Effect of Six-Minute Walk Test and Incremental Exercise on Inspiratory Capacity, Ventilatory Constraints, Breathlessness and Exercise Performance in Sedentary Male Smokers without Airway Obstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Protocol
2.3. Body Composition
2.4. Pulmonary Function Tests
2.5. Maximal Inspiratory Pressure
2.6. Six Minutes Walk Test (6-MWT)
2.7. Cardiopulmonary Exercise Testing (CPET)
2.8. Flow-Volume Measurements
2.9. Data Analysis
3. Results
3.1. Subjects
3.2. Pulmonary Function
3.3. Six Minutes Walk Test (6-MWT)
3.4. Cardiopulmonary Exercise Testing (CPET)
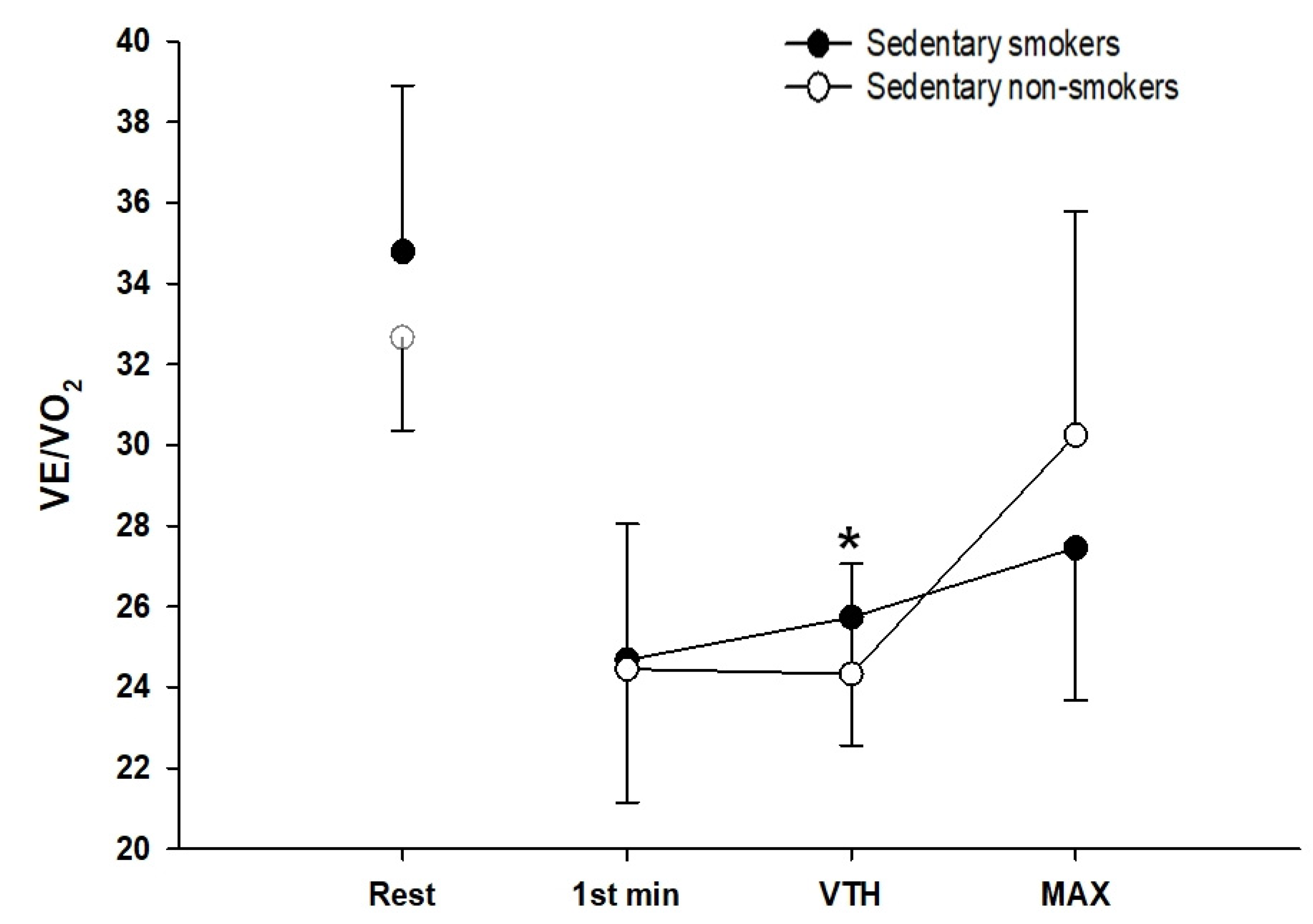
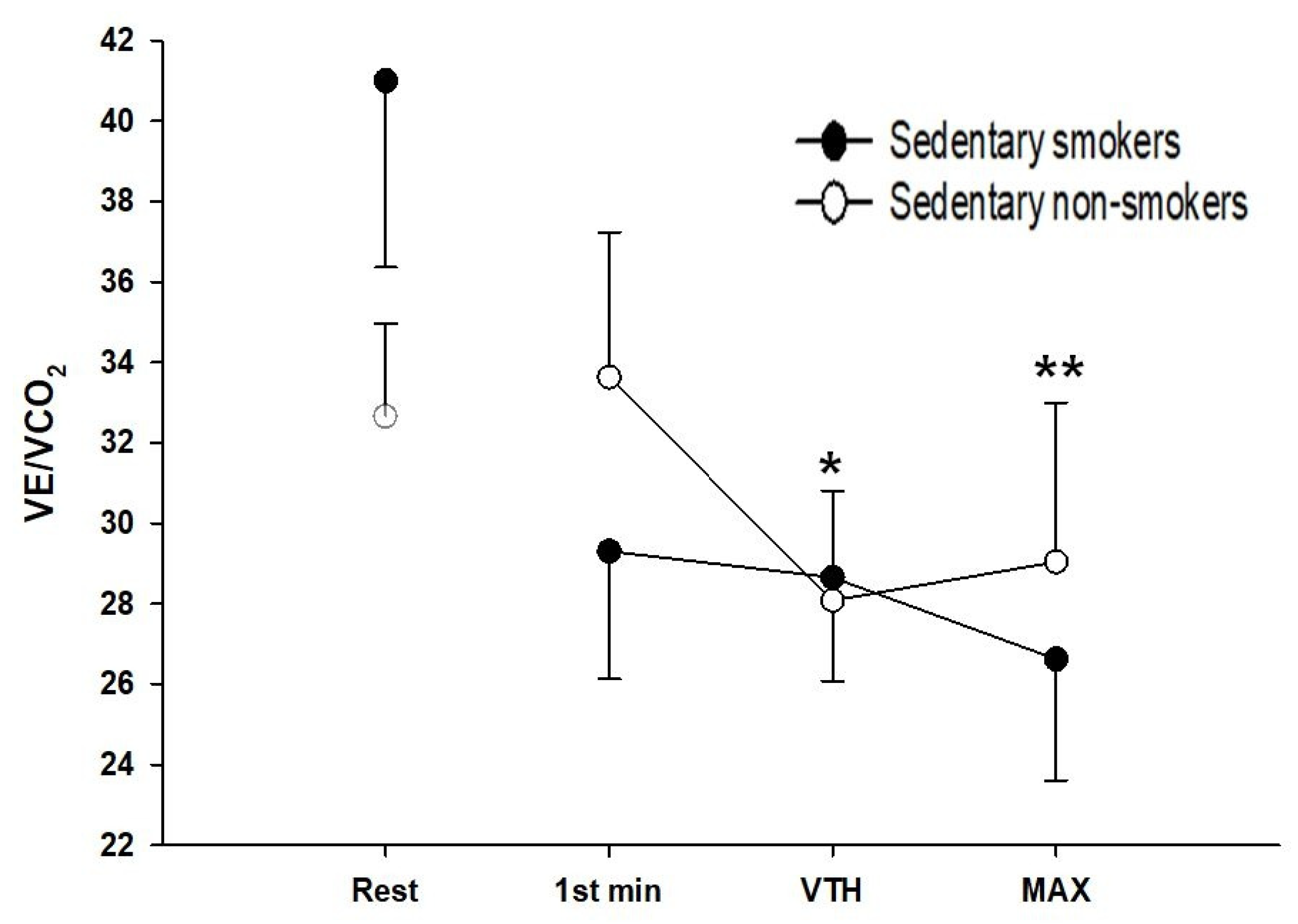
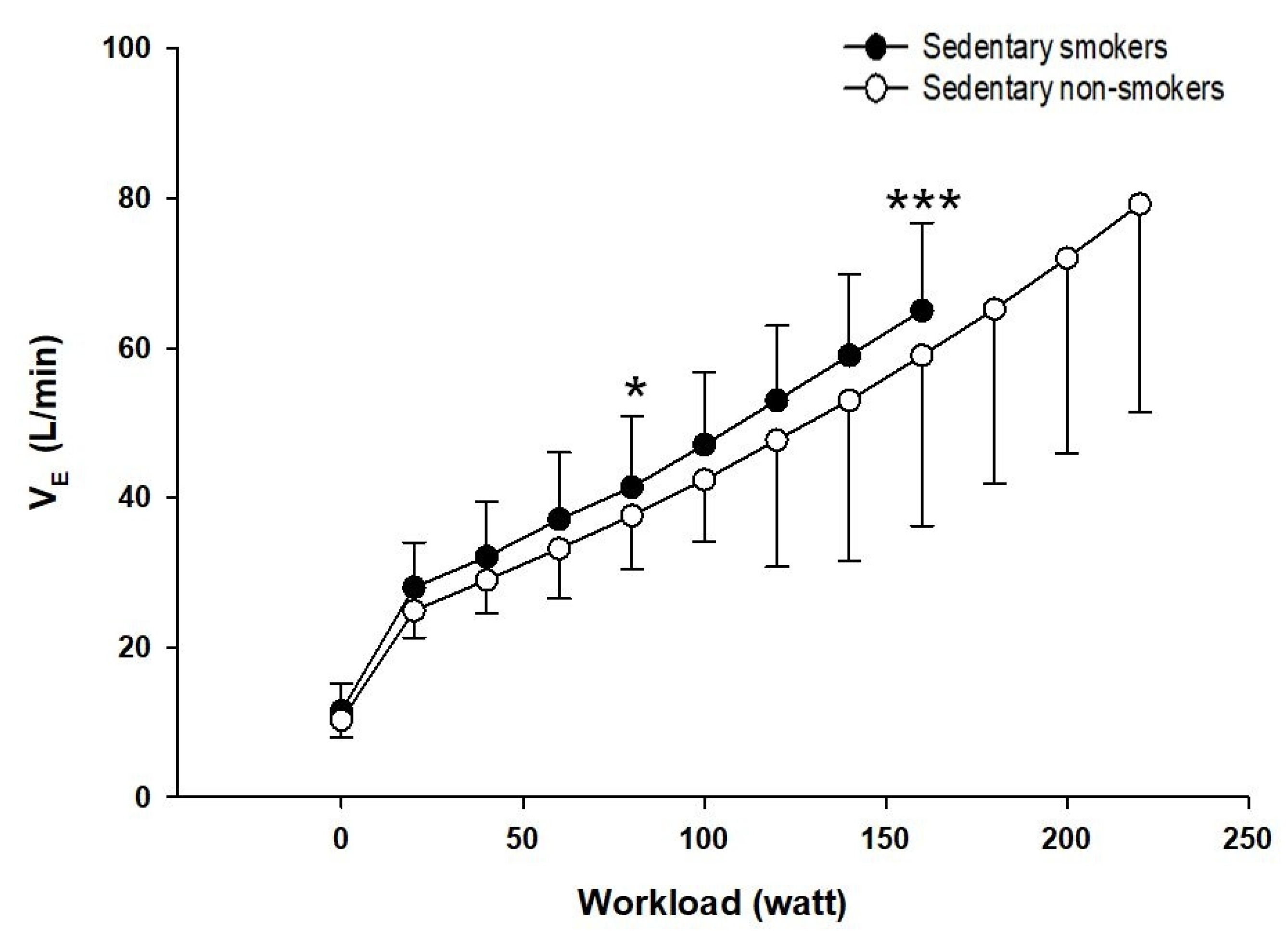
3.5. Ventilatory Responses to Exercise
3.6. Breathing Mechanics
3.7. Maximal Inspiratory Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mackenbach, J.P.; Damhuis, R.A.; Been, J.V. The effects of smoking on health: Growth of knowledge reveals even grimmer picture. Ned. Tijdschr. Voor Geneeskd. 2017, 160, D869. [Google Scholar]
- Brito-Mutunayagam, R.; Appleton, S.L.; Wilson, D.H.; Ruffin, R.E.; Adams, R.J. Global Initiative for Chronic Obstructive Lung Disease stage 0 is associated with excess FEV(1) decline in a representative population sample. Chest 2010, 138, 605–613. [Google Scholar] [CrossRef]
- Hess, M.W. The 2017 Global Initiative for Chronic Obstructive Lung Disease Report and Practice Implications for the Respiratory Therapist. Respir Care 2017, 62, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Vozoris, N.T.; O’Donnell, D.E. Smoking, activity level and exercise test outcomes in a young population sample without cardiopulmonary disease. J. Sports Med. Phys. Fit. 2015, 55, 787–796. [Google Scholar]
- ATS. Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Doutreleau, S.; Di Marco, P.; Talha, S.; Charloux, A.; Piquard, F.; Geny, B. Can the six-minute walk test predict peak oxygen uptake in men with heart transplant? Arch. Phys. Med. Rehabil. 2009, 90, 51–57. [Google Scholar] [CrossRef] [PubMed]
- ATS; ACCP. Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Johnson, B.D.; Weisman, I.M.; Zeballos, R.J.; Beck, K.C. Emerging concepts in the evaluation of ventilatory limitation during exercise: The exercise tidal flow-volume loop. Chest 1999, 116, 488–503. [Google Scholar] [CrossRef]
- Faisal, A.; Alghamdi, B.J.; Ciavaglia, C.E.; Elbehairy, A.F.; Webb, K.A.; Ora, J.; Neder, J.A.; O’Donnell, D.E. Common Mechanisms of Dyspnea in Chronic Interstitial and Obstructive Lung Disorders. Am. J. Respir. Crit. Care Med. 2016, 193, 299–309. [Google Scholar] [CrossRef]
- Dueck, R. Assessment and monitoring of flow limitation and other parameters from flow/volume loops. J. Clin. Monit. Comput. 2000, 16, 425–432. [Google Scholar] [CrossRef]
- DeLorey, D.S.; Babb, T.G. Progressive mechanical ventilatory constraints with aging. Am. J. Respir. Crit. Care Med. 1999, 160, 169–177. [Google Scholar] [CrossRef]
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting: The population health science of sedentary behavior. Exerc. Sport Sci. Rev. 2010, 38, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pleasants, R.A.; Rivera, M.P.; Tilley, S.L.; Bhatt, S.P. Both Duration and Pack-Years of Tobacco Smoking Should Be Used for Clinical Practice and Research. Ann. Am. Thorac. Soc. 2020, 17, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Later, W.; Hitze, B.; Sato, T.; Kossel, E.; Gluer, C.C.; Heller, M.; Muller, M.J. Accuracy of bioelectrical impedance consumer devices for measurement of body composition in comparison to whole body magnetic resonance imaging and dual X-ray absorptiometry. Obes. Facts 2008, 1, 319–324. [Google Scholar] [CrossRef]
- ATS; ERS. Standardization of Lung Function Testing: Standardization of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar]
- Cooper, B.G.; Stocks, J.; Hall, G.L.; Culver, B.; Steenbruggen, I.; Carter, K.W.; Thompson, B.R.; Graham, B.L.; Miller, M.R.; Ruppel, G.; et al. The Global Lung Function Initiative (GLI) Network: Bringing the world’s respiratory reference values together. Breathe (Sheff. Engl.) 2017, 13, e56–e64. [Google Scholar] [CrossRef]
- Clausen, J.L. Measurement of lung volumes in humans: Review and recommendations from an ATS/ERS workshop. Eur. Respir. J. 1997, 10, 1205–1206. [Google Scholar] [CrossRef] [Green Version]
- Black, L.F.; Hyatt, R.E. Maximal respiratory pressures: Normal values and relationship to age and sex. Am. Rev. Respir. Dis. 1969, 99, 696–702. [Google Scholar]
- ATS; ERS. ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Poole, D.C.; Jones, A.M. Measurement of the maximum oxygen uptake Vo2max: Vo2peak is no longer acceptable. J. Appl. Physiol. 2017, 122, 997–1002. [Google Scholar] [CrossRef]
- Caiozzo, V.J.; Davis, J.A.; Ellis, J.F.; Azus, J.L.; Vandagriff, R.; Prietto, C.A.; McMaster, W.C. A comparison of gas exchange indices used to detect the anaerobic threshold. J. Appl. Physiol. 1982, 53, 1184–1189. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, K.; Stringer, W.W.; Casaburi, R.; Koike, A.; Cooper, C.B. Determination of the anaerobic threshold by gas exchange: Biochemical considerations, methodology and physiological effects. Z. Kardiol. 1994, 83 (Suppl. 3), 1–12. [Google Scholar]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, M.; Agostoni, P.; Durigato, A.; Santoriello, C.; Farina, S.; Brusasco, V.; Pellegrino, R. Do maximum flow-volume loops collected during maximum exercise test alter the main cardiopulmonary parameters? Chest 2009, 135, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Ting, E.Y.; Giuffrida, J.G. The effect of changes in the body position obsese patients on pulmonary volume and ventilatory function. Bull. N. Y. Acad. Med. 1976, 52, 830–837. [Google Scholar] [PubMed]
- Younes, M.; Kivinen, G. Respiratory mechanics and breathing pattern during and following maximal exercise. J. Appl. Physiol. 1984, 57, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [Green Version]
- Sengbusch, J.R.; Tiernan, D.L.; Tamulevicius, N.; Martinasek, M.P. The Impact of Smoking on Maximum Oxygen Uptake. Respir. Care 2021, 66, 857–861. [Google Scholar] [CrossRef]
- Watai, K.; Sekiya, K.; Hayashi, H.; Fukutomi, Y.; Taniguchi, M. Effects of short-term smoking on lung function and airway hyper-responsiveness in young patients with untreated intermittent adult-onset asthma: Retrospective cross-sectional study at a primary-tertiary care hospital in Japan. BMJ Open 2019, 9, e023450. [Google Scholar] [CrossRef]
- Rubin, B.K.; Dhand, R.; Ruppel, G.L.; Branson, R.D.; Hess, D.R. Respiratory care year in review 2010: Part 1. asthma, COPD, pulmonary function testing, ventilator-associated pneumonia. Respir. Care 2011, 56, 488–502. [Google Scholar] [CrossRef] [Green Version]
- Allinson, J.; Hardy, R.; Shaheen, S.; Donaldson, G.; Kuh, D.; Wedzicha, J. Combined impact of smoking and early life exposures on adult lung function. Am. J. Respir. Crit. Care Med. 2017, 196, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Carsin, A.E.; Keidel, D. Health-related quality of life and risk factors associated with spirometric restriction. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef] [Green Version]
- Dugral, E.; Balkanci, D. Effects of smoking and physical exercise on respiratory function test results in students of university: A cross-sectional study. Medicine 2019, 98, e16596. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takeuchi, T.; Hosoi, T.; Loeppky, J.A. Effects of habitual smoking on cardiorespiratory responses to sub-maximal exercise. J. Physiol. Anthropol. Appl. Hum. Sci. 2004, 23, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Frazão, M.; Silva, P.E.; Frazão, W.; da Silva, V.Z.M.; Correia, M.A.V., Jr.; Neto, M.G. Dynamic Hyperinflation Impairs Cardiac Performance During Exercise in COPD. J. Cardiopulm. Rehabil. Prev. 2019, 39, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Soffler, M.I.; Hayes, M.M.; Schwartzstein, R.M. Respiratory Sensations in Dynamic Hyperinflation: Physiological and Clinical Applications. Respir. Care 2017, 62, 1212–1223. [Google Scholar] [CrossRef] [Green Version]
- Sinderby, C.; Spahija, J.; Beck, J.; Kaminski, D.; Yan, S.; Comtois, N.; Sliwinski, P. Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 163, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Bertley, J.C.; Chau, L.K.; Webb, K.A. Qualitative aspects of exertional breathlessness in chronic airflow limitation: Pathophysiologic mechanisms. Am. J. Respir. Crit. Care Med. 1997, 155, 109–115. [Google Scholar] [CrossRef]
- Calverley, P.M.; Koulouris, N.G. Flow limitation and dynamic hyperinflation: Key concepts in modern respiratory physiology. Eur. Respir. J. 2005, 25, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, G. Maximal oxygen consumption in healthy humans: Theories and facts. Eur. J. Appl. Physiol. 2014, 114, 2007–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, P.T.; Barbosa, G.W.; O’Donnell, D.E.; Neder, J.A. Cardiopulmonary and Muscular Interactions: Potential Implications for Exercise (In)tolerance in Symptomatic Smokers Without Chronic Obstructive Pulmonary Disease. Front. Physiol. 2019, 10, 859. [Google Scholar] [CrossRef]
- Naeije, R. Lung mechanics and exertional dyspnea in pulmonary arterial hypertension. Respir. Int. Rev. Thorac. Dis. 2014, 88, 16–17. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Neder, J.A.; Elbehairy, A.F. Physiological impairment in mild COPD. Respirol. (Carlton Vic.) 2016, 21, 211–223. [Google Scholar] [CrossRef]
- Pellegrino, R.; Brusasco, V.; Rodarte, J.R.; Babb, T.G. Expiratory flow limitation and regulation of end-expiratory lung volume during exercise. J. Appl. Physiol. 1993, 74, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Poole-Wilson, P.A.; Coats, A.J. Exercise limitation in chronic heart failure: Central role of the periphery. J. Am. Coll. Cardiol. 1996, 28, 1092–1102. [Google Scholar] [CrossRef] [Green Version]


| Sedentary Smokers (n = 25) | Sedentary Non-Smokers (n = 25) | |
