Effects of High-Intensity Interval Training on Inflammatory Biomarkers in Patients with Type 2 Diabetes. A Systematic Review
Abstract
1. Introduction
1.1. Approach to the Problem
1.2. Objectives of the Study
2. Materials and Methods
2.1. Search Strategy
- P: Diabetes Mellitus Type 2.
- I: High-Intensity Interval Training.
- C: Alternative treatment or control.
- O: Values of body composition and inflammatory biomarkers.
2.2. Selection Criteria
2.3. Paper Selection and Data Abstraction
2.4. Quality Assessment
3. Results
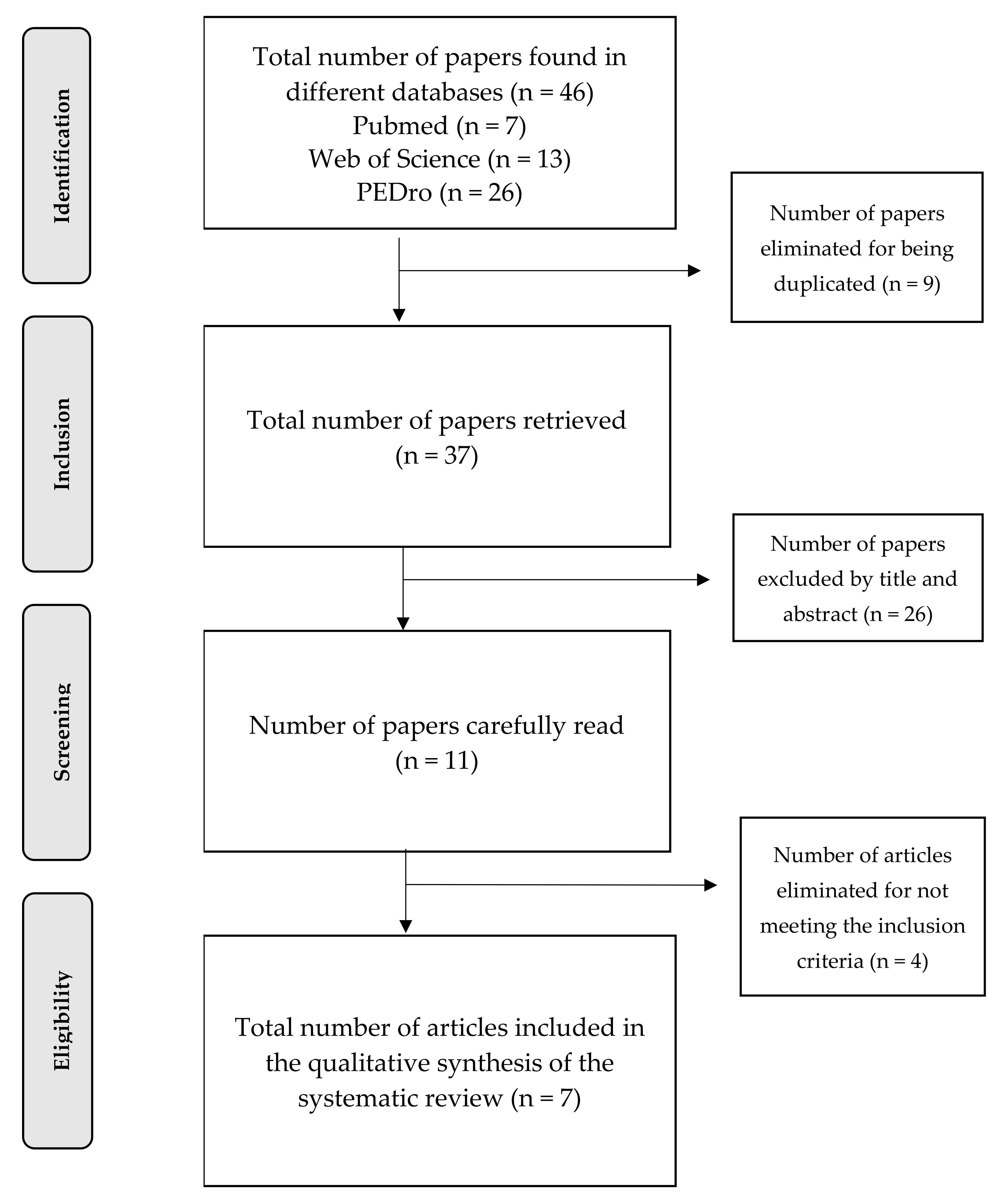
3.1. Selection of Studies
3.2. Evaluation of the Quality of the Studies
3.3. Data Abstraction
3.4. General Description of Data Obtained
3.5. Characteristics of HIIT, Values of Body Composition and Biomarkers
4. Discussion
4.1. HIIT and Inflammatory Biomarkers
4.2. HIIT and Values of Body Composition
4.3. Values of Body Composition and Biomarkers
4.4. HIIT, Biomarkers and Diet
4.5. Effects on Inflammatory Biomarkers of the Different Intervention Modalities
4.6. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batista, T.M.; Haider, N.; Kahn, C.R. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia 2021, 64, 994–1006. [Google Scholar] [CrossRef]
- International Diabetes Federation. In IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; pp. 1–176.
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef] [PubMed]
- Waugh, N.R.; Shyangdan, D.; Taylor-Phillips, S.; Suri, G.; Hall, B. Screening for type 2 diabetes: A short report for the National Screening Committee. Health Technol. Assess. 2013, 17, 1–89. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, N. Risk factors for type 2 diabetes. U.S. Pharm. 2007, 32, 61–63. [Google Scholar]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Smith, S.C.; Sowers, J.R. Diabetes and cardiovascular disease: A statement for healthcare professionals from the american heart association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef]
- Haffner, S.M. Obesity and the metabolic syndrome: The San Antonio Heart Study. Br. J. Nutr. 2000, 83, 67–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Disease, C. Diabetes mellitus: A major risk factor for cardiovascular disease. Circulation 1999, 100, 1132–1133. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Thomas, D.; Elliott, E.J.; Naughton, G.A. Exercise for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2006, 2017, 1–42. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–610. [Google Scholar] [CrossRef]
