Per- and Polyfluoroalkyl Substances (PFAS) in Integrated Crop–Livestock Systems: Environmental Exposure and Human Health Risks
Abstract
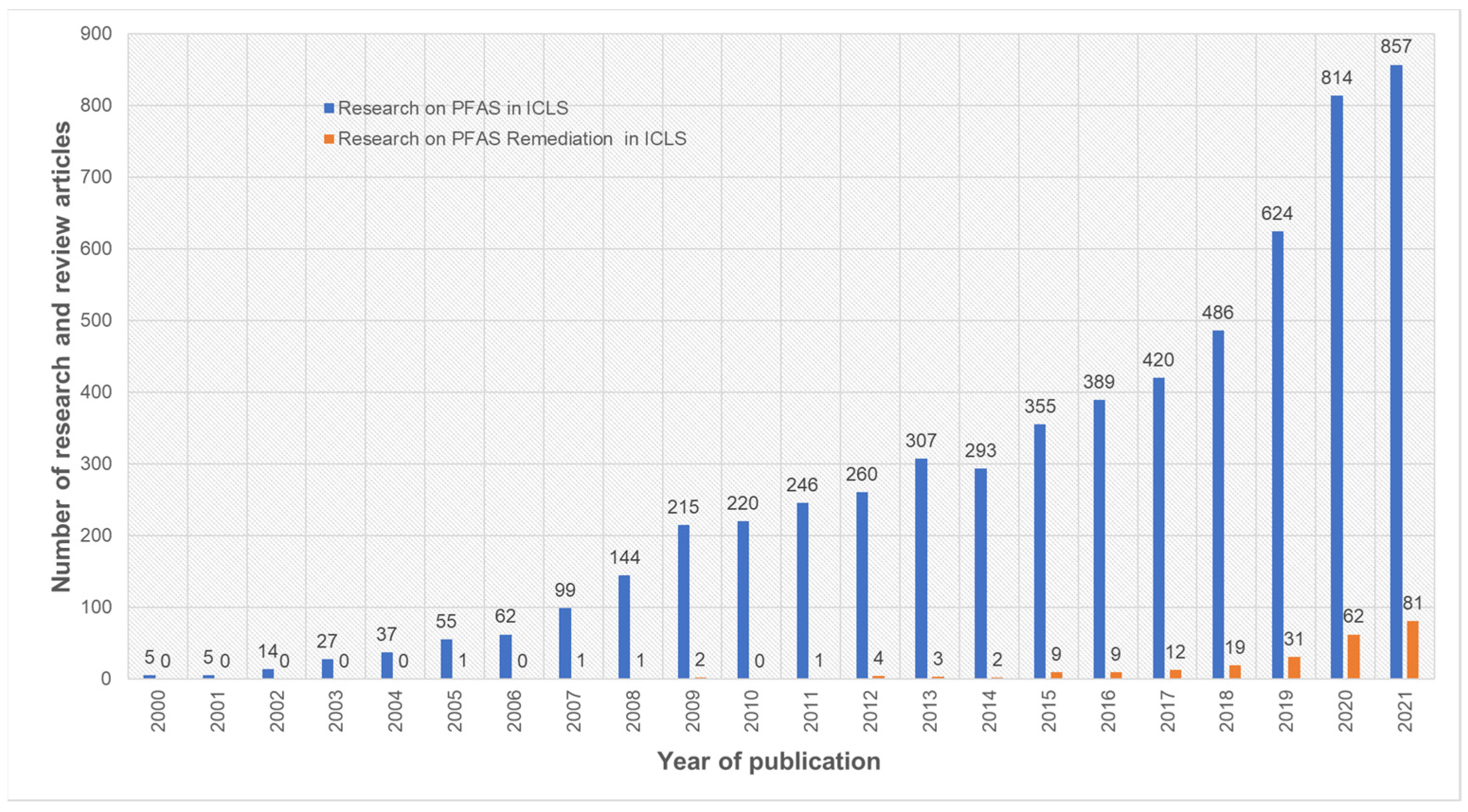
:1. Introduction
2. “Forever Chemicals”: Persistence and Mobility
3. Human Health Impacts and Exposure
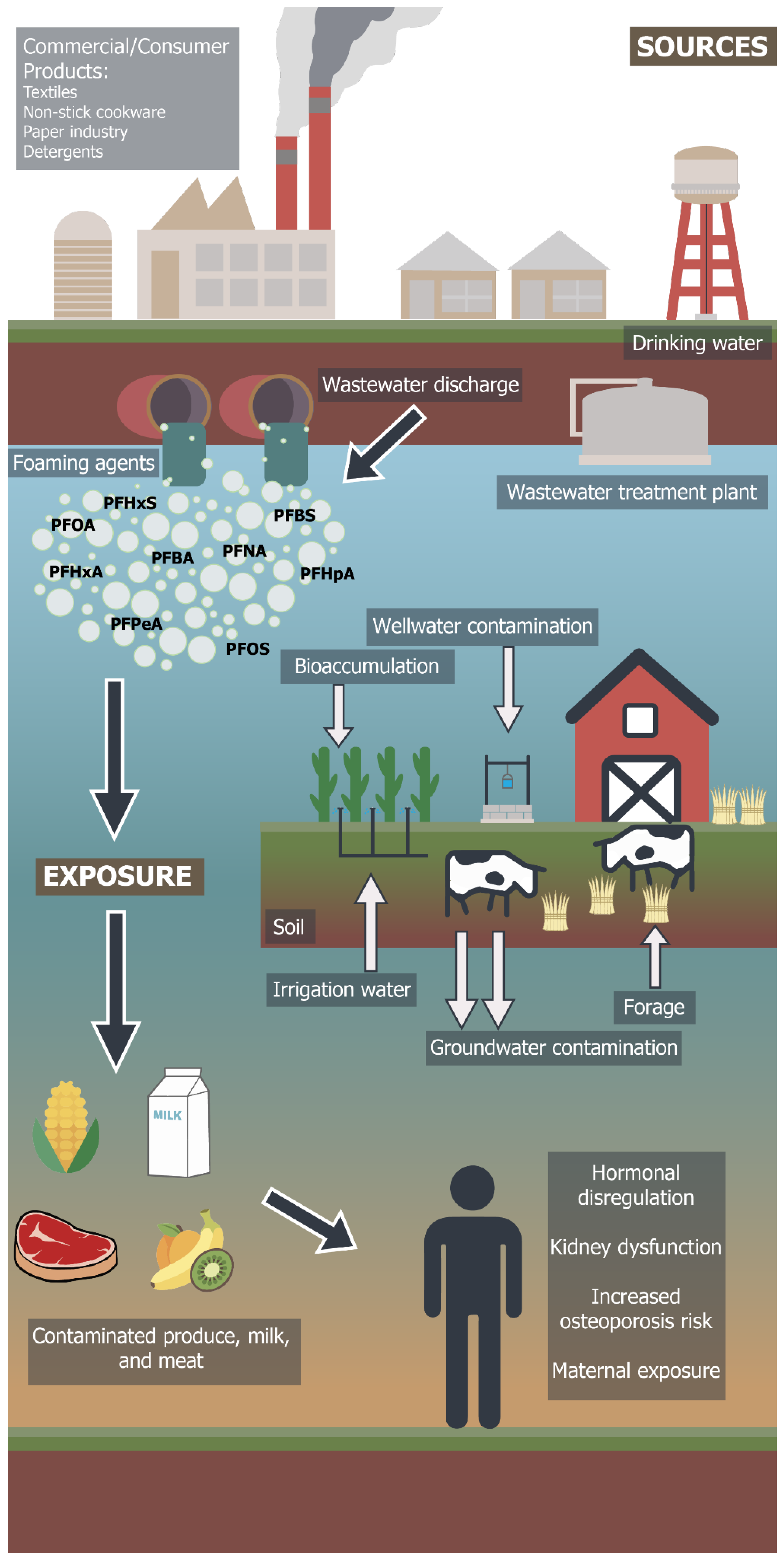
4. Fate of PFAS Compounds in Integrated Crop–Livestock Systems
5. Limited Global Regulations and Standards to Address Environmental Concerns
6. Remediation and Preventive Strategies
6.1. Soil and Sediment Remediation Methods
6.2. Water Remediation Methods
6.3. Microbial and Phytoremediation
7. Exposure and Equity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haukås, M.; Berger, U.; Hop, H.; Gulliksen, B.; Gabrielsen, G.W. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ. Pollut. 2007, 148, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Smithwick, M.M.; Braune, B.M.; Hoekstra, P.F.; Muir, D.C.G.; Mabury, S.A. Identification of Long-Chain Perfluorinated Acids in Biota from the Canadian Arctic. Environ. Sci. Technol. 2004, 38, 373–380. [Google Scholar] [CrossRef]
- Taniyasu, S.; Kannan, K.; Horii, Y.; Hanari, N.; Yamashita, N. A Survey of Perfluorooctane Sulfonate and Related Perfluorinated Organic Compounds in Water, Fish, Birds, and Humans from Japan. Environ. Sci. Technol. 2003, 37, 2634–2639. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.G.; Jones, K.C.; Sweetman, A.J. A First Global Production, Emission, And Environmental Inventory For Perfluorooctane Sulfonate. Environ. Sci. Technol. 2009, 43, 386–392. [Google Scholar] [CrossRef]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef]
- Boiteux, V.; Dauchy, X.; Bach, C.; Colin, A.; Hemard, J.; Sagres, V.; Rosin, C.; Munoz, J.-F. Concentrations and patterns of perfluoroalkyl and polyfluoroalkyl substances in a river and three drinking water treatment plants near and far from a major production source. Sci. Total Environ. 2017, 583, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.H.; Cho, C.-R.; Kannan, K.; Cho, H.-S. A nationwide survey of perfluorinated alkyl substances in waters, sediment and biota collected from aquatic environment in Vietnam: Distributions and bioconcentration profiles. J. Hazard. Mater. 2017, 323, 116–127. [Google Scholar] [CrossRef]
- Loganathan, B.G.; Sajwan, K.S.; Sinclair, E.; Senthil Kumar, K.; Kannan, K. Perfluoroalkyl sulfonates and perfluorocarboxylates in two wastewater treatment facilities in Kentucky and Georgia. Water Res. 2007, 41, 4611–4620. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, W.; Wang, L.; Zhang, Y.; Zhang, Y.; Wang, M.; Wang, Y.; Li, P. A review of sources, multimedia distribution and health risks of novel fluorinated alternatives. Ecotoxicol. Environ. Saf. 2019, 182, 109402. [Google Scholar] [CrossRef]
