non-clinical sample of older adults. Int. Psychogeriatr. 2015, 27, 1089–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forlani, M.; Morri, M.; Murri, M.B.; Bernabei, V.; Moretti, F.; Attili, T.; Biondini, A.; De Ronchi, D.; Atti, A.R. Anxiety Symptoms in 74+ Community-Dwelling Elderly: Associations with Physical Morbidity, Depression and Alcohol Consumption. PLoS ONE 2014, 9, e89859. [Google Scholar] [CrossRef]
- Brauns, H.; Steinmann, S. Educational reform in France, West-Germany and the United Kingdom: Updating the CASMIN ed-ucational classification. Zuma Nachr. 1999, 23, 7–44. [Google Scholar]
- Sheikh, J.I.; Yesavage, J.A. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar]
- Gauggel, S.; Birkner, B. Validity and reliability of a German version of the Geriatric Depression Scale (GDS). Z. Klin. Psychol.-Forsch. Prax. 1999, 28, 18–27. [Google Scholar] [CrossRef]
- Weyerer, S.; Eifflaender-Gorfer, S.; Wiese, B.; Luppa, M.; Pentzek, M.; Bickel, H.; Bachmann, C.; Scherer, M.; Maier, W.; Riedel-Heller, S.G. Incidence and predictors of depression in non-demented primary care attenders aged 75 years and older: Results from a 3-year follow-up study. Age Ageing 2013, 42, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerlings, M.I.; Jonker, C.; Bouter, L.M.; Adèr, H.J.; Schmand, B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am. J. Psychiatry 1999, 156, 531–537. [Google Scholar] [CrossRef]
- Cooper, B.; Helgason, T. Epidemiology and the Prevention of Mental Disorders; Routledge: London, UK, 2021. [Google Scholar]
- de Graaf, R.; Have, M.T.; Tuithof, M.; van Dorsselaer, S. First-incidence of DSM-IV mood, anxiety and substance use disorders and its determinants: Results from the Netherlands Mental Health Survey and Incidence Study-2. J. Affect. Disord. 2013, 149, 100–107. [Google Scholar] [CrossRef]
- Chou, K.-L.; MacKenzie, C.S.; Liang, K.; Sareen, J. Three-Year Incidence and Predictors of First-Onset of DSM-IV Mood, Anxiety, and Substance Use Disorders in Older Adults. J. Clin. Psychiatry 2011, 72, 144–155. [Google Scholar] [CrossRef]
- Kenbubpha, K.; Higgins, I.; Chan, S.W.-C.; Wilson, A. Promoting active ageing in older people with mental disorders living in the community: An integrative review. Int. J. Nurs. Pr. 2018, 24, e12624. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.P.; Blank, M.B. Diagnosis and treatment of depression and anxiety in rural and nonrural primary care: National survey results. Psychiatr. Serv. 2010, 61, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.A.; Fortes, S.; Tófoli, L.F.; Campos, M.R.; Mari, J.D.J. Determinants of Common Mental Disorders Detection by General Practitioners in the Primary Health Care in Brazil. Int. J. Psychiatry Med. 2011, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.C.; Coelho, C.M.; Byrne, G.J. The use of healthcare services for mental health problems by middle-aged and older adults. Arch. Gerontol. Geriatr. 2014, 59, 393–397. [Google Scholar] [CrossRef] [Green Version]
- Garrido, M.M.; Kane, R.L.; Kaas, M.; Kane, R.A. Use of Mental Health Care by Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2011, 59, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamers, F.; Van Oppen, P.; Comijs, H.C.; Smit, J.H.; Spinhoven, P.; Van Balkom, A.J.L.M.; Nolen, W.A.; Zitman, F.G.; Beekman, A.T.F.; Penninx, B.W.J.H. Comorbidity Patterns of Anxiety and Depressive Disorders in a Large Cohort Study. J. Clin. Psychiatry 2011, 72, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, T.E.; Harrington, H.; Caspi, A.; Kim-Cohen, J.; Goldberg, D.; Gregory, A.M.; Poulton, R. Depression and Generalized Anxiety Disorder. Arch. Gen. Psychiatry 2007, 64, 651–660. [Google Scholar] [CrossRef]
- Grant, B.F.; Goldstein, R.; Chou, S.P.; Huang, B.; Stinson, F.S.; Dawson, D.A.; Saha, T.D.; Smith, S.M.; Pulay, A.J.; Pickering, R.P.; et al. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: Results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol. Psychiatry 2008, 14, 1051–1066. [Google Scholar] [CrossRef]
- Butters, M.A.; Bhalla, R.K.; Andreescu, C.; Wetherell, J.L.; Mantella, R.; Begley, A.E.; Lenze, E.J. Changes in neuropsychological functioning following treatment for late-life generalised anxiety disorder. Br. J. Psychiatry 2011, 199, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Mantella, R.C.; Butters, M.A.; Dew, M.A.; Mulsant, B.H.; Begley, A.E.; Tracey, B.; Shear, M.K.; Reynolds, C.F.; Lenze, E.J. Cognitive Impairment in Late-Life Generalized Anxiety Disorder. Am. J. Geriatr. Psychiatry 2007, 15, 673–679. [Google Scholar] [CrossRef]
- Delphin-Combe, F.; Bathsavanis, A.; Rouch, I.; Liles, T.; Vannier-Nitenberg, C.; Fantino, B.; Dauphinot, V.; Krolak-Salmon, P. Relationship between anxiety and cognitive performance in an elderly population with a cognitive complaint. Eur. J. Neurol. 2016, 23, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Coen, R.; Kilroy, D.; Belinski, K.; Bruce, I.; Coakley, D.; Walsh, B.; Cunningham, C.; Lawlor, B.A. Anxiety and behavioural disturbance as markers of prodromal Alzheimer’s disease in patients with mild cognitive impairment. Int. J. Geriatr. Psychiatry 2011, 26, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.M.; Ganguli, M.; Yaffe, K.; Hanlon, J.T.; Lopez, O.L.; Wilson, J.W.; Cauley, J.A.; Osteoporotic Fractures in Men (MrOS) Study Research Group. Anxiety symptoms and risk of cognitive decline in older community-dwelling men. Int. Psychogeriatr. 2017, 29, 1137–1145. [Google Scholar] [CrossRef]
- Gulpers, B.; Ramakers, I.; Hamel, R.; Köhler, S.; Voshaar, R.O.; Verhey, F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am. J. Geriatr. Psychiatry 2016, 24, 823–842. [Google Scholar] [CrossRef]
- Price, R.B.; Eldreth, D.A.; Mohlman, J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: An fMRI investigation. Transl. Psychiatry 2011, 1, e46. [Google Scholar] [CrossRef] [Green Version]
- Mantella, R.C.; Butters, M.A.; Amico, J.A.; Mazumdar, S.; Rollman, B.L.; Begley, A.E.; Reynolds, C.F.; Lenze, E.J. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology 2008, 33, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Biringer, E.; Mykletun, A.; Dahl, A.A.; Smith, A.D.; Engedal, K.; Nygaard, H.A.; Lund, A. The association between depression, anxiety, and cognitive function in the elderly general population—the Hordaland Health Study. Int. J. Geriatr. Psychiatry 2005, 20, 989–997. [Google Scholar] [CrossRef]
- Balash, Y.; Mordechovich, M.; Shabtai, H.; Giladi, N.; Gurevich, T.; Korczyn, A.D. Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurol. Scand. 2013, 127, 344–350. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current Concepts in Mild Cognitive Impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.A.; Clare, L.; Woods, R.T.; Cfas, M. Subjective memory complaints, mood and MCI: A follow-up study. Aging Ment. Heal. 2017, 21, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Shear, M.K.; Skritskaya, N.A. Bereavement and Anxiety. Curr. Psychiatry Rep. 2012, 14, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Floyd, F.J.; Seltzer, M.M.; Greenberg, J.S.; Hong, J. Long-Term Effects of Child Death on Parents’ Health-Related Quality of Life: A Dyadic Analysis. Fam. Relations 2010, 59, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittkowski, J.; Scheuchenpflug, R.; Information, R. Trauern in Abhängigkeit vom Verwandtschaftsverhältnis zum Verstorbenen und der Todesart. Z. Gesundh. 2016, 24, 107–118. [Google Scholar] [CrossRef]
- Burton, A.M.; Haley, W.; Small, B. Bereavement after caregiving or unexpected death: Effects on elderly spouses. Aging Ment. Heal. 2006, 10, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Keyes, K.M.; Pratt, C.; Galea, S.; McLaughlin, K.; Koenen, K.C.; Shear, M.K. The Burden of Loss: Unexpected Death of a Loved One and Psychiatric Disorders Across the Life Course in a National Study. Am. J. Psychiatry 2014, 171, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G.A.; Wortman, C.B.; Lehman, D.R.; Tweed, R.G.; Haring, M.; Sonnega, J.; Carr, D.; Nesse, R.M. Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss. J. Pers. Soc. Psychol. 2002, 83, 1150–1164. [Google Scholar] [CrossRef]
- Cole, M.G.; Dendukuri, N. Risk factors for depression among elderly community subjects: A systematic review and me-ta-analysis. Am. J. Psychiatry 2003, 160, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Karger, A. Geschlechtsspezifische Aspekte bei depressiven Erkrankungen. Bundesgesundheitsblatt Gesundh. Gesundh. 2014, 57, 1092–1098. [Google Scholar] [CrossRef]
| Study Sample (N = 702) | |
|---|---|
| Age, years | |
| Mean, SD | 86.4 (2.8) |
| Range | 81–97 |
| Gender, n (%) | |
| Male | 236 (33.6) |
| Female | 466 (66.4) |
| Education a, n (%) | |
| High | 108 (15.4) |
| Middle | 213 (30.3) |
| Low | 381 (54.3) |
| Marital status, n (%) | |
| Married | 211 (30.1) |
| Widowed/divorced | 445 (63.4) |
| Single | 46 (6.5) |
| Living situation, n (%) | |
| Alone | 371 (52.8) |
| Not alone | 331 (47.2) |
| GAI-SF, mean (SD) | 0.67 (0.77) |
| MMSE, mean (SD) | 28.2 (1.4) |
| Depressive symptoms b n (%) | 54 (7.7) |
| Recent loss experience, n (%) | 202 (28.8) |
| Functional impairments, n (%) | |
| Mobility impairment | 376 (53.6) |
| Vision impairment | 159 (22.6) |
| Hearing impairment | 337 (48.0) |
| Subjective memory complaints, n (%) | 116 (16.5) |
| n | No. of New Cases | Sum of Risk Years | Incidence per 1000 Person-Years | (95% CI) | |
|---|---|---|---|---|---|
| Total | 702 | 77 | 1501 | 51.3 | (41.2–64.1) |
| Men | 236 | 19 | 509 | 37.3 | (23.6–58.3) |
| Women | 466 | 58 | 992 | 58.5 | (43.2–72.4) |
| Age | |||||
| 81 to 85 | 312 | 39 | 679 | 57.4 | (41.8–78.6) |
| 86 to 90 | 320 | 35 | 673 | 52.0 | (35.7–72.8) |
| 91 and over | 70 | 3 | 149 | 20.1 | (6.7–62.4) |
| Men | |||||
| 81 to 85 | 127 | 16 | 273 | 58.6 | (35.9–95.9) |
| 86 to 90 | 89 | 3 | 194 | 15.5 | (5.1–47.9) |
| 91 and over | 20 | 0 | 42 | - | - |
| Women | |||||
| 81 to 85 | 185 | 23 | 406 | 56.6 | (37.7–85.2) |
| 86 to 90 | 231 | 32 | 479 | 66.8 | (47.2–94.6) |
| 91 and over | 50 | 3 | 107 | 28.0 | (8.9–86.9) |
| Overall Sample (N = 702) | Subsample without Depression at FU5 (N = 648) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables at FU5 | Incident Anxiety n/N | Multivariable HR (95% CI) | Wald F | SE | p-Value | Incident Anxiety n/N | Multivariable HR (95% CI) | Wald F | SE | p-Value |
| Age, every additional year | 77/702 | 0.93 (0.85–1.01) | 2.960 | 0.044 | 0.088 | 61/648 | 0.92 (0.84–1.00) | 3.332 | 0.047 | 0.070 |
| Gender | ||||||||||
| Female | 58/466 | 1 | 44/425 | 1 | ||||||
| Male | 19/236 | 0.70 (0.42–1.19) | 1.763 | 0.264 | 0.187 | 17/223 | 0.78 (0.44–1.39) | 0.707 | 0.292 | 0.402 |
| Depressive symptoms a | ||||||||||
| No | 61/648 | 1 | - | - | - | - | - | |||
| Yes | 16/54 | 3.20 (1.46–7.01) | 8.675 | 0.395 | 0.004 | - | - | - | - | - |