- Gillen, J.B.; Gibala, M.J. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 2014, 39, 409–412. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle: Part I: Cardiopulmonary emphasis. Sport. Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Wahl, P.; Bloch, W.; Proschinger, S. The Molecular Signature of High-intensity Training in the Human Body. Int. J. Sports Med. 2021, 1–15. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation the link between insulin resistance. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef]
- Fernandez, M.; Gonzalez, R.; Subirats, A. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. Eur. J. Teach. Educ. 1988, 11, 123–130. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J. Am. Med. Assoc. 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zulet, M.A.; Puchau, B.; Navarro, C.; Martí, A.; Martínez Hernández, J.A. Biomarcadores del estado inflamatorio: Nexo de unión con la obesidad y complicaciones asociadas. Nutr. Hosp. 2007, 22, 511–527. [Google Scholar] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Pankow, J.S.; Bang, H.; Couper, D.; Ballantyne, C.M.; Hoogeveen, R.C.; Heiss, G. Adiponectin and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes 2004, 53, 2473–2478. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Porzia, A.; Mainiero, F.; Costantino, C.; Bertoccini, L.; Ceccarelli, V.; Morini, S.; Baroni, M.G.; Lenzi, A.; et al. Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol. 2017, 54, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.B.; Thakur, S.; Muddapur, M.V. Evaluation of serum interleukin-10 levels as a predictor of glycemic alteration in chronic periodontitis and type 2 diabetes mellitus. J. Indian Soc. Periodontol. 2015, 19, 388–392. [Google Scholar] [CrossRef]
- Møller, H.J.; Frikke-Schmidt, R.; Moestrup, S.K.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Serum soluble CD163 predicts risk of type 2 diabetes in the general population. Clin. Chem. 2011, 57, 291–297. [Google Scholar] [CrossRef]
- Vega-Robledo, G.B.; Rico-Rosillo, M.G. Adipose tissue: Immune function and alterations caused by obesity. Rev. Alerg. Mex. 2019, 66, 340–353. [Google Scholar]
- Banitalebi, E.; Kazemi, A.R.; Faramarzi, M.; Nasiri, S.; Haghighi, M.M. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. 2019, 217, 101–109. [Google Scholar] [CrossRef]
- Larsen, C.M.; Faulenbach, M.; Vaag, A.; Vølund, A.; Ehses, J.A.; Seifert, B.; Mandrup-Poulsen, T.; Donath, M.Y. Interleukin-1–Receptor Antagonist in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2007, 356, 1517–1526. [Google Scholar] [CrossRef]
- Larsen, C.; Faulenbach, M.; Vaag, A.; Ehses, J.; Donath, M.Y.; Mandrup-Poulsen, T. Antagonist Treatment in Type 2 Diabetes. Emerg. Treat. Technol. 2009, 32, 1663–1668. [Google Scholar] [CrossRef]
- Siewko, K.; MacIulewski, R.; Zielinska-Maciulewska, A.; Poplawska-Kita, A.; Szumowski, P.; Wawrusiewicz-Kurylonek, N.; Lipinska, D.; Milewski, R.; Gorska, M.; Kretowski, A.; et al. Interleukin-6 and Interleukin-15 as Possible Biomarkers of the Risk of Autoimmune Diabetes Development. Biomed Res. Int. 2019, 2019, 7–9. [Google Scholar] [CrossRef]
- Kuczyński, S.; Winiarska, H.; Abramczyk, M.; Szczawińska, K.; Wierusz-Wysocka, B.; Dworacka, M. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2005, 69, 231–236. [Google Scholar] [CrossRef]
- Luz María Trujillo, G.; Daniela García, L.; Astrid Von Oetinger, G. Actualizaciones sobre “Irisina”: La nueva mioquina. Rev. Chil. Nutr. 2016, 43, 308–314. [Google Scholar] [CrossRef][Green Version]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The effects of LY2405319, an FGF21 Analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef]
- Wu, D.; Li, N.; Yang, M.; Liu, H.; Yang, G. Elevated plasma levels of SPARC in patients with newly diagnosed type 2 diabetes mellitus. Eur. J. Endocrinol. 2011, 165, 597–601. [Google Scholar] [CrossRef]
- McCulloch, L.J.; Bramwell, L.R.; Knight, B.; Kos, K. Circulating and tissue specific transcription of angiopoietin-like protein 4 in human Type 2 diabetes. Metabolism 2020, 106, 154192. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Leggate, M.; Nowell, M.A.; Jones, S.A.; Nimmo, M.A. The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperones 2010, 15, 827–833. [Google Scholar] [CrossRef]
- Wadley, A.J.; Chen, Y.W.; Lip, G.Y.H.; Fisher, J.P.; Aldred, S. Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J. Sports Sci. 2016, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elmer, D.J.; Laird, R.H.; Barberio, M.D.; Pascoe, D.D. Inflammatory, lipid, and body composition responses to interval training or moderate aerobic training. Eur. J. Appl. Physiol. 2016, 116, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, D.; Werlen, C.; Bulfaro, S.; Chenevière, X.; Borrani, F. Effect of high-intensity interval exercise on lipid oxidation during postexercise recovery. Med. Sci. Sports Exerc. 2009, 41, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, F.; Jelinek, H.F.; Perkins, S.; Al-Aubaidy, H.A.; Dejong, B.; Butkowski, E. Acute-Phase Inflammatory Response to Single-Bout HIIT and Endurance Training: A Comparative Study. Mediators Inflamm. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cabral-Santos, C.; Gerosa-Neto, J.; Inoue, D.S.; Panissa, V.L.G.; Gobbo, L.A.; Zagatto, A.M.; Campos, E.Z.; Lira, F.S. Similar Anti-Inflammatory Acute Responses from Moderate-Intensity Continuous and High-Intensity Intermittent Exercise. J. Sports Sci. Med. 2015, 14, 849. [Google Scholar] [PubMed]