- Hoffman, K.; Webster, T.F.; Weisskopf, M.G.; Weinberg, J.; Vieira, V.M. Exposure to Polyfluoroalkyl Chemicals and Attention Deficit/Hyperactivity Disorder in U.S. Children 12–15 Years of Age. Environ. Health Perspect. 2010, 118, 1762–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calafat, A.M.; Wong, L.-Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Scharf, S.; Weiss, S.; Gans, O.; Scheffknecht, C. Emissions of perfluorinated alkylated substances (PFAS) from point sources—identification of relevant branches. Water Sci. Technol. 2008, 58, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Shahsavari, E.; Rouch, D.; Khudur, L.S.; Thomas, D.; Aburto-Medina, A.; Ball, A.S. Challenges and Current Status of the Biological Treatment of PFAS-Contaminated Soils. Front. Bioeng. Biotechnol. 2021, 8, 1493. [Google Scholar] [CrossRef]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Li, L.Y.; Grace, J.R. Review of the fate and transformation of per- and polyfluoroalkyl substances (PFASs) in landfills. Environ. Pollut. 2018, 235, 74–84. [Google Scholar] [CrossRef]
- Hepburn, E.; Madden, C.; Szabo, D.; Coggan, T.L.; Clarke, B.; Currell, M. Contamination of groundwater with per- and polyfluoroalkyl substances (PFAS) from legacy landfills in an urban re-development precinct. Environ. Pollut. 2019, 248, 101–113. [Google Scholar] [CrossRef]
- Curtzwiler, G.W.; Silva, P.; Hall, A.; Ivey, A.; Vorst, K. Significance of Perfluoroalkyl Substances (PFAS) in Food Packaging. Integr. Environ. Assess. Manag. 2021, 17, 7–12. [Google Scholar] [CrossRef]
- Pontius, F. Regulation of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) in Drinking Water: A Comprehensive Review. Water 2019, 11, 2003. [Google Scholar] [CrossRef] [Green Version]
- Hamid, H.; Li, L. Role of wastewater treatment plant in environmental cycling of poly- and perfluoroalkyl substances. Ecocycles 2016, 2, 43–53. [Google Scholar] [CrossRef]
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 2019, 169, 326–341. [Google Scholar] [CrossRef]
- Higgins, C.; Field, J.; Criddle, C.; Luthy, R. Quantitative Determination of Perfluorochemicals in Sediments and Domestic Sludge. Environ. Sci. Technol. 2005, 39, 3946–3956. [Google Scholar] [CrossRef]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Langberg, H.A.; Arp, H.P.H.; Breedveld, G.D.; Slinde, G.A.; Høiseter, Å.; Grønning, H.M.; Jartun, M.; Rundberget, T.; Jenssen, B.M.; Hale, S.E. Paper product production identified as the main source of per- and polyfluoroalkyl substances (PFAS) in a Norwegian lake: Source and historic emission tracking. Environ. Pollut. 2021, 273, 116259. [Google Scholar] [CrossRef]
- Boone, J.S.; Vigo, C.; Boone, T.; Byrne, C.; Ferrario, J.; Benson, R.; Donohue, J.; Simmons, J.E.; Kolpin, D.W.; Furlong, E.T.; et al. Per- and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci. Total Environ. 2019, 653, 359–369. [Google Scholar] [CrossRef]
- Backe, W.J.; Day, T.C.; Field, J.A. Zwitterionic, Cationic, and Anionic Fluorinated Chemicals in Aqueous Film Forming Foam Formulations and Groundwater from U.S. Military Bases by Nonaqueous Large-Volume Injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47, 5226–5234. [Google Scholar] [CrossRef]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hundal, L.S.; Kumar, K.; Lau, C.; Mills, M.A.; Harris, K.M.; Higgins, C.P. Perfluoroalkyl Acid Distribution in Various Plant Compartments of Edible Crops Grown in Biosolids-Amended soils. Environ. Sci. Technol. 2014, 48, 7858–7865. [Google Scholar] [CrossRef]
- Parsons, J.R.; Sáez, M.; Dolfing, J.; de Voogt, P. Biodegradation of Perfluorinated Compounds. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer US: New York, NY, USA, 2008; pp. 53–71. ISBN 978-0-387-78444-1. [Google Scholar]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Scheringer, M.; Wang, Z. The high persistence of PFAS is sufficient for their management as a chemical class. Environ. Sci. Process. Impacts 2020, 22, 2307–2312. [Google Scholar] [CrossRef]
- Martin, J.W.; Asher, B.J.; Beesoon, S.; Benskin, J.P.; Ross, M.S. PFOS or PreFOS? Are perfluorooctane sulfonate precursors (PreFOS) important determinants of human and environmental perfluorooctane sulfonate (PFOS) exposure? J. Environ. Monit. 2010, 12, 1979–2004. [Google Scholar] [CrossRef]
- Wackett, L.P.; Robinson, S.L. The ever-expanding limits of enzyme catalysis and biodegradation: Polyaromatic, polychlorinated, polyfluorinated, and polymeric compounds. Biochem. J. 2020, 477, 2875–2891. [Google Scholar] [CrossRef]
- Huang, S.; Jaffé, P.R. Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, K.; Li, Z.; Ren, C.; Chen, J.; Lin, Y.-H.; Liu, J.; Men, Y. Microbial Cleavage of C–F Bonds in Two C 6 Per- and Polyfluorinated Compounds via Reductive Defluorination. Environ. Sci. Technol. 2020, 54, 14393–14402. [Google Scholar] [CrossRef]
- Wei, C.; Song, X.; Wang, Q.; Hu, Z. Sorption kinetics, isotherms and mechanisms of PFOS on soils with different physicochemical properties. Ecotoxicol. Environ. Saf. 2017, 142, 40–50. [Google Scholar] [CrossRef]
