Insulin Resistance Is Associated with Multiple Chemical Sensitivity in a Danish Population-Based Study—DanFunD
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- The participant needed to have experienced symptoms upon being exposed to at least 2 of 11 common odors and airborne chemicals; and
- The parcipant needed to have experienced at least one symptom from the central nervous system and at least one symptom from another organ system in response to the inhalation of airborne odors or chemicals; and
- The participant needed to report significant lifestyle or functional impairment due to the symptoms related to the inhalation of airborne chemicals and odor, which could be defined as responding affirmably to the statements (A) symptoms have influenced my choice of products used for personal hygiene, products used for cleaning at home, and/or the choice of places where I do my daily shopping and either (B) symptoms have negatively influenced my social lifestyle (i.e., limited my possibility to use public transport, dine at restaurants, go to the cinema/theatre, participate in meetings/social events) or (C) symptoms have negatively influenced my occupational condition (i.e., have had to go on temporary sick leave, discontinued education/employment, been unable to hold a job position, or unable to complete my education).
2.2. Assessment of Biomarkers
2.3. Lifestyle Factors and Physiological Assessments
2.4. Statistical Analyses
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, N.D.; Linneberg, A.; Dirksen, A.; Elberling, J. Prevalence of Self-Reported Symptoms and Consequences Related to Inhalation of Airborne Chemicals in a Danish General Population. Int. Arch. Occup. Environ. Health 2008, 81, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Dantoft, T.M.; Nordin, S.; Andersson, L.; Petersen, M.W.; Skovbjerg, S.; Jørgensen, T. Multiple Chemical Sensitivity Described in the Danish General Population: Cohort Characteristics and the Importance of Screening for Functional Somatic Syndrome Comorbidity-The DanFunD Study. PLoS ONE 2021, 16, e0246461. [Google Scholar] [CrossRef]
- Kreutzer, R. MCS: The Status of Population-Based Research. Int. J. Hyg. Environ. Health 2002, 205, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Anne, S. International Prevalence of Chemical Sensitivity, Co-Prevalences with Asthma and Autism, and Effects from Fragranced Consumer Products. Air Qual. Atmos. Health 2019, 12, 519–527. [Google Scholar] [CrossRef]
- Park, J.; Gilmour, H. Medically Unexplained Physical Symptoms (MUPS) among Adults in Canada: Comorbidity, Health Care Use and Employment. Health Rep. 2017, 28, 3–8. [Google Scholar]