|---|---|---|
| Age, yr | 24 ± 1 | 24 ± 2 |
| Ht, cm | 176.3 ± 6.8 | 178.3 ± 3 |
| Wt, kg | 70.5 ± 9.9 | 71.6 ± 8.5 |
| BMI, kg/m2 | 22.8 ± 3.1 | 22.4 ± 2.2 |
| BF (%) | 14.48 ± 6.55 | 13.52 ± 5.06 |
| Pack years | 4 ± 1 | 0 |
| FEV1 (L) | 4.2 ± 0.7 | 4.5 ± 0.6 |
| FEV1 (% predicted) | 93.7 ± 13.7 | 97.5 ± 9.2 |
| FVC (L) | 5.2 ± 0.7 | 5.3 ± 0.8 |
| FEV1/FVC (%) | 79.6 ± 4.5 * | 83.9 ± 5.5 |
| PEF (L/sec) | 6.7 ± 2.3 ** | 9.8 ± 2 |
| PEF (% predicted) | 63.6 ± 12.5 * | 71.6 ± 5.04 |
| TLC (L) | 7.2 ± 1.1 | 7.8 ± 0.6 |
| TLC (% predicted) | 102.8 ± 12.3 * | 107.6 ± 6.8 |
| FRC (L) | 4.2 ± 0.9 | 4.4 ± 0.6 |
| FRC (% predicted) | 129.8 ± 22.4 | 132.2 ± 16.4 |
| RV (L) | 2 ± 0.7 | 2.8 ± 0.4 |
| RV (% predicted) | 124.4 ± 38.6 | 152.2 ± 27.5 |
| Sedentary Smokers (n = 25) | Sedentary Non-Smokers (n = 25) | |
|---|---|---|
| Distance (m) | 584.8 ± 31.7 ** | 636.1 ± 52.2 |
| HR baseline (beats/min) | 72 ± 9 | 70 ± 8 |
| HR peak (beats/min) | 115 ± 14 | 104 ± 11 |
| SBP. DBP baseline (mmHg) | 112.73 ± 5.5 | 112.74 ± 7.2 |
| SBP. DBP peak (mmHg) | 113.76 ± 8.5 | 112.79 ± 9.7 |
| SpO2 baseline (%) | 98 ± 1 | 98 ± 1 |
| SpO2 peak (%) | 95 ± 1 *** | 97 ± 1 |
| Δ SpO2 (%) | −1.2 ± 1.2 | −1.8 ± 1.7 |
| ΔMIP (cmH2O) | −5.6 ± 2.13 | −0.4 ± 1.52 |
| ΔIC from rest, (L) | −0.2 ± 0.3 * | −0.1 ± 0.2 |
| ΔIC (%) | 6.1 ± 9 | 1.2 ± 5.3 |
| Dyspnea baseline (Borg) | 0 | 0 |
| Dyspnea peak (Borg) | 1 | 1 |
| Sedentary Smokers (n = 25) | Sedentary Non-Smokers (n = 25) | |
|---|---|---|
| Duration (min) | 8 ± 1 * | 10 ± 1 |
| Work Rate (Watt) | 160 ± 20 * | 210 ± 10 |
| VO2 (L/min) | 2.2 ± 0.3 | 2.4 ± 0.5 |
| VO2 (mL/Kg/min) | 35.2 ± 5 * | 42.3 ± 2.5 |
| HR (beats/min) | 172 ± 18 | 181 ± 7 |
| HR (%predicted) | 89 ± 8 | 91 ± 5 |
| VO2/HR (mL/beats/min) | 13.4 ± 2.1 ** | 15.1 ± 3.1 |
| RER | 1 ± 0.15 | 1 ± 0.1 |
| RPE (6–20) | 18 ± 2 | 18 ± 1 |
| RPB (0–10) | 9 ± 2 | 6 ± 3 |
| ∆ SpO2 | −3 ± 2 * | −2 ± 1 |
| EELV/TLC (%) | EILV/TLC (%) | |||||
|---|---|---|---|---|---|---|
| Rest | VTH | Max | Rest | VTH | Max | |
| Sedentary Smokers (n = 25) | 59.3 ± 6.5 * | 57 ± 3.4 * | 61.8 ± 6.8 | 68 ± 6.9 * | 73 ± 0.2 * | 91 ± 5.9 |
| Sedentary Non-Smokers (n = 25) | 64.2 ± 4.9 | 62 ± 5.1 | 61.9 ± 6.6 | 72.2 ± 5.9 | 77 ± 6.3 | 87.4 ± 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melliti, W.; Kammoun, R.; Masmoudi, D.; Ahmaidi, S.; Masmoudi, K.; Alassery, F.; Hamam, H.; Chlif, M. Effect of Six-Minute Walk Test and Incremental Exercise on Inspiratory Capacity, Ventilatory Constraints, Breathlessness and Exercise Performance in Sedentary Male Smokers without Airway Obstruction. Int. J. Environ. Res. Public Health 2021, 18, 12665. https://doi.org/10.3390/ijerph182312665
Melliti W, Kammoun R, Masmoudi D, Ahmaidi S, Masmoudi K, Alassery F, Hamam H, Chlif M. Effect of Six-Minute Walk Test and Incremental Exercise on Inspiratory Capacity, Ventilatory Constraints, Breathlessness and Exercise Performance in Sedentary Male Smokers without Airway Obstruction. International Journal of Environmental Research and Public Health. 2021; 18(23):12665. https://doi.org/10.3390/ijerph182312665
Chicago/Turabian StyleMelliti, Wassim, Rim Kammoun, Donies Masmoudi, Said Ahmaidi, Kaouthar Masmoudi, Fawaz Alassery, Habib Hamam, and Mehdi Chlif. 2021. "Effect of Six-Minute Walk Test and Incremental Exercise on Inspiratory Capacity, Ventilatory Constraints, Breathlessness and Exercise Performance in Sedentary Male Smokers without Airway Obstruction" International Journal of Environmental Research and Public Health 18, no. 23: 12665. https://doi.org/10.3390/ijerph182312665
APA StyleMelliti, W., Kammoun, R., Masmoudi, D., Ahmaidi, S., Masmoudi, K., Alassery, F., Hamam, H., & Chlif, M. (2021). Effect of Six-Minute Walk Test and Incremental Exercise on Inspiratory Capacity, Ventilatory Constraints, Breathlessness and Exercise Performance in Sedentary Male Smokers without Airway Obstruction. International Journal of Environmental Research and Public Health, 18(23), 12665. https://doi.org/10.3390/ijerph182312665