- Bolan, N.; Sarkar, B.; Yan, Y.; Li, Q.; Wijesekara, H.; Kannan, K.; Tsang, D.C.W.; Schauerte, M.; Bosch, J.; Noll, H.; et al. Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils—To mobilize or to immobilize or to degrade? J. Hazard. Mater. 2021, 401, 123892. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Anderson, R.H.; Guo, B. PFAS concentrations in soils: Background levels versus contaminated sites. Sci. Total Environ. 2020, 740, 140017. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Heyn, J.; Thiele, H.; Hüther, J.; Failing, K.; Georgii, S.; Brunn, H. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plants. Arch. Environ. Contam. Toxicol. 2009, 57, 289–298. [Google Scholar] [CrossRef]
- Lechner, M.; Knapp, H. Carryover of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) from Soil to Plant and Distribution to the Different Plant Compartments Studied in Cultures of Carrots (Daucus carota ssp. Sativus), Potatoes (Solanum tuberosum), and Cucumbers (Cucumis Sativus). J. Agric. Food Chem. 2011, 59, 11011–11018. [Google Scholar] [CrossRef] [PubMed]
- Meijer, S.N.; Ockenden, W.A.; Sweetman, A.; Breivik, K.; Grimalt, J.O.; Jones, K.C. Global Distribution and Budget of PCBs and HCB in Background Surface Soils: Implications for Sources and Environmental Processes. Environ. Sci. Technol. 2003, 37, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Naile, J.E.; Khim, J.S.; Wang, T.; Chen, C.; Luo, W.; Kwon, B.-O.; Park, J.; Koh, C.-H.; Jones, P.D.; Lu, Y.; et al. Perfluorinated compounds in water, sediment, soil and biota from estuarine and coastal areas of Korea. Environ. Pollut. 2010, 158, 1237–1244. [Google Scholar] [CrossRef]
- Sepulvado, J.G.; Blaine, A.C.; Hundal, L.S.; Higgins, C.P. Occurrence and Fate of Perfluorochemicals in Soil Following the Land Application of Municipal Biosolids. Environ. Sci. Technol. 2011, 45, 8106–8112. [Google Scholar] [CrossRef] [PubMed]
- Washington, J.W.; Henderson, W.M.; Ellington, J.J.; Jenkins, T.M.; Evans, J.J. Analysis of perfluorinated carboxylic acids in soils II: Optimization of chromatography and extraction. J. Chromatogr. A 2008, 1181, 21–32. [Google Scholar] [CrossRef]
- Yoo, H.; Washington, J.W.; Ellington, J.J.; Jenkins, T.M.; Neill, M.P. Concentrations, Distribution, and Persistence of Fluorotelomer Alcohols in Sludge-Applied Soils near Decatur, Alabama, USA. Environ. Sci. Technol. 2010, 44, 8397–8402. [Google Scholar] [CrossRef]
- Rankin, K.; Mabury, S.A.; Jenkins, T.M.; Washington, J.W. A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence. Chemosphere 2016, 161, 333–341. [Google Scholar] [CrossRef]
- Washington, J.W.; Yoo, H.; Ellington, J.J.; Jenkins, T.M.; Libelo, E.L. Concentrations, Distribution, and Persistence of Perfluoroalkylates in Sludge-Applied Soils near Decatur, Alabama, USA. Environ. Sci. Technol. 2010, 44, 8390–8396. [Google Scholar] [CrossRef]
- US EPA. Basic Information on PFAS. Available online: https://www.epa.gov/pfas/basic-information-pfas (accessed on 29 September 2021).