- Hausteiner, C.; Bornschein, S.; Hansen, J.; Zilker, T.; Förstl, H. Self-Reported Chemical Sensitivity in Germany: A Population-Based Survey. Int. J. Hyg. Environ. Health 2005, 208, 271–278. [Google Scholar] [CrossRef]
- Skovbjerg, S.; Elberling, J.; Brorson, S.; Rasmussen, A.; Johansen, J.D. Impact of Self-Reported Multiple Chemical Sensitivity on Everyday Life: A Qualitative Study. Scand. J. Public Health 2009, 37, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Caress, S.M.; Steinemann, A.C. A Review of a Two-Phase Population Study of Multiple Chemical Sensitives. Environ. Health Perspect. 2003, 111, 1490–1497. [Google Scholar] [CrossRef]
- Petersen, M.W.; Schroder, A.; Jorgensen, T.; Ornbol, E.; Dantoft, T.M.; Eliasen, M.; Thuesen, B.H.; Fink, P. The Unifying Diagnostic Construct of Bodily Distress Syndrome (BDS) Was Confirmed in the General Population. J. Psychosom. Res. 2019, 128, 109868. [Google Scholar] [CrossRef]
- Azuma, K.; Uchiyama, I.; Katoh, T.; Ogata, H.; Arashidani, K.; Kunugita, N. Prevalence and Characteristics of Chemical Intolerance: A Japanese Population-Based Study. Arch. Environ. Occup. Health 2015, 70, 341–353. [Google Scholar] [CrossRef]
- Steinemann, A. Prevalence and Effects of Multiple Chemical Sensitivities in Australia. Prev. Med. Rep. 2018, 10, 191–194. [Google Scholar] [CrossRef]
- Caress, S.M.; Steinemann, A.C. Prevalence of Multiple Chemical Sensitivities: A Population-Based Study in the Southeastern United States. Am. J. Public Health 2004, 94, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S. The Compelling Anomaly of Chemical Intolerance. Ann. N. Y. Acad. Sci 2001, 933, 1–23. [Google Scholar] [CrossRef]
- De Luca, C.; Scordo, M.G.; Cesareo, E.; Pastore, S.; Mariani, S.; Maiani, G.; Stancato, A.; Loreti, B.; Valacchi, G.; Lubrano, C.; et al. Biological Definition of Multiple Chemical Sensitivity from Redox State and Cytokine Profiling and Not from Polymorphisms of Xenobiotic-Metabolizing Enzymes. Toxicol. Appl. Pharmacol. 2010, 248, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, M.; Laurino, C.; Corazzari, V.; Palmieri, B. A Proposal for Clinical Biomarkers in Multiple Chemical Sensitivity. Clin. Ter. 2020, 171, e149–e155. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.J.; McKeown-Eyssen, G.E.; Riley, N.; Cole, D.E.; Marshall, L.; Loescher, B.; Jazmaji, V. Case-Control Study of Multiple Chemical Sensitivity, Comparing Haematology, Biochemistry, Vitamins and Serum Volatile Organic Compound Measures. Occup. Med. 2004, 54, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Loevinger, B.L.; Muller, D.; Alonso, C.; Coe, C.L. Metabolic Syndrome in Women with Chronic Pain. Metabolism 