| Recent loss experience | ||||||||||
| No | 54/500 | 1 | 42/463 | 1 | ||||||
| Yes | 23/202 | 0.96 (0.58–1.62) | 0.018 | 0.260 | 0.893 | 19/185 | 1.07 (0.61–1.88) | 0.052 | 0.286 | 0.820 |
| Subjective memory complaints (SMC) | ||||||||||
| No | 55/586 | 1 | 45/549 | 1 | ||||||
| Yes | 22/116 | 2.03 (1.16–3.57) | 6.202 | 0.285 | 0.014 | 16/99 | 1.92 (1.09–3.39) | 5.170 | 0.287 | 0.025 |
| Mobility impairment | ||||||||||
| No | 27/326 | 1 | 25/318 | 1 | ||||||
| Yes | 50/376 | 1.42 (0.87–2.30) | 2.045 | 0.245 | 0.155 | 36/330 | 1.43 (0.87–2.34) | 2.049 | 0.249 | 0.155 |
| Vision impairment | ||||||||||
| No | 55/543 | 1 | 42/505 | 1 | ||||||
| Yes | 22/159 | 1.21 (0.72–2.05) | 0.533 | 0.266 | 0.467 | 19/143 | 1.58 (0.93–2.69) | 2.951 | 0.267 | 0.088 |
| Hearing impairment | ||||||||||
| No | 45/365 | 1 | 33/331 | 1 | ||||||
| Yes | 32/337 | 0.79 (0.47–1.34) | 0.746 | 0.263 | 0.389 | 28/317 | 0.86 (0.48–1.54) | 0.245 | 0.293 | 0.621 |
| Depression*SMC | - | 0.59 (0.18–1.94) | 0.751 | 0.596 | 0.388 | - | - | - | - | - |
| FU5 | FU6 | FU7 | N (%) a | |
|---|---|---|---|---|
| OCT/2010-NOV/2012 | JAN/2012- FEB/2014 | JAN/2014- FEB/2015 | ||
| no anxiety | no anxiety | no anxiety | 508 (72.4) | no anxiety |
| no anxiety | anxiety | 29 (4.1) | temporary anxiety | |
| anxiety | no anxiety | 22 (3.1) | ||
| anxiety | anxiety | 17 (2.4) | persistent anxiety |
| No Anxiety (N = 508) | Temporary Anxiety (N = 51) | Persistent Anxiety (N = 17) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 86.45 (2.9) | 85.8 (2.5) | 86.0 (2.7) |
| N (%) | N (%) | N (%) | |
| Female sex | 331 (65.2) | 37 (72.5) | 13 (76.5) |
| Education | |||
| High | 82 (16.1) | 6 (11.8) | 2 (11.8) |
| Middle | 162 (31.9) | 12 (23.5) | 4 (23.5) |
| Low | 264 (52.0) | 33 (64.7) | 11 (64.7) |
| Depressive symptoms | 27 (5.3) | 7 (13.7) * | 5 (29.4) ** |
| Subjective memory complaints | 70 (13.8) | 13 (25.5) | 3 (17.6) |
| Recent loss experience | 155 (30.5) | 14 (27.5) | 8 (47.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welzel, F.D.; Luppa, M.; Pabst, A.; Pentzek, M.; Fuchs, A.; Weeg, D.; Bickel, H.; Weyerer, S.; Werle, J.; Wiese, B.; et al. Incidence of Anxiety in Latest Life and Risk Factors. Results of the AgeCoDe/AgeQualiDe Study. Int. J. Environ. Res. Public Health 2021, 18, 12786. https://doi.org/10.3390/ijerph182312786
Welzel FD, Luppa M, Pabst A, Pentzek M, Fuchs A, Weeg D, Bickel H, Weyerer S, Werle J, Wiese B, et al. Incidence of Anxiety in Latest Life and Risk Factors. Results of the AgeCoDe/AgeQualiDe Study. International Journal of Environmental Research and Public Health. 2021; 18(23):12786. https://doi.org/10.3390/ijerph182312786
Chicago/Turabian StyleWelzel, Franziska Dinah, Melanie Luppa, Alexander Pabst, Michael Pentzek, Angela Fuchs, Dagmar Weeg, Horst Bickel, Siegfried Weyerer, Jochen Werle, Birgitt Wiese, and et al. 2021. "Incidence of Anxiety in Latest Life and Risk Factors. Results of the AgeCoDe/AgeQualiDe Study" International Journal of Environmental Research and Public Health 18, no. 23: 12786. https://doi.org/10.3390/ijerph182312786
APA StyleWelzel, F. D., Luppa, M., Pabst, A., Pentzek, M., Fuchs, A., Weeg, D., Bickel, H., Weyerer, S., Werle, J., Wiese, B., Oey, A., Brettschneider, C., König, H.-H., Heser, K., van den Bussche, H., Eisele, M., Maier, W., Scherer, M., Wagner, M., & Riedel-Heller, S. G. (2021). Incidence of Anxiety in Latest Life and Risk Factors. Results of the AgeCoDe/AgeQualiDe Study. International Journal of Environmental Research and Public Health, 18(23), 12786. https://doi.org/10.3390/ijerph182312786