- Greathouse, J.A.; Cygan, R.T.; Fredrich, J.T.; Jerauld, G.R. Adsorption of Aqueous Crude Oil Components on the Basal Surfaces of Clay Minerals: Molecular Simulations Including Salinity and Temperature Effects. J. Phys. Chem. C 2017, 121, 22773–22786. [Google Scholar] [CrossRef]
- Jeddy, Z.; Tobias, J.H.; Taylor, E.V.; Northstone, K.; Flanders, W.D.; Hartman, T.J. Prenatal concentrations of perfluoroalkyl substances and bone health in British girls at age 17. Arch. Osteoporos. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Coperchini, F.; Croce, L.; Ricci, G.; Magri, F.; Rotondi, M.; Imbriani, M.; Chiovato, L. Thyroid Disrupting Effects of Old and New Generation PFAS. Front. Endocrinol. 2020, 11, 612320. [Google Scholar] [CrossRef]
- van Gerwen, M.; Alpert, N.; Alsen, M.; Ziadkhanpour, K.; Taioli, E.; Genden, E. The Impact of Smoking on the Association between Perfluoroalkyl Acids (PFAS) and Thyroid Hormones: A National Health and Nutrition Examination Survey Analysis. Toxics 2020, 8, 116. [Google Scholar] [CrossRef]
- Christensen, K.Y.; Raymond, M.; Meiman, J. Perfluoroalkyl substances and metabolic syndrome. Int. J. Hyg. Environ. Health 2019, 222, 147–153. [Google Scholar] [CrossRef]
- Preston, E.V.; Rifas-Shiman, S.L.; Hivert, M.-F.; Zota, A.R.; Sagiv, S.K.; Calafat, A.M.; Oken, E.; James-Todd, T. Associations of Per- and Polyfluoroalkyl Substances (PFAS) With Glucose Tolerance During Pregnancy in Project Viva. J. Clin. Endocrinol. Metab. 2020, 105, e2864–e2876. [Google Scholar] [CrossRef]
- Stanifer, J.W.; Stapleton, H.M.; Souma, T.; Wittmer, A.; Zhao, X.; Boulware, L.E. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health: A Scoping Review. Clin. J. Am. Soc. Nephrol. 2018, 13, 1479–1492. [Google Scholar] [CrossRef] [Green Version]
- Stern, J.E.; Son, S.; Biancardi, V.C.; Zheng, H.; Sharma, N.; Patel, K.P. Astrocytes Contribute to Angiotensin II Stimulation of Hypothalamic Neuronal Activity and Sympathetic Outflow. Hypertens. 2016, 68, 1483–1493. [Google Scholar] [CrossRef] [Green Version]
- Steenland, K.; Winquist, A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ. Res. 2021, 194, 110690. [Google Scholar] [CrossRef]
- Schmidt, C.W. Reduced Bone Mineral Density in Children: Another Potential Health Effect of PFAS. Environ. Health Perspect. 2020, 128, 044002. [Google Scholar] [CrossRef]
- Khalil, N.; Chen, A.; Lee, M.; Czerwinski, S.A.; Ebert, J.R.; DeWitt, J.C.; Kannan, K. Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009–2010. Environ. Health Perspect. 2016, 124, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Di Nisio, A.; De Rocco Ponce, M.; Giadone, A.; Rocca, M.S.; Guidolin, D.; Foresta, C. Perfluoroalkyl substances and bone health in young men: A pilot study. Endocrine 2020, 67, 678–684. [Google Scholar] [CrossRef]
- Gardener, H.; Sun, Q.; Grandjean, P. PFAS concentration during pregnancy in relation to cardiometabolic health and birth outcomes. Environ. Res. 2021, 192, 110287. [Google Scholar] [CrossRef]
- Liew, Z.; Luo, J.; Nohr, E.A.; Bech, B.H.; Bossi, R.; Arah, O.A.; Olsen, J. Maternal Plasma Perfluoroalkyl Substances and Miscarriage: A Nested Case–Control Study in the Danish National Birth Cohort. Environ. Health Perspect. 2020, 128, 047007. [Google Scholar] [CrossRef] [Green Version]
- Preston, E.V.; Webster, T.F.; Claus Henn, B.; McClean, M.D.; Gennings, C.; Oken, E.; Rifas-Shiman, S.L.; Pearce, E.N.; Calafat, A.M.; Fleisch, A.F.; et al. Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the Project Viva Cohort: A mixtures approach. Environ. Int. 2020, 139, 105728. [Google Scholar] [CrossRef]
- Manea, S.; Salmaso, L.; Lorenzoni, G.; Mazzucato, M.; Russo, F.; Mantoan, D.; Martuzzi, M.; Fletcher, T.; Facchin, P. Exposure to PFAS and small for gestational age new-borns: A birth records study in Veneto Region (Italy). Environ. Res. 2020, 184, 109282. [Google Scholar] [CrossRef]