2007, 56, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.M.; Boneva, R.S.; Lin, J.M.S.; Reeves, W.C. Chronic Fatigue Syndrome Is Associated with Metabolic Syndrome: Results from a Case-Control Study in Georgia. Metabolism 2010, 59, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Niu, K.; Momma, H.; Kobayashi, Y.; Chujo, M.; Otomo, A.; Fukudo, S.; Nagatomi, R. Irritable Bowel Syndrome Is Positively Related to Metabolic Syndrome: A Population-Based Cross-Sectional Study. PLoS ONE 2014, 9, e112289. [Google Scholar] [CrossRef] [PubMed]
- Dantoft, T.M.; Ebstrup, J.F.; Linneberg, A.; Skovbjerg, S.; Madsen, A.L.; Mehlsen, J.; Brinth, L.; Eplov, L.; Carstensen, T.; Schroder, A.; et al. Cohort Description: The Danish Study of Functional Disorders. Clin. Epidemiol. 2017, 9, 127–139. [Google Scholar] [CrossRef]
- Petersen, M.W.; Schroder, A.; Jorgensen, T.; Ornbol, E.; Meinertz Dantoft, T.; Eliasen, M.; Benros, M.E.; Fink, P. Irritable Bowel, Chronic Widespread Pain, Chronic Fatigue and Related Syndromes Are Prevalent and Highly Overlapping in the General Population: DanFunD. Sci. Rep. 2020, 10, 3273. [Google Scholar] [CrossRef]
- Lacour, M.; Zunder, T.; Schmidtke, K.; Vaith, P.; Scheidt, C. Multiple Chemical Sensitivity Syndrome (MCS)—Suggestions for an Extension of the US MCS-Case Definition. Int. J. Hyg. Environ. Health 2005, 208, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Read, D. Multiple chemical sensitivity: A 1999 consensus. Arch Environ Health. 1999, 54, 147–149. [Google Scholar] [CrossRef]
- White, K.P.; Harth, M.; Speechley, M.; ØStbye, T. Testing an Instrument to Screen for Fibromyalgia Syndrome in General Population Studies: The London Fibromyalgia Epidemiology Study Screening Questionnaire. J. Rheumatol. 1999, 26, 880–884. [Google Scholar]
- Cella, M.; Chalder, T. Measuring Fatigue in Clinical and Community Settings. J. Psychosom. Res. 2010, 69, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a Fatigue Scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Kay, L.; Jørgensen, T.; Schultz-Larsen, K.; Davidsen, M. Irritable Bowel Syndrome and Upper Dyspepsia among the Elderly: A Study of Symptom Clusters in a Random 70 Year Old Population. Eur. J. Epidemiol. 1996, 12, 199–204. [Google Scholar] [CrossRef]
- Kasch, H.; Qerama, E.; Kongsted, A.; Bendix, T.; Jensen, T.S.; Bach, F.W. Clinical Assessment of Prognostic Factors for Long-Term Pain and Handicap after Whiplash Injury: A 1-Year Prospective Study. Eur. J. Neurol. 2008, 15, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Aadahl, M.; Kjær, M.; Kristensen, J.H.; Mollerup, B.; Jørgensen, T. Self-Reported Physical Activity Compared with Maximal Oxygen Uptake in Adults. Eur. J. Prev. Cardiol. 2007, 14, 422–428. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Olsen, L.R.; Mortensen, E.L.; Bech, P. The SCL-90 and SCL-90R Versions Validated by Item Response Models in a Danish Community Sample. Acta Psychiatr. Scand. 2004, 110, 225–229. [Google Scholar] [CrossRef]
- American Heart Association. Symptoms and Diagnosis of Metabolic Syndrome. Available online: https://www.heart.org/en/health-topics/metabolic-syndrome/symptoms-and-diagnosis-of-metabolic-syndrome (accessed on 2 September 2021).
- Wahrenberg, H.; Hertel, K.; Leijonhufvud, B.M.; Persson, L.G.; Toft, E.; Arner, P. Use of Waist Circumference to Predict Insulin Resistance: Retrospective Study. Br. Med. J. 2005, 330, 1363–1364. [Google Scholar] [CrossRef]
- Racette, S.B.; Evans, E.M.; Weiss, E.P.; Hagberg, J.M.; Holloszy, J.O. Abdominal Adiposity Is a Stronger Predictor of Insulin Resistance than Fitness among 50–95 Year Olds. Diabetes Care 2006, 29, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Tsao, Y.C.; Tzeng, I.S.; Chuang, H.H.; Li, W.C.; Tung, T.H.; Chen, J.Y. Body Mass Index and Waist Circumference Are Better Predictors of Insulin Resistance than Total Body Fat Percentage in Middle-Aged and Elderly Taiwanese. Medicine 2017, 96, e8126. [Google Scholar] [CrossRef]
- Xing, L.W.; Zhang, L.; Youker, K.; Zhang, M.X.; Wang, J.; LeMaire, S.A.; Coselli, J.S.; Shen, Y.H. Free Fatty Acids Inhibit Insulin Signaling-Stimulated Endothelial Nitric Oxide Synthase Activation through Upregulating PTEN or Inhibiting Akt Kinase. Diabetes 2006, 55, 2301–2310. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, N.; Song, L.; Xie, H.; Zhao, C.; Li, S.; Zhao, W.; Zhao, Y.; Gao, C.; Xu, G. Adipokines and Free Fatty Acids Regulate Insulin Sensitivity by Increasing MicroRNA-21 Expression in Human Mature Adipocytes. Mol. Med. Rep. 2017, 16, 2254–2258. [Google Scholar] [CrossRef] [PubMed]
- Fabricio, G.; Malta, A.; Chango, A.; De Freitas Mathias, P.C. Environmental Contaminants and Pancreatic Beta-Cells. JCRPE J. Clin. Res. Pediatric Endocrinol. 2016, 8, 257–263. [Google Scholar] [CrossRef]
- Papalou, O.; Kandaraki, E.A.; Papadakis, G.; Diamanti-Kandarakis, E. Endocrine Disrupting Chemicals: An Occult Mediator of Metabolic Disease. Front. Endocrinol. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Newbold, R.; Schug, T.T. Endocrine Disruptors and Obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Salmon, A.B. Oxidative Stress in the Etiology of Age-Associated Decline in Glucose Metabolism. Longev. Health 2012, 1, 7. [Google Scholar] [CrossRef]
- De Luca, C.; Raskovic, D.; Pacifico, V.; Thai, J.C.S.; Korkina, L. The Search for Reliable Biomarkers of Disease in Multiple Chemical Sensitivity and Other Environmental Intolerances. Int. J. Environ. Res. Public Health 2011, 8, 2770–2797. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Silman, A.J. Psychological Stress and Fibromyalgia: A Review of the Evidence Suggesting a Neuroendocrine Link. Arthritis Res. Ther. 2004, 6, 98–106. [Google Scholar] [CrossRef]
- Qin, H.Y.; Cheng, C.W.; Tang, X.D.; Bian, Z.X. Impact of Psychological Stress on Irritable Bowel Syndrome. World J. Gastroenterol. 2014, 20, 14126–14131. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Tsigos, C. Stress Hormones: Physiological Stress and Regulation of Metabolism. Curr. Opin. Pharmacol. 2009, 9, 787–793. [Google Scholar] [CrossRef]
- Petersen, M.W.; Schroder, A.; Jorgensen, T.; Ornbol, E.; Dantoft, T.M.; Eliasen, M.; Carstensen, T.W.; Falgaard Eplov, L.; Fink, P. Prevalence of Functional Somatic Syndromes and Bodily Distress Syndrome in the Danish Population: The DanFunD Study. Scand. J. Public Health 2019, 48, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Dantoft, T.M.; Andersson, L.; Nordin, S.; Skovbjerg, S. Chemical Intolerance. Curr. Rheumatol. Rev. 2015, 11, 167–184. [Google Scholar] [CrossRef]
- Schovsbo, S.U.; Dantoft, T.M.; Thuesen, B.H.; Leth-Moller, K.B.; Eplov, L.F.; Petersen, M.W.; Jorgensen, T.; Osler, M. Social Position and Functional Somatic Disorders. The DanFunD Study. Scand. J. Public Health 2021. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Alessandrini, M.; Caccamo, D.; Cormano, A.; Guzzi, G.; Mazzatenta, A.; Micarelli, A.; Migliore, A.; Piroli, A.; Bianca, M.; et al. Italian Expert Consensus on Clinical and Therapeutic Management of Multiple Chemical Sensitivity (MCS). Int. J. Environ. Res. Public Health 2021, 18, 11294. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.W.; Ørnbøl, E.; Dantoft, T.M.; Fink, P. Assessment of Functional Somatic Disorders in Epidemiological Research: Self-Report Questionnaires versus Diagnostic Interviews. J. Psychosom. Res. 2021, 146, 110491. [Google Scholar] [CrossRef] [PubMed]
| Controls (n = 7791) | MCS + FSD (n = 188) | p-Value | MCS ÷ FSD (n = 109) | p-Value | |
|---|---|---|---|---|---|
| Age | 54 (26–70) | 56 (25–70) | 0.23 | 57 (24–70) | 0.04 |
| Sex (% women) | 51 | 67 | <0.0001 | 61 | 0.03 |
| Anthropometry | |||||
| Weight (kg) | 75.9 (54.7–105) | 74.6 (52.7–112) | 0.98 | 73.1 (52.0–111) | 0.44 |
| Height (cm) (mean, SD) | 172 (9.4) | 169 (0.08) | <0.001 | 170 (7.8) | 0.004 |
| BMI (kg/m2) (mean, SD) | 25.9 (4.4) | 27.3 (5.78) | 0.001 | 26.4 (5.0) | 0.35 |
| Waist circumference (cm) | 88.0 (69.5–112) | 90.0 (67.8–120) | 0.06 | 88.6 (67.8–115) | 0.40 |