- Wen, D.; Utesch, T.; Wu, J.; Robertson, S.; Liu, J.; Hu, G.; Chen, H. Effects of different protocols of high intensity interval training for VO2max improvements in adults: A meta-analysis of randomised controlled trials. J. Sci. Med. Sport 2019, 22, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R. Worldwide Survey of Fitness Trends for 2020. ACSM’s Heal. Fit. J. 2019, 23, 10–18. [Google Scholar] [CrossRef]
- Laursen, P.B.; Jenkins, D.G. The Scientific Basis for High-Intensity Interval Training. Sport. Med. 2002, 32, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Magalhães, J.P.; Santos, D.A.; Correia, I.R.; Hetherington-Rauth, M.; Ribeiro, R.; Raposo, J.F.; Matos, A.; Bicho, M.D.; Sardinha, L.B. Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: A secondary analysis from a 1-year randomized controlled trial. Cardiovasc. Diabetol. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Afrasyabi, S.; Marandi, S.M.; Kargarfard, M. The effects of high intensity interval training on appetite management in individuals with type 2 diabetes: Influenced by participants weight. J. Diabetes Metab. Disord. 2019, 18, 107–117. [Google Scholar] [CrossRef]
- Mallard, A.R.; Hollekim-Strand, S.M.; Coombes, J.S.; Ingul, C.B. Exercise intensity, redox homeostasis and inflammation in type 2 diabetes mellitus. J. Sci. Med. Sport 2017, 20, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Dünnwald, T.; Melmer, A.; Gatterer, H.; Salzmann, K.; Ebenbichler, C.; Burtscher, M.; Schobersberger, W.; Grander, W. Supervised Short-term High-intensity Training on Plasma Irisin Concentrations in Type 2 Diabetic Patients. Int. J. Sports Med. 2019, 40, 158–164. [Google Scholar] [CrossRef]
- Madsen, S.M.; Thorup, A.C.; Bjerre, M.; Jeppesen, P.B. Does 8 weeks of strenuous bicycle exercise improve diabetes-related inflammatory cytokines and free fatty acids in type 2 diabetes patients and individuals at high-risk of metabolic syndrome? Arch. Physiol. Biochem. 2015, 121, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Asle Mohammadi Zadeh, M.; Kargarfard, M.; Marandi, S.M.; Habibi, A. Diets along with interval training regimes improves inflammatory & anti-inflammatory condition in obesity with type 2 diabetes subjects. J. Diabetes Metab. Disord. 2018, 17, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Organization W.H. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity. Available online: https://www.google.com/search?q=Obesity%3A+preventing+and+managing+theglobal+epidemic.+Report+of+a+WHO+consultation+on+obesity.+Geneva%2CJune+3–5%2C+1997&oq=Obesity%3A+preventing++and++managing++theglobal+epidemic.+Report+of+a+WHO+consultation+on+obesity (accessed on 29 January 2021).