- Wen, L.-L.; Lin, C.-Y.; Chou, H.-C.; Chang, C.-C.; Lo, H.-Y.; Juan, S.-H. Perfluorooctanesulfonate Mediates Renal Tubular Cell Apoptosis through PPARgamma Inactivation. PLoS ONE 2016, 11, e0155190. [Google Scholar] [CrossRef]
- Hu, W.; Jones, P.D.; Upham, B.L.; Trosko, J.E.; Lau, C.; Giesy, J.P. Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol. Sci. 2002, 68, 429–436. [Google Scholar] [CrossRef]
- Aimuzi, R.; Luo, K.; Chen, Q.; Wang, H.; Feng, L.; Ouyang, F.; Zhang, J. Perfluoroalkyl and polyfluoroalkyl substances and fetal thyroid hormone levels in umbilical cord blood among newborns by prelabor caesarean delivery. Environ. Int. 2019, 130, 104929. [Google Scholar] [CrossRef]
- Li, J.; Cai, D.; Chu, C.; Li, Q.; Zhou, Y.; Hu, L.; Yang, B.; Dong, G.; Zeng, X.; Chen, D. Transplacental Transfer of Per- and Polyfluoroalkyl Substances (PFASs): Differences between Preterm and Full-Term Deliveries and Associations with Placental Transporter mRNA Expression. Environ. Sci. Technol. 2020, 54, 5062–5070. [Google Scholar] [CrossRef]
- Spratlen, M.J.; Perera, F.P.; Lederman, S.A.; Robinson, M.; Kannan, K.; Herbstman, J.; Trasande, L. The Association Between Perfluoroalkyl Substances and Lipids in Cord Blood. J. Clin. Endocrinol. Metab. 2020, 105, 43–54. [Google Scholar] [CrossRef]
- National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/onehealth/basics/index.html (accessed on 15 November 2021).
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Dairy Production and Products: Production. Available online: http://www.fao.org/dairy-production-products/production/en/ (accessed on 4 October 2021).
- Wang, Z. An Assessment Report on Issues of Concern: Chemicals and Waste Issues Posing Risks to Human Health and the Environment; United Nations Environment Programme: Nairobi, Kenya, 2020. [Google Scholar]
- Rajan, A.; Shah, T. Impact of Irrigation on India’s Dairy Economy. Agriculture 2020, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Larssen, T.; Bečanová, J.; Karásková, P.; Whitehead, P.G.; Futter, M.N.; Butterfield, D.; Nizzetto, L. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human exposure. Environ. Pollut. 2016, 208, 704–713. [Google Scholar] [CrossRef]
- Yeung, L.W.Y.; Stadey, C.; Mabury, S.A. Simultaneous analysis of perfluoroalkyl and polyfluoroalkyl substances including ultrashort-chain C2 and C3 compounds in rain and river water samples by ultra performance convergence chromatography. J. Chromatogr. A 2017, 1522, 78–85. [Google Scholar] [CrossRef]
- Yeung, L.W.Y.; Yamashita, N.; Taniyasu, S.; Lam, P.K.S.; Sinha, R.K.; Borole, D.V.; Kannan, K. A survey of perfluorinated compounds in surface water and biota including dolphins from the Ganges River and in other waterbodies in India. Chemosphere 2009, 76, 55–62. [Google Scholar] [CrossRef] [PubMed]
- EWG Interactive Map. PFAS Contamination in the U.S. Available online: http://www.ewg.org/interactive-maps/pfas_contamination/map/ (accessed on 29 September 2021).
- Fourth National Report on Human Exposure to Environmental Chemicals Update; Centers for Disease Control and Prevention: Atlanta, Georgia, 2021.
- Buttarazzi, D. Maine Dairy Farmer’s Blood Tests High for ‘Forever Chemicals’ from Toxic Sludge. Available online: https://pfasproject.com/2019/08/16/maine-dairy-farmers-blood-tests-high-for-forever-chemicals-from-toxic-sludge/ (accessed on 29 September 2021).
- FDA. Analytical Results of Testing Food for PFAS from Environmental Contamination. Available online: https://www.fda.gov/food/chemical-contaminants-food/analytical-results-testing-food-pfas-environmental-contamination (accessed on 4 October 2021).
- USDA ARS. Search for an ARS Project. Available online: https://www.ars.usda.gov/research/project/?accnNo=436179 (accessed on 29 September 2021).
- Madison Water Utility. Perfluorinated Compounds. Available online: https://www.cityofmadison.com/water/water-quality/water-quality-testing/perfluorinated-compounds (accessed on 29 September 2021).
- Li, J.; Sun, J.; Li, P. Exposure routes, bioaccumulation and toxic effects of per- and polyfluoroalkyl substances (PFASs) on plants: A critical review. Environ. Int. 2022, 158, 106891. [Google Scholar] [CrossRef] [PubMed]
- Ingelido, A.M.; Abballe, A.; Gemma, S.; Dellatte, E.; Iacovella, N.; De Angelis, G.; Marra, V.; Russo, F.; Vazzoler, M.; Testai, E.; et al. Serum concentrations of perfluorinated alkyl substances in farmers living in areas affected by water contamination in the Veneto Region (Northern Italy). Environ. Int. 2020, 136, 105435. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.W.; Gunderson, K.G.; Green, L.A.; Rediske, R.R.; Steinman, A.D. Perfluoroalkylated Substances (PFAS) Associated with Microplastics in a Lake Environment. Toxics 2021, 9, 106. [Google Scholar] [CrossRef]
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per- and polyfluoroalkyl substances (PFAS) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Ehlers, S.; Oberhausen, A.; Tischer, M.; Fürst, P.; Schafft, H.; Lahrssen-Wiederholt, M. Absorption, Distribution, and Milk Secretion of the Perfluoroalkyl Acids PFBS, PFHxS, PFOS, and PFOA by Dairy Cows Fed Naturally Contaminated Feed. J. Agric. Food Chem. 2013, 61, 2903–2912. [Google Scholar] [CrossRef]
- Vestergren, R.; Orata, F.; Berger, U.; Cousins, I.T. Bioaccumulation of perfluoroalkyl acids in dairy cows in a naturally contaminated environment. Environ. Sci. Pollut. Res. 2013, 20, 7959–7969. [Google Scholar] [CrossRef]
- van Asselt, E.D.; Kowalczyk, J.; van Eijkeren, J.C.H.; Zeilmaker, M.J.; Ehlers, S.; Fürst, P.; Lahrssen-Wiederholt, M.; van der Fels-Klerx, H.J. Transfer of perfluorooctane sulfonic acid (PFOS) from contaminated feed to dairy milk. Food Chem. 2013, 141, 1489–1495. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Shi, Y.; Wang, P.; Jones, K.; Sweetman, A.J.; Johnson, A.C.; Zhang, M.; Zhou, Y.; Lu, X.; et al. Crop bioaccumulation and human exposure of perfluoroalkyl acids through multi-media transport from a mega fluorochemical industrial park, China. Environ. Int. 2017, 106, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Jin, Y.; Quan, X.; Sasaki, K.; Saito, N.; Nakayama, S.F.; Sato, I.; Tsuda, S. Perfluorosulfonates and perfluorocarboxylates in snow and rain in Dalian, China. Environ. Int. 2009, 35, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, D.; Lu, M.; Zhou, S.; Peng, T.; Yue, Z.; Zhou, Y. QuEChERs Combined with Online Interference Trapping LC-MS/MS Method for the Simultaneous Determination of 20 Polyfluoroalkane Substances in Dietary Milk. J. Agric. Food Chem. 2015, 63, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Lu, J.; Liu, Z.; Li, S.; Wang, G.; Wang, X. Occurrence of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Milk and Yogurt and Their Risk Assessment. Int. J. Environ. Res. Public. Health 2016, 13, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macheka, L.R.; Olowoyo, J.O.; Mugivhisa, L.L.; Abafe, O.A. Determination and assessment of human dietary intake of per and polyfluoroalkyl substances in retail dairy milk and infant formula from South Africa. Sci. Total Environ. 2021, 755, 142697. [Google Scholar] [CrossRef] [PubMed]
- Tittlemier, S.A.; Pepper, K.; Seymour, C.; Moisey, J.; Bronson, R.; Cao, X.-L.; Dabeka, R.W. Dietary Exposure of Canadians to Perfluorinated Carboxylates and Perfluorooctane Sulfonate via Consumption of Meat, Fish, Fast Foods, and Food Items Prepared in Their Packaging. J. Agric. Food Chem. 2007, 55, 3203–3210. [Google Scholar] [CrossRef]
- Guruge, K.S.; Manage, P.M.; Yamanaka, N.; Miyazaki, S.; Taniyasu, S.; Yamashita, N. Species-specific concentrations of perfluoroalkyl contaminants in farm and pet animals in Japan. Chemosphere 2008, 73, S210–S215. [Google Scholar] [CrossRef]
- Health Canada. HC (Health Canada) Updates to Health Canada Soil Screening Values for Perfluoroalkylated Substances (PFAS); Health Canada: Ottawa, Canada, 2019; pp. 1–2.
- UNEP. Fifteenth meeting of the Persistent Organic Pollutants Review Committee (POPRC.15). Available online: http://chm.pops.int/theconvention/popsreviewcommittee/meetings/poprc15/overview/tabid/8052/default.aspx (accessed on 5 October 2021).
- EEA relevance. Commission Regulation (EU) 2017/1000 of 13 June 2017 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards perfluorooctanoic acid (PFOA), its salts and PFOA-related substances. Off. J. Eur. Union. 2017, 150, 14. [Google Scholar]
- EEA. Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (integrated pollution prevention and control), Off. J. Eur. Union 2010, 337, 17.
- US EPA. Drinking Water Health Advisories for PFOA and PFOS. Available online: https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos (accessed on 11 October 2021).
- US EPA. PFAS Fact Sheets and Infographics. Available online: https://www.epa.gov/pfas/pfas-fact-sheets-and-infographics (accessed on 29 September 2021).
- Action Levels for PFOS in Cow’s Milk. Available online: https://www.maine.gov/dep/spills/topics/pfas/Derivation-of-Action-Levels-for-PFOS-in-Cows-Milk-03.28.17.pdf (accessed on 12 October 2021).
- Maine Department of Environmental Protection: Remedial Action Guidelines for Contaminated Sites (RAGs). Available online: https://www.maine.gov/dep/spills/publications/guidance/rags/Maine-Remedial-Action-Guidelines-2021-05-01.pdf (accessed on 12 October 2021).
- Bundesinstitut Für Risikobewertung. New Health-Based Guidance Values for the Industrial Chemicals PFOS and PFOA.; German Federal Institute for Risk Assessment: Berlin, Germany, 2019. [CrossRef]
- Country information—OECD Portal on Per and Poly Fluorinated Chemicals. Available online: https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/countryinformation/european-union.htm (accessed on 11 October 2021).
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of PFASs. Remediat. J. 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Lu, D.; Sha, S.; Luo, J.; Huang, Z.; Zhang Jackie, X. Treatment train approaches for the remediation of per- and polyfluoroalkyl substances (PFAS): A critical review. J. Hazard. Mater. 2020, 386, 121963. [Google Scholar] [CrossRef]
- Schröder, H.F.R.; José, H.J.; Gebhardt, W.; Moreira, R.F.P.M.; Pinnekamp, J. Biological wastewater treatment followed by physicochemical treatment for the removal of fluorinated surfactants. Water Sci. Technol. 2010, 61, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nanosci. Technol. 2007, 2, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- West, J.L.; Halas, N.J. Engineered nanomaterials for biophotonics applications: Improving sensing, imaging, and therapeutics. Annu. Rev. Biomed. Eng. 2003, 5, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, D.; Liang, Y. Nanotechnology in remediation of water contaminated by poly- and perfluoroalkyl substances: A review. Environ. Pollut. 2019, 247, 266–276. [Google Scholar] [CrossRef]
- CRC CARE. Assessment, Management and Remediation for PFOS and PFOA Part 5: Management and Remediation of PFOS and PFOA.; CRC for Contamination Assessment and Remediation of the Environment: Newcastle, Australia, 2017. [Google Scholar]
- Darlington, R.; Barth, E.; McKernan, J. The Challenges of PFAS Remediation. Mil. Eng. 2018, 110, 58–60. [Google Scholar]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef]
- Merino, N.; Qu, Y.; Deeb, R.A.; Hawley, E.L.; Hoffmann, M.R.; Mahendra, S. Degradation and Removal Methods for Perfluoroalkyl and Polyfluoroalkyl Substances in Water. Environ. Eng. Sci. 2016, 33, 615–649. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, Z.K. Activated Carbon; Marsh, H., Rodríguez-Reinoso, F., Eds.; Elsevier Science Ltd: Oxford, UK, 2006; ISBN 978-0-08-044463-5. [Google Scholar]
- De Bruecker, T. Status Report PFOS Remediation. DEC Environ. Solut. 2015, 12, 11–14. [Google Scholar]
- PFAS Remediation. Available online: https://www.ventia.com/capabilities/pfas-remediation (accessed on 5 October 2021).
- Sarsby, R.W. Environmental Geotechnics, 2nd ed.; Ice Publishing: London, UK, 2013; ISBN 978-0-7277-4187-5. [Google Scholar]
- Australian Research Council. Selection Report: PFAS Remediation Research Program (SR18) Round One. Available online: https://www.arc.gov.au/grants/grant-outcomes/selection-outcome-reports/selection-report-pfas-remediation-research-program-sr18-round-one (accessed on 5 October 2021).
- PFAS Management Area Plan—RAAF Base Williamtown. Available online: https://defence.gov.au/Environment/PFAS/docs/Williamtown/Reports/201907WilliamtownPMAP.pdf (accessed on 5 October 2021).
- PFAS Management Area Plan—Army Aviation Centre Oakey. Available online: https://defence.gov.au/Environment/PFAS/Docs/Oakey/Reports/AACOakeyPMAP.pdf (accessed on 5 October 2021).
- Sara, S. PFAS in the Baltic Sea Region. Available online: https://portal.helcom.fi/meetings/PRESSURE%207-2017-462/MeetingDocuments/7-7_Att%20Information%20on%20PFAS%20in%20the%20Baltc%20Sea%20Region.pdf (accessed on 5 October 2021).
- Environment Protection Authority Victoria. PFAS National Environmental Management Plan|Environment Protection Authority Victoria. Available online: https://www.epa.vic.gov.au/for-community/environmental-information/pfas/pfas-national-environmental-management-plan (accessed on 22 July 2021).
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical Decomposition of Perfluorooctane Sulfonate and Perfluorooctanoic Acid. Environ. Sci. Technol. 2005, 39, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical