Hospitalization Trends for Acute Appendicitis in Spain, 1998 to 2017
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castleton, K.B.; Peustow, C.B.; Sauer, D. Is appendicitis decreasing in frequency. AMA Arch. Surg. 1959, 78, 794–798, discussion 799–801. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.A.; Polymeros, D.; Kateri, M.; Tzathas, C.; Koutras, M.; Ladas, S.D. Dramatic decline of acute appendicitis in Greece over 30 years: Index of improvement of socioeconomic conditions or diagnostic aids? Dig. Dis. 2008, 26, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, D.P.; Xavier, C.; Samarasekera, D.N. The Worldwide Epidemiology of Acute Appendicitis: An Analysis of the Global Health Data Exchange Dataset. World J. Surg. 2021, 45, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Ferris, M.; Quan, S.; Kaplan, B.S.; Molodecky, N.; Ball, C.G.; Chernoff, G.W.; Bhala, N.; Ghosh, S.; Dixon, E.; Ng, S.; et al. The Global Incidence of Appendicitis: A Systematic Review of Population-based Studies. Ann. Surg. 2017, 266, 237–241. [Google Scholar] [CrossRef]
- Addis, D.G.; Shaffer, N.; Fowler, B.S.; Tauxe, R.V. The epidemiology of appendicitis and appendectomy in the United States. Am. J. Epidemiol. 1990, 132, 910–925. [Google Scholar] [CrossRef]
- Rautava, L.; Rautava, P.; Sipilä, J.; Kytö, V. Occurrence and Treatment of Pediatric Appendicitis in Finland 2004. J. Surg. Res. 2018, 232, 33–38. [Google Scholar] [CrossRef]
- Zavras, N.; Vaos, G. Management of complicated acute appendicitis in children: Still an existing controversy. World J. Gastrointest Surg. 2020, 27, 129–137. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Domjanović, J.; Jukić, M.; Poklepović Peričić, T. Acute Appendicitis in Children Younger than Five Years of Age: Diagnostic Challenge for Pediatric Surgeons. Surg. Infect. (Larchmt) 2020, 21, 239–245. [Google Scholar] [CrossRef]
- Primatesta, P.; Goldacre, M.J. Appendicectomy for acute appendicitis and for other conditions: An epidemiological study. Int. J. Epidemiol. 1994, 23, 155–160. [Google Scholar] [CrossRef]
- Livingston, E.H.; Fomby, T.B.; Woodward, W.A.; Haley, R.W. Epidemiological similarities between appendicitis and diverticulitis suggesting a common underlying pathogenesis. Arch. Surg. 2011, 146, 308–314. [Google Scholar] [CrossRef]
- Al-Omran, M.; Mamdani, M.; McLeod, R.S. Epidemiologic features of acute appendicitis in Ontario, Canada. Can. J. Surg. 2003, 46, 263. [Google Scholar] [PubMed]
- Klingler, P.J.; Seelig, M.H.; DeVault, K.R.; Wetscher, G.J.; ·Floch, N.R.; ·Branton, S.A.; Hinder, R.A. Ingested foreign bodieswithin the appendix: A 100-year review of the literature. Dig. Dis. 1998, 16, 308–314. [Google Scholar] [CrossRef]
- Carr, N.J. The pathology of acute appendicitis. Ann. Diag. Pathol. 2000, 4, 46–58. [Google Scholar] [CrossRef]
- Fitz, R.h. Perforating information of the vermiform appendix, with special reference to its early diagnosis and treatment. Am. J. Med. Sci. 1886, 92, 321–346. [Google Scholar]
- Howell, J.M.; Eddy, O.L.; Lukens, T.W.; Thiessen, M.E.; Weingart, S.D.; Decker, W.W. American College of Emergency Physicians. Clinical policy: Critical issues in the evaluation and management of emergency department patients with suspected appendicitis. Ann. Emerg. Med. 2010, 55, 71–116. [Google Scholar] [CrossRef] [PubMed]
- Heineman, J. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. BET 1: An evaluation of the Alvarado score as a diagnostic tool for appendicitis in children. Emerg. Med. J. 2012, 29, 1013–10134. [Google Scholar] [CrossRef] [PubMed]
- Jose, T.; Rajesh, P.S. Appendicitis Inflammatory Response Score in Comparison to Alvarado Score in Acute Appendicitis. Surg. J. 2021, 19, e127–e131. [Google Scholar] [CrossRef]
- Alvarado, A. A practical score for the early diagnosis of acute appendicitis. Ann. Emerg. Med. 1986, 15, 557–564. [Google Scholar] [CrossRef]
- Yu, C.W.; Juan, L.I.; Wu, M.H.; Shen, C.J.; Wu, J.Y.; Lee, C.C. Systematic review and meta-analysis of the diagnostic accuracy of procalcitonin, C-reactive protein and white blood cell count for suspected acute appendicitis. Br. J. Surg. 2013, 100, 322–329. [Google Scholar] [CrossRef]
- Stoker, J.; van Randen, A.; Laméris, W.; Boermeester, M.A. Imaging patients with acute abdominal pain. Radiology 2009, 253, 31–46. [Google Scholar] [CrossRef]
- Menoch, M.J.; Hirsh, D.A.; Khan, N.S.; Simon, H.K.; Sturm, J.J. Trends in computed tomography utilization in the pediatric emergency department. Pediatrics 2012, 129, e690–e697. [Google Scholar] [CrossRef]
- Doria, A.S. Optimizing the role of imaging in appendicitis. Pediatr. Radiol. 2009, 39 (Suppl. 2), S144–S148. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, C.E.; Simianu, V.V.; Carnell, J.; Hippe, D.S.; Bhargava, P.; Flum, D.R.; Davidson, G.H. Use of Computed Tomography to Determine Perforation in Patients With Acute Appendicitis. Curr. Probl. Diagn. Radiol. 2018, 47, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Fdez Lobato, R. Imaging tests and acute appendicitis: Importance for quality of life. Rev. Calid. Asist. 2010, 25, 183. [Google Scholar] [CrossRef]
- Schuler, J.G.; Shortsleeve, M.J.; Goldenson, R.S.; Perez-Rosello, J.; Perlmutter, R.; Thorsen, A. Is there a role for abdominal computed tomographic scans in appendicitis? Arch. Surg. 1998, 133, 373–377. [Google Scholar] [CrossRef]
- Rodríguez Cuellar, E.; Gutiérrez Andreu, M.; Gómez Rodríguez, P.; Alcalde Escribano, J.; De La Cruz Vigo, F. Impact of imaging tests on the negative appendectomy rate. Rev. Calid. Asist. 2010, 25, 188. (In Spanish) [Google Scholar] [CrossRef]
- Cho, H.W.; Koo, Y.J.; Min, K.J.; Hong, J.H.; Lee, J.K. Pelvic Inflammatory Disease in Virgin Women With Tubo-ovarian Abscess: A Single-Center Experience and Literature Review. J. Pediatr. Adolesc. Gynecol. 2017, 30, 203–208. [Google Scholar] [CrossRef]
- El Hentour, K.; Millet, I.; Pages-Bouic, E.; Curros-Doyon, F.; Molinari, N.; Taourel, P. How to differentiate acute pelvic inflammatory disease from acute appendicitis? A decision tree based on CT findings. Eur. Radiol. 2018, 28, 673–682. [Google Scholar] [CrossRef]
- Louyer-Villermay, J.B. Observations pour servir à l’histoire des inflammations de l’appendice du caecum. Arch. Générales Médecine 1824, 5, 246–250. [Google Scholar]
- McBurney, C. Experience with early operative interference in cases of vermiform appendix disease. N. Y. Med. J. 1889, 50, 676–684. [Google Scholar]