- Pedersen, L.R.; Olsen, R.H.; Anholm, C.; Astrup, A.; Eugen-Olsen, J.; Fenger, M.; Simonsen, L.; Walzem, R.L.; Haugaard, S.B.; Prescott, E. Effects of 1 year of exercise training versus combined exercise training and weight loss on body composition, low-grade inflammation and lipids in overweight patients with coronary artery disease: A randomized trial. Cardiovasc. Diabetol. 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Zoppini, G.; Targher, G.; Zamboni, C.; Venturi, C.; Cacciatori, V.; Moghetti, P.; Muggeo, M. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 543–549. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Timothy Garvey, W.; Karen Lau, K.H.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Papamichou, D.; Panagiotakos, D.B.; Itsiopoulos, C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Re-Examining High-Fat Diets for Sports Performance: Did We Call the “Nail in the Coffin” Too Soon? Sports Med. 2015, 45 Suppl. 1, 33–49. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Lei, B.; Li, J.; Wang, R. Effects of a low-carbohydrate high-fat diet combined with high-intensity interval training on body composition and maximal oxygen uptake: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 740. [Google Scholar] [CrossRef] [PubMed]
| Downs and Black Checklist (Modified) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Papers | Items | TOTAL | |||||||||||||||||||||||||
| 1 | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. | 20. | 21. | 22. | 23. | 24. | 25. | 26. | 26 ITEMS | |
| Magalhães et al., 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 0 | 1 | - | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 1 | 1 | 21/26 |
| Mallard AR et al., 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | - | - | 0 | 1 | - | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 1 | - | 18/26 |
| Banitalebi E et al., 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | - | - | 0 | 1 | - | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 0 | 1 | 18/26 |
| Asle M et al., 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | - | - | 0 | - | - | 1 | 1 | 1 | 1 | 1 | - | 1 | 0 | 1 | 1 | 18/26 |
| Dünnwald T et al., 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | - | - | 0 | - | - | 1 | 1 | 1 | 1 | 1 | - | 0 | 0 | 1 | 1 | 16/26 |
| Madsen SM et al., 2015 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | - | - | 1 | 1 | 1 | 1 | 1 | - | 0 | 0 | 1 | - | 16/26 |
| Afrasyabi, S et al., 2019 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | - | - | 0 | - | - | 1 | 0 | - | 1 | 1 | - | 1 | 0 | 0 | - | 11/26 |
| Study Reference | Country | Downs and Black | Type of Study | Profile | Intervention Duration | Women/Men | Age range | Weight Conditions | Level of Activity at Study Inception | Groups Included in the Study | Intervention in Each Group |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Magalhães JP et al., 2020 [50] | Portugal | 21/26 | RCT HIIT vs MCT vs control (all T2D) | T2D | 12 months | Women:38/80 (47.5%) when starting. Hombres: 42/80 (52.5%) when starting. | 30–75 | BMI < 48 kg/m2 | - | MCT Group N = 16 | MCT Group + RT in final stages = cycle ergometer |
| Control Group N = 22 | Control Group = Initial recommendation on standard and non-structured physical activity in one session | ||||||||||
| HIIT Group N = 13 | HIIT Group + RT in final stages = cycle ergometer | ||||||||||
| Mallard AR et al., 2017 [52] | Australia | 18/26 | RCT parallel design. HIIT vs MICT (both T2D) | T2D | 12 weeks | Women: 14/36 (38.88%) Men: 22/36 (61.11%) | 44–65 | HIIT = BMI 30.2 ± 2.7 kg/m2 MICT = BMI 29.6 ± 3.6 kg/m2 | Less than 210 min/week | HIIT Group N = 20 | HIIT Group: treadmill |
| MICT Group N = 16 | MICT Group: home based exercise | ||||||||||
| Banitalebi E et al., 2019 [28] | Iran | 18/26 | RCT SIT vs Aerobic + resistance vs Control (all T2D) | T2D | 10 weeks | Women: 42 (100%) | 30–65 | BMI > 25 kg/m2 | Sedentary (no more than 20 min of structured exercise of any kind for the 6 months before the study or no sprint interval exercise). | Group A + R Training N = 14 | Group A + R Training: Participants free to choose cycle ergometer or treadmill. |
| Control Group N = 14 | Control Group: asked to keep their physical activity levels during the study. | ||||||||||
| SIT Group N = 14 | SIT Group: cycle ergometer | ||||||||||
| Asle M et al., 2018 [55] | Iran | 18/26 | RCT Types of diets + HIIT vs Normal diet + HIIT (All T2D) | T2D | 24 weeks | - | 36–58 | BMI between 30 and 39 kg/m2 | Sedentary (no regular exercise more than one day a week) | Experimental HIIT Group + Low carbohydrates N = 10 | Experimental Group + low carbohydrates: cycle ergometer |
| Experimental HIIT Group + Low fat N =10 | Experimental HIIT Group + low fat: cycle ergometer | ||||||||||
| Experimental Group = HIIT + high fat N = 10 | Experimental Group = HIIT + high fat: cycle ergometer | ||||||||||
| Control HIIT Group + Normal diet N = 9 | Control HIIT Group + normal diet: cycle ergometer | ||||||||||
| Dünnwald T et al., 2019 [53] | Austria | 16/26 | CT HIIT vs CMT (all T2D) | T2D | 4 weeks | - | 50–65 | HIIT = BMI 27.8 ± 2.8 kg/m2 CMT = BMI 31.8 ± 4.6 kg/m2 | - | HIIT Group N = 8 | HIIT Group: cycle ergometer |
| CMT Group N = 6 | CMT Group: cycle ergometer | ||||||||||
| Madsen SM et al., 2015 [54] | Denmark | 16/26 | CT T2D vs Healthy (all doing HIIT) | T2D and Healthy | 8 weeks | Women 15/23 (65.22%) Hombres 8/23 (34.78%) | 52 ± 2 | - | - | Experimental T2D Group N = 10 | Experimental T2D Group N = 10 |
| Healthy Control Group N = 13 | Healthy Control Group N = 13 | ||||||||||
| Afrasyabi S et al., 2019 [51] | Iran | 11/26 | RCT HIIT vs Control (In both groups there are T2D and healthy subgroups. 8 groups in total) | T2D and Healthy | 12 weeks | - | 40 ± 10 | BMI ≥ 30 kg/m2 for obese ≤ 20 kg/m2 for slim | Less than moderate intensity exercise > 1.5 h per week | HIIT(O-ND-T) N = 10 HIIT (O-D-T) N = 10 HIIT (N-ND-T) N = 10 HIIT (N-D-T) N = 10 Control (O-ND-C) N = 10 Control (O-D-C) N = 10 Control (N-ND-C) N = 10 Control (N-D-C) N = 10 | HIIT (O-ND-T) = Running HIIT (O-D-T) = Running HIIT (N-ND-T) = Running HIIT (N-D-T) = Running Control (O-ND-C) = Running Control (O-D-C) = Running Control (N-ND-C) = Running Control (N-D-C) = Running |
| Study Reference | HIIT Group Size | HIIT Intensity | HIIT Duration (Weeks) |
Frecuency (Session/Week) | Series | Working-Out Time (Sec) | Rest Time (Sec) | Working Intervals | Volume per Session | Training Periods | Body Composition | Inflammatory Biomarkers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Magalhães JP et al., 2020 [50] | 13/51 | 70% to 90%Hrmax | 52 weeks | 3 | 1 | 120 s to 60 s | 60 s | LI-HIIT) to (MI-HIIT | LV-HIIT | LT-HIIT | BMI (kg/m2) WC Waist circumference (cm) WBFI whole-body fat index (kg/m2) AFI android fat index (kg/m2) Weight (kg) | TNF-α IL-6, CRP sCD163 |
| Mallard AR et al., 2017 [52] | 16/36 | 90%–95% Hrmax | 12 weeks | 3 | 4 | 240 s | 180 s | LI-HIIT | HV-HIIT | LT-HIIT | BMI (kg/m2) Waist circumference (cm) | TNF-α IL-8 IL-6 IL-10 |
| Banitalebi E et al., 2019 [28] | 14/42 | All out | 10 weeks | 3 | 4 | 30 s | 120 s at 50W | SI-HIIT | MV-HIIT | MT-HIIT | BMI (kg/m2) WC Waist circumference (cm) Weight (kg) LBM lean body mass (kg) Body fat (%) | Irisin IL-15 FGF21 IL-6 ANGPTL4 SPARC |
| Asle M et al., 2018 [55] | 39/39 | 75% to 90% Hrmax | 24 weeks | 3 | 10 | 60 s | 60 s 30% Hrmax | SIT-HIIT | LV-HIIT | LT-HIIT | BMI (kg/m2) WC Waist circumference (cm) Weight (kg) Height (cm) | TNF-α IL-6, Leptin Resistin Adiponectin FGF21 |
| Dünnwald T et al., 2019 [53] | 8/14 | 90%–95% Hrmax | 4 weeks | 3 | 5 | 240 s | 180 s (70%Hrmax) | LI-HIIT | HV-HIIT | ST-HIIT | IMC (kg/m2) Adipose Tissue Mass (kg) Fat-free mass (kg) | TNF-α Leptin Adiponectin Irisin |
| Madsen SM et al., 2015 [54] | 10/23 | 90% Hrmax | 8 weeks | 3 | 10 | 60 s | 60 s active recovery | MI_HIIT | HV-HIIT | MT-HIIT | Weight (kg) Abdominal fat (kg) | TNF-α IL-6, IL-1, Leptin CRP |
| Afrasyabi S et al., 2019 [51] | 40/80 | 85%–95% Hrmax | 3 week 12 weeks | 3 | 6 to 12 | 60 s | 60 s | MI_HIIT | HV-HIIT | LT-HIIT | BMI (kg/m2) Weight (kg) Height (cm) | TNF-α |
| Paper | Results |
|---|---|
| Magalhães et al., 2020 [50] | No significant results found for the HIIT group. However, there was a significant reduction in the MCT (−3.6 ± 16.4 pg/mL) Group compared with the control (7.0 ± 17.3 pg/mL) (p = 0.047) for the IL-6. There is no information on post-treatment to determine if they recorded significant changes for the body composition variables. |
| Mallard AR et al., 2017 [52] | No significant results were found (p < 0.05) for biomarkers IL-10, IL-6, IL-8, TNF-α within any of the intervention groups (HIIT and MCT) throughout 12 weeks. There is no information on post-treatment to determine if they recorded significant changes for the body composition variables. |
| Banitalebi E et al., 2019 [28] | A significant reduction was recorded for biomarkers IL-15 (p = 0.02) and IL-6 (p = 0.002) after the treatment period in each of the intervention groups; it was greater within the SIT group (−0.23 and −0.67 mean difference) than in those of A+R (−0.21 and −0.52 mean difference) and control (−0.07 and −0.23 mean difference). Significant differences recorded among different groups after having received treatment for biomarker Irisin (SIT = 42.15, A + R = 68.57, Control = 17.15 ng/mL mean difference) (p = 0.009). Regarding the values of body composition, significant differences were found among groups for BMI (p = 0.01), weight (p = 0.02), LBM (p = 0.01) values. |
| Asle M et al., 2018 [55] | The TNF-α showed a significant reduction after intervention (p < 0.001) and among groups (p < 0.001). (Low CHO-18.69, Low Fat-48.06, High Fat 0.66, and Control 11.69 percentage of change)The IL-6 did not show a significant reduction after intervention, but it did show it among groups (p < 0.001). (Low CHO-32.10, Low Fat-24.97, High Fat-18.67, and Control-4.23 percentage of change)The leptin showed a significant reduction after intervention (p < 0.001) and among groups (p < 0.001). (Low CHO-53.92, Low Fat-30.26, High Fat 0.11, and Control-1.99 percentage of change)Adiponectin showed a significant increase after intervention (p < 0.001) and among groups (p< 0.001). (Low CHO 18.10, Low Fat-42.32, High Fat 1.84, and Control 4.89 percentage of change) The FGF21 showed a significant increase after intervention (p < 0.006) and among groups (p < 0.001). (Low CHO 55.86, Low Fat 52.30, High Fat-13.43, and Control 21.66 percentage of change)Resistin: it showed a significant reduction after intervention (p < 0.006) and among groups (p < 0.001). (Low CHO-28.29, Low Fat-15.27, High Fat-9.18, and Control-1.30 percentage of change)Reductions were more remarkable in groups with low carbohydrates and low-fat diet.There is no information on post-treatment to determine if they recorded significant changes for the body composition variables. |