| Fat percentage | 28.6 (15.4–44.4) | 33.9 (18.0–48.3) | <0.0001 | 31.6 (17.2–46.3) | 0.003 |
| BMI groups (%) | <0.0001 | 0.04 | |||
| Normal weight | 47.0 | 41.0 | 46.8 | ||
| Overweight | 37.2 | 30.8 | 29.4 | ||
| Obese (class I-III) | 15.7 | 28.2 | 23.8 | ||
| Physical activity METS (mean, SD) | 39.9 (4.28) | 40.2 (4.66) | 0.39 | 40.9 (4.88) | 0.01 |
| Cohens Stress scale | 9 (1–19) | 13 (3–25) | <0.0001 | 12 (2–22) | 0.0001 |
| SCL Anxiety | 1 (0–7) | 4 (0–16) | <0.0001 | 2 (0–13) | <0.0001 |
| SCL Depression | 2 (0–12) | 6 (0–27) | <0.0001 | 4 (0–19) | <0.0001 |
| High self-perceived health (%) | 56 | 28 | <0.0001 | 38 | 0.0003 |
| High self-perceived fitness (%) | 44 | 26 | <0.0001 | 38 | 0.21 |
| Biochemical markers | |||||
| Total cholesterol (mmol/L) (mean, SD) | 5.32 (1.05) | 5.32 (1.14) | 0.99 | 5.48 (1.18) | 0.13 |
| HDL-cholesterol (mmol/L) | 1.41 (0.89–2.25) | 1.42 (0.85–2.24) | 0.94 | 1.47 (0.85–2.21) | 0.26 |
| Non-HDL-cholesterol (mmol/L) (mean, SD) | 3.86 (1.05) | 3.84 (1.16) | 0.86 | 3.97 (1.25) | 0.33 |
| Triglycerides (mmol/L) | 1.03 (0.54–2.59) | 1.09 (0.56–2.66) | 0.09 | 1.08 (0.54–2.54) | 0.30 |
| Glucose (mmol/L) | 5.40 (4.6–6.8) | 5.4 (4.6–7.2) | 0.29 | 5.4 (4.6–7.2) | 0.66 |
| HbA1c (mmol/mol) (mean, SD) | 35.9 (5.39) | 36.5 (6.05) | 0.23 | 36.6 (7.04) | 0.33 |
| Insulin (pmol/L) | 48.9 (19.0–150) | 57.8 (21.3–202) | 0.005 | 52.9 (23.2–176) | 0.16 |
| HOMA-IR a | 1.70 (0.61–6.04) | 1.90 (0.65–7.77) | 0.017 | 1.84 (0.69–6.99) | 0.21 |
| Individuals with diabetes b (%) | 4 | 3 | 0.29 | 3 | 0.63 |
| Individuals with metabolic syndrome c (%) | 19 | 28 | 0.002 | 23 | 0.27 |
| n Cases/Total n | % Difference (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | ||
| Total cholesterol | 186/7950 | −1 (−4, 2) | −1 (−4, 1) |
| HDL-cholesterol | 186/7952 | −2 (−6, 1) | 1 (−3, 4) |
| Non HDL-cholesterol | 183/7890 | 0 (−4, 4) | −2 (−6, 1) |
| Triglycerides | 186/7952 | 12 (2, 22) * | 3 (−5, 12) |
| Glucose | 179/7590 | 2 (0, 4) * | 1 (−1, 3) |
| HbA1c | 178/7419 | 2 (1, 4) * | 1 (0, 3) |
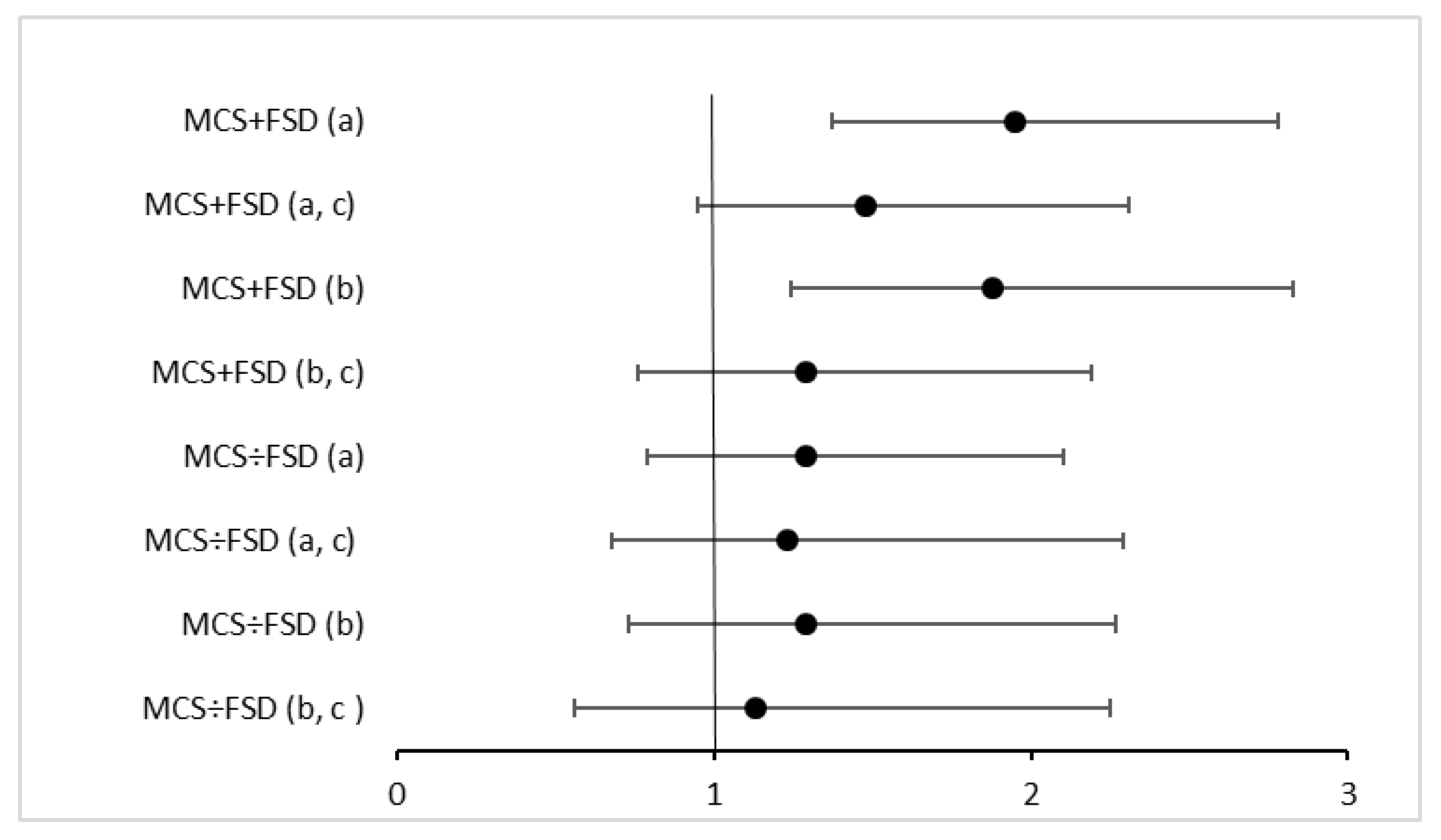
| Insulin | 172/7314 | 42 (28, 57) * | 23 (14, 34) * |
| HOMA-IR | 171/7295 | 53 (37, 71) * | 33 (21, 46) * |
| n Cases/Total n | % Difference (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | ||
| Total cholesterol | 157/6581 | 0 (−3, 3) | −1 (−4, 2) |
| HDL-cholesterol | 157/6582 | −3 (−7, 1) | −1 (−4, 3) |
| Non HDL-cholesterol | 155/6543 | 1 (−2, 5) | −1 (−5, 3) |
| Triglycerides | 157/6582 | 12 (2, 23) * | 6 (−3, 16) |
| Glucose | 155/6562 | 1 (−1, 3) | 1 (−1, 2) |
| HbA1c | 155/6414 | 2 (0, 4) * | 1 (0, 3) |
| Insulin | 149/6322 | 24 (10, 39) * | 5 (−5, 15) |
| HOMA-IR | 148/6308 | 29 (13, 46) * | 9 (−2, 21) |
| n Cases/Total n | % Difference (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | ||
| Total cholesterol | 108/7872 | 2 (−2, 5) | 1 (−3, 4) |
| HDL-cholesterol | 108/7874 | 0 (−5, 5) | 1 (−4, 5) |
| Non HDL-cholesterol | 106/7813 | 3 (−2, 8) | 1 (−4, 6) |
| Triglycerides | 108/7874 | 7 (−5, 20) | 5 (−6,16) |
| Glucose | 105/7516 | 2 (0, 4) | 2 (0, 4) |
| HbA1C | 103/7529 | 2 (0, 4) * | 2 (0, 4) |
| Insulin | 99/7241 | 20 (4, 38) * | 13 (0, 28) * |
| HOMA-IR | 99/7223 | 27 (8, 48) * | 25 (10, 42) * |
| n Cases/Total n | % Difference (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | ||
| Total cholesterol | 96/6677 | 2 (−1, 6) | 1 (−2, 5) |
| HDL-cholesterol | 96/6378 | −1 (−6, 4) | −1 (−5, 4) |
| Non HDL-cholesterol | 94/6637 | 4 (−1, 9) | 2 (−2, 7) |
| Triglycerides | 96/6678 | 9 (−4, 24) | 8 (−3, 21) |
| Glucose | 96/6658 | 2 (0, 5) * | 2 (0, 5) * |
| HbA1c | 95/6666 | 3 (1, 5) * | 3 (1, 5) * |
| Insulin | 91/6413 | 24 (7, 42) * | 15 (2, 30) * |
| HOMA-IR | 91/6399 | 32 (13, 54) * | 29 (14, 46) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjerregaard, A.A.; Petersen, M.W.; Gormsen, L.K.; Skovbjerg, S.; Jørgensen, N.R.; Linneberg, A.; Cedeño-Laurent, J.G.; Jørgensen, T.; Dantoft, T.M. Insulin Resistance Is Associated with Multiple Chemical Sensitivity in a Danish Population-Based Study—DanFunD. Int. J. Environ. Res. Public Health 2021, 18, 12654. https://doi.org/10.3390/ijerph182312654
Bjerregaard AA, Petersen MW, Gormsen LK, Skovbjerg S, Jørgensen NR, Linneberg A, Cedeño-Laurent JG, Jørgensen T, Dantoft TM. Insulin Resistance Is Associated with Multiple Chemical Sensitivity in a Danish Population-Based Study—DanFunD. International Journal of Environmental Research and Public Health. 2021; 18(23):12654. https://doi.org/10.3390/ijerph182312654
Chicago/Turabian StyleBjerregaard, Anne A., Marie W. Petersen, Lise Kirstine Gormsen, Sine Skovbjerg, Niklas R. Jørgensen, Allan Linneberg, José G. Cedeño-Laurent, Torben Jørgensen, and Thomas M. Dantoft. 2021. "Insulin Resistance Is Associated with Multiple Chemical Sensitivity in a Danish Population-Based Study—DanFunD" International Journal of Environmental Research and Public Health 18, no. 23: 12654. https://doi.org/10.3390/ijerph182312654
APA StyleBjerregaard, A. A., Petersen, M. W., Gormsen, L. K., Skovbjerg, S., Jørgensen, N. R., Linneberg, A., Cedeño-Laurent, J. G., Jørgensen, T., & Dantoft, T. M. (2021). Insulin Resistance Is Associated with Multiple Chemical Sensitivity in a Danish Population-Based Study—DanFunD. International Journal of Environmental Research and Public Health, 18(23), 12654. https://doi.org/10.3390/ijerph182312654