- Wagner, M.; Tubre, D.J.; Asensio, J.A. Evolution and Current Trends in the Management of Acute Appendicitis. Surg. Clin. N. Am. 2018, 98, 1005–1023. [Google Scholar] [CrossRef] [PubMed]
- Drake, F.T.; Flum, D.R. Improvement in the diagnosis of appendicitis. Adv. Surg. 2013, 47, 299–328. [Google Scholar] [CrossRef]
- Townsend, C.M.; Beauchamp, R.D.; Evers, B.M.; Mattox, K.L. Sabiston Textbook of Surgery, 18th ed.; Philadelphia, Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1333–1347. [Google Scholar]
- Garg, C.P.; Vaidya, B.B.; Chengalath, M.M. Efficacy of laparoscopy in complicated appendicitis. Int. J. Surg. 2009, 7, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.; García-Alcalá, H.; Domínguez-Cocco, A.; Justo-Janeiro, J.M. Comparative study of laparoscopic appendectomy vs open appendectomy. Rev. Gastroenterol. Mex. 1997, 62, 254. (In Spanish) [Google Scholar]
- Masoomi, H.; Mills, S.; Dolich, M.O.; Ketana, N.; Carmichael, J.C.; Nguyen, N.T.; Stamos, M.J. Does laparoscopic appendectomy impart an advantage over open appendectomy in elderly patients? World J. Surg. 2012, 36, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Wijkerslooth, E.M.L.; Bakas, J.M.; van Rosmalen, J.; van den Boom, A.L.; Wijnhoven, B.P.L. Same-day discharge after appendectomy for acute appendicitis: A systematic review and meta-analysis. Int. J. Colorectal. Dis. 2021, 36, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Pimentel, M.; Leite, G.G.S.; Celly, S.; Villanueva-Millan, M.J.; Lacsina, I.; Chuang, B.; Parodi, G.; Morales, W.; Weitsman, S.; et al. Acute appendicitis is associated with appendiceal microbiome changes including elevated Campylobacter jejuni levels. BMJ Open Gastroenterol. 2020, 7, e000412. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.R.; Kallehave, F.L.; Andersen, H.K. Antibiotics versus placebo for prevention of postoperative infection after appendicectomy. Cochrane Database Syst. Rev. 2005, 2009, CD001439. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, K.; Juhlin, C.; Pahlman, L. The use of pre- or postoperative antibiotics in surgery for appendicitis: A systematic review. Scand. J. Surg. 2014, 103, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kasatpibal, N.; Nørgaard, M.; Sørensen, H.T.; Schønheyder, H.C.; Jamulitrat, S.; Chongsuvivatwong, V. Risk of surgical site infection and efficacy of antibiotic prophylaxis: A cohort study of appendectomy patients in Thailand. BMC Infect. Dis. 2006, 6, 111. [Google Scholar] [CrossRef]
- Burkitt, D.P. Related disease—Related cause? Lancet 1969, 6, 1229–1231. [Google Scholar] [CrossRef]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Minimum Basic Data Set of Hospital Discharges. Health Information Institute Healthcare Information and Statistics Area Ministry of Health, Social Services and Equality Paseo del Prado18–20 28071 Madrid. Spain. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/cmbdhome.htm (accessed on 20 January 2020).
- Continuous Register Statistics. Available online: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736177012&menu=ultiDatos&idp=1254734710990 (accessed on 27 April 2021).
- National Cancer Institute, Joinpoint Regresion Program, Version 4.6.Bethesda: Nacional Cancer Institute. Available online: http://surveillance.cancer.gov/joinpoint/ (accessed on 20 January 2020).
- Körner, H.; Söreide, J.A.; Pedersen, E.J.; Bru, T.; Söndenaa, K.; Vatten, L. Stability in incidence of acute appendicitis. A population-based longitudinal study. Dig. Surg. 2001, 18, 61–66. [Google Scholar] [CrossRef]
- Ilves, I.; Paajanen, H.E.K.; Herzig, K.H.; Fagerström, A.; Miettinen, P.J. Changing incidence of acute appendicitis and nonspecific abdominal pain between 1987 and 2007 in Finland. World J. Surg. 2011, 35, 731. [Google Scholar] [CrossRef] [PubMed]
- Coward, S.; Kareemi, H.; Clement, F.; Zimmer, S.; Dixon, E.; Ball, C.G.; Heitman, S.J.; Swain, M.; Ghosh, S.; Kaplan, G.G. Incidence of Appendicitis over Time: A Comparative Analysis of an Administrative Healthcare Database and a Pathology-Proven Appendicitis Registry. PLoS ONE 2016, 11, e0165161. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Consumption-Carlos III Health Institute; Health Technology Assessment Agency (AETS). Ultrasound in Primary Care; AETS-Carlos III Health Institute: Madrid, Spain, 1998. [Google Scholar]
- Spanish Society of General and Family Physicians Spanish School of Clinical Ultrasound. Available online: http://www.semg.es/formacion_continuada/ecografia/escuela_espanola_ecografia.html./ (accessed on 4 May 2021).
- Spanish Society of Primary Care Physicians Working Group on Ultrasound. Available online: https://www.semergen.es/index.php?seccion=grupos&subSeccion=detalleGrupo&idG=70 (accessed on 5 April 2021).
- Ultrasound and Primary Care. Spanish Society of Family and Community Medicine. 2017. Available online: https://www.semfyc.es/ecografia-y-atencion-primaria/ (accessed on 10 January 2018).
- Calvo Cebrián, A.; López García-Franco, A.; Short Apellaniz, J. Point of Care Ultrasound in Primary Care. Is it a high resolution tool? Aten Primaria 2018, 50, 500. [Google Scholar] [CrossRef]
- Mowbray, N.G.; Hurt, L.; Powell-Chandler, A.; Reeves, N.; Chandler, S.; Walters, E.; Cornish, J. Where have all the appendicectomies gone? Ann. R. Coll. Surg. Engl. 2021, 103, 250–254. [Google Scholar] [CrossRef]
- Yeh, C.C.; Wu, S.C.; Liao, C.C.; Su, L.T.; Hsieh, C.H.; Li, T.C. Laparoscopic appendectomy for acute appendicitis is more favorable for patients with comorbidities, the elderly, and those with complicated appendicitis: A nationwide population-based study. Surg. Endosc. 2011, 25, 2932–2942. [Google Scholar] [CrossRef]
- Barker, D.J.; Morris, J.; Nelson, M. Vegetable consumption and acute appendicitis in 59 areas in England and Wales. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 927–930. [Google Scholar] [CrossRef]
- Morris, J.; Barker, D.J.; Nelson, M. Diet, infection, and acute appendicitis in Britain and Ireland. J. Epidemiol. Community Health 1987, 41, 44–49. [Google Scholar] [CrossRef]
- García-Fernández, E.; Rico-Cabanas, L.; Rosgaard, N.; Estruch, R.; Bach-Faig, A. Mediterranean diet and cardiodiabesity: A review. Nutrients 2014, 6, 3474–3500. [Google Scholar] [CrossRef] [PubMed]
- Viniol, A.; Keunecke, C.; Biroga, T.; Stadje, R.; Dornieden, K.; Bösner, S.; Donner-Banzhoff, N.; Haasenritter, J.; Becker, A. Studies of the symptom abdominal pain--a systematic review and meta-analysis. Fam. Pract. 2014, 31, 517–529. [Google Scholar] [CrossRef] [PubMed]

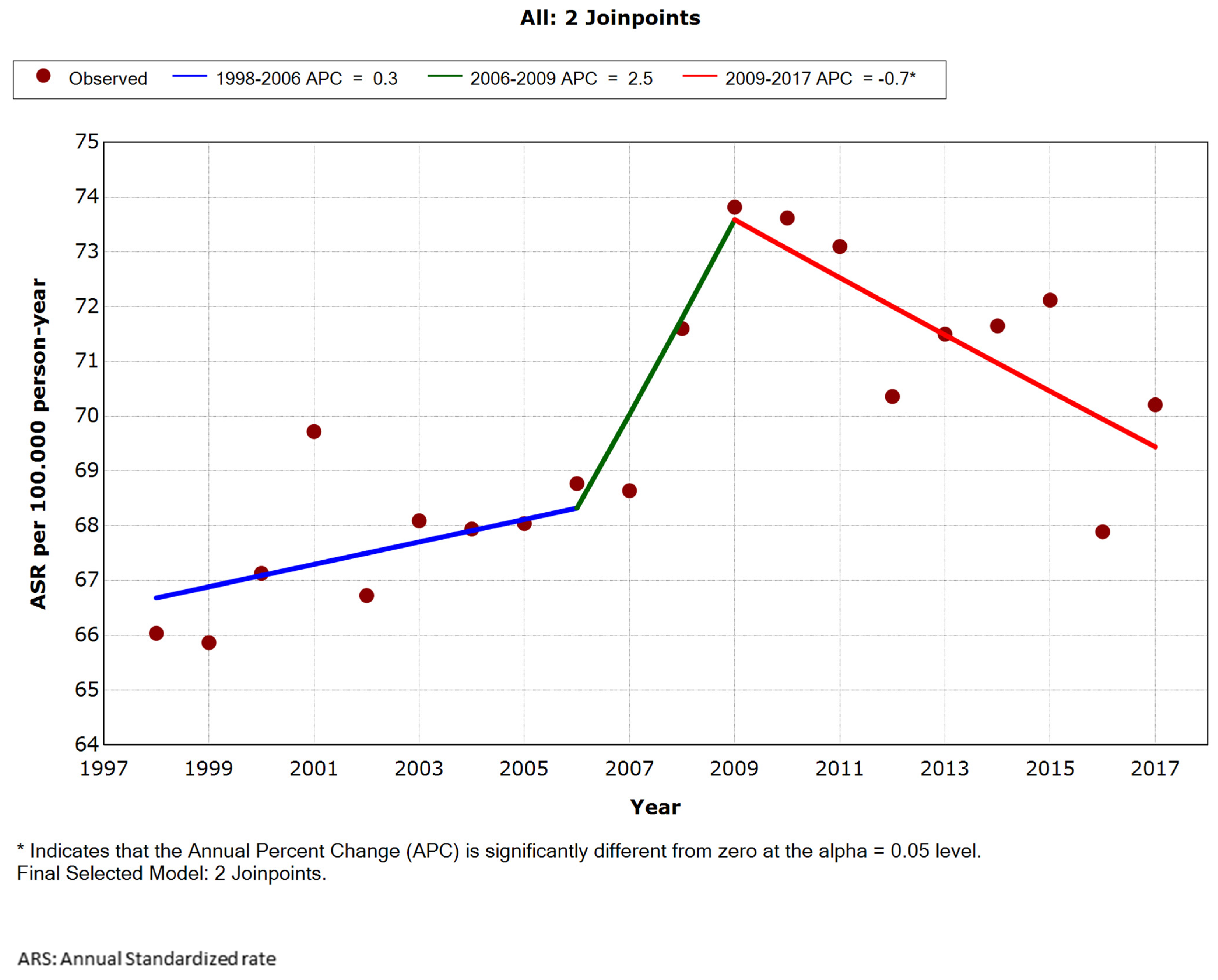
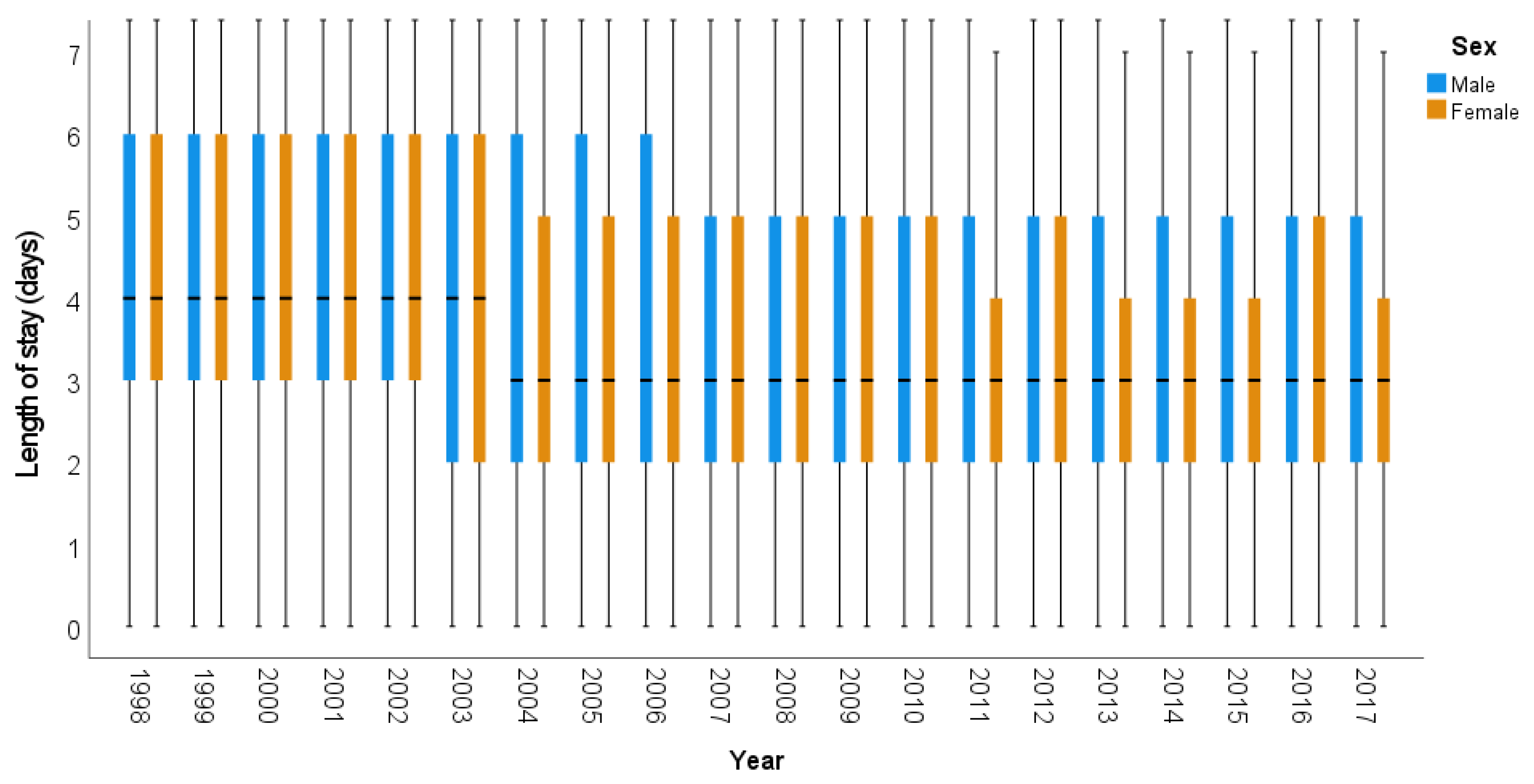
| Gender | Age Group | Time Segment | APC | p-Value | 95% CI | AAPC | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|---|
| Male | All | 1998–2009 | 0.6 | <0.001 | (0.4, 0.8) | −0.1 | (−0.3, 0.1) | 0.340 |
| 2009–2017 | −1.0 | <0.001 | (−1.4, −0.7) | |||||
| 0–14 years | 1998–2013 | 0.1 | 0.399 | (−0.1, 0.4) | −0.7 | (−1.2, −0.3) | 0.001 | |
| 2013–2017 | −3.8 | 0.001 | (−5.8, −1.8) | |||||
| 15–34 years | 1998–2003 | 2.1 | <0.001 | (1.6, 2.6) | 0.0 | (−0.5, 0.5) | 0.985 | |
| 2003–2009 | 0.4 | 0.059 | (−0.0, 0.9) | |||||
| 2009–2012 | −2.4 | 0.134 | (−5.6, 0.9) | |||||
| 2012–2017 | −1.1 | 0.001 | (−1.6, −0.6) | |||||
| 35–44 years | 1998–2005 | 1.5 | 0.001 | (0.7, 2.2) | 0.5 | (0.2, 0.8) | 0.001 | |
| 2005–2017 | −0.1 | 0.440 | (−0.4, 0.2) | |||||
| 45–64 years | 1998–2017 | 0.3 | 0.004 | (0.1, 0.6) | 0.3 | (0.1, 0.6) | 0.004 | |
| ≥65 years | 1998–2006 | −0.9 | 0.157 | (−2.2, 0.4) | 0.2 | (−0.4, 0.8) | 0.533 | |
| 2006–2017 | 1.0 | 0.010 | (0.3, 1.7) | |||||
| Female | All | 1998–2006 | 0.3 | 0.176 | (−0.2, 0.8) | 0.2 | (−0.7, 1.1) | 0.640 |
| 2006–2009 | 2.5 | 0.391 | (−3.5, 8.9) | |||||
| 2009–2017 | −0.7 | 0.003 | (−1.2, −0.3) | |||||
| 0–14 years | 1998–2010 | −0.1 | 0.771 | (−0.5, 0.4) | −1.1 | (−1.5, −0.7) | <0.001 | |
| 2010–2017 | −2.8 | <0.001 | (−3.8, −1.8) | |||||
| 15–34 years | 1998–2006 | 0.8 | 0.001 | (0.4, 1.1) | 0.4 | (−0.5, 1.2) | 0.403 | |
| 2006–2009 | 3.3 | 0.246 | (−2.5, 9.5) | |||||
| 2009–2017 | −1.1 | <0.001 | (−1.5, −0.7) | |||||
| 1998–2010 | 1.8 | <0.001 | (1.2, 2.3) | 1.2 | (0.7, 1.7) | <0.001 | ||
| 35–44 years | 2010–2017 | 0.3 | 0.561 | (−0.8, 1.4) | ||||
| 45–64 years | 1998–2017 | 1.5 | <0.001 | (1.3, 1.7) | 1.5 | (1.3, 1.7) | <0.001 | |
| ≥65 years | 1998–2006 | −1.0 | 0.086 | (−2.2, 0.2) | 0.4 | (−0.2, 1.0) | 0.179 | |
| 2006–2017 | 1.4 | <0.001 | (0.8, 2.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carratalá-Munuera, C.; Pilco, J.d.R.; Orozco-Beltrán, D.; Compañ, A.; Quesada, J.A.; Nouni-García, R.; Gil-Guillén, V.F.; García-Ortíz, L.; López-Pineda, A. Hospitalization Trends for Acute Appendicitis in Spain, 1998 to 2017. Int. J. Environ. Res. Public Health 2021, 18, 12718. https://doi.org/10.3390/ijerph182312718
Carratalá-Munuera C, Pilco JdR, Orozco-Beltrán D, Compañ A, Quesada JA, Nouni-García R, Gil-Guillén VF, García-Ortíz L, López-Pineda A. Hospitalization Trends for Acute Appendicitis in Spain, 1998 to 2017. International Journal of Environmental Research and Public Health. 2021; 18(23):12718. https://doi.org/10.3390/ijerph182312718
Chicago/Turabian StyleCarratalá-Munuera, Concepción, Jessica del Rocio Pilco, Domingo Orozco-Beltrán, Antonio Compañ, Jose A. Quesada, Rauf Nouni-García, Vicente F. Gil-Guillén, Luis García-Ortíz, and Adriana López-Pineda. 2021. "Hospitalization Trends for Acute Appendicitis in Spain, 1998 to 2017" International Journal of Environmental Research and Public Health 18, no. 23: 12718. https://doi.org/10.3390/ijerph182312718
APA StyleCarratalá-Munuera, C., Pilco, J. d. R., Orozco-Beltrán, D., Compañ, A., Quesada, J. A., Nouni-García, R., Gil-Guillén, V. F., García-Ortíz, L., & López-Pineda, A. (2021). Hospitalization Trends for Acute Appendicitis in Spain, 1998 to 2017. International Journal of Environmental Research and Public Health, 18(23), 12718. https://doi.org/10.3390/ijerph182312718