| Dünnwald T et al., 2019 [53] | A significant increase was recorded within the HIIT group for the biomarker irisin (0.57 ± 0.04 pre, 0.61 ± 0.06 post (ug/mL) compared to CMT group (0.60 ± 0.06 pre, 0.58 ± 0.07 post) (p ≤ 0.05). Moreover, a significant increase was recorded within the CMT group for the fat-free mass anthropometric variable (p ≤ 0.05). |
| Madsen SM et al., 2015 [54] | HIIT did not lead to significant variations in inflammatory biomarkers in patients with T2D. However, a significant increase was observed in the group of healthy people with respect to the IL-1Ra biomarker (p = 0.03). In relation to the values of body composition, a significant reduction in weight (p < 0.01) and abdominal fat mass (p < 0.01) was observed after treatment within the T2D group and in weight (p < 0.001) and abdominal fat mass (p < 0.05) within the control group (healthy subjects). |
| Afrasyabi S et al., 2019 [51] | The TNF-α was significantly diminished after treatment with HIIT (p = 0.001) in groups N-ND-T, O-D-T and O-ND-T, and there were significant differences among groups, the O-D-T recording the greatest reduction (p = 0.001).There is no information on post-treatment to determine if they recorded significant changes for the body composition variables. |
| Working Out Interval | Close to Maximum (All-Out) Intensity: ≥ 90 VO2max / ≥ 95% Hrmax / ≥120% v/pVO2max | ||
|---|---|---|---|
| ≤10s RST | 10–30s (SIT) | ||
| Second Máximum Intensity: 80–90% VO2max / 85–85% Hrmax / 90–120% v/pVO2max | |||
| ≤ 30 s (SI-HIIT) | 30 s to 2 min (MI-HIIT) | ≥ 2 min (LI-HIIT) | |
| Volume per Session (Duration x Repetition) | ≤5 min (LV-HIIT) | 5 to 15 min (MV-HIIT) | ≥15 min (HV-HIIT) |
| Training Period (Intervention Duration) | ≤4 weeks (ST-HIIT) | 4 to 12 weeks (MT-HIIT) | ≥12 weeks (LT-HIIT) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva-Valderrama, J.M.; Montes-de-Oca-Garcia, A.; Opazo-Diaz, E.; Ponce-Gonzalez, J.G.; Molina-Torres, G.; Velázquez-Díaz, D.; Galán-Mercant, A. Effects of High-Intensity Interval Training on Inflammatory Biomarkers in Patients with Type 2 Diabetes. A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12644. https://doi.org/10.3390/ijerph182312644
Leiva-Valderrama JM, Montes-de-Oca-Garcia A, Opazo-Diaz E, Ponce-Gonzalez JG, Molina-Torres G, Velázquez-Díaz D, Galán-Mercant A. Effects of High-Intensity Interval Training on Inflammatory Biomarkers in Patients with Type 2 Diabetes. A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(23):12644. https://doi.org/10.3390/ijerph182312644
Chicago/Turabian StyleLeiva-Valderrama, José Manuel, Adrián Montes-de-Oca-Garcia, Edgardo Opazo-Diaz, Jesus G. Ponce-Gonzalez, Guadalupe Molina-Torres, Daniel Velázquez-Díaz, and Alejandro Galán-Mercant. 2021. "Effects of High-Intensity Interval Training on Inflammatory Biomarkers in Patients with Type 2 Diabetes. A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 23: 12644. https://doi.org/10.3390/ijerph182312644
APA StyleLeiva-Valderrama, J. M., Montes-de-Oca-Garcia, A., Opazo-Diaz, E., Ponce-Gonzalez, J. G., Molina-Torres, G., Velázquez-Díaz, D., & Galán-Mercant, A. (2021). Effects of High-Intensity Interval Training on Inflammatory Biomarkers in Patients with Type 2 Diabetes. A Systematic Review. International Journal of Environmental Research and Public Health, 18(23), 12644. https://doi.org/10.3390/ijerph182312644









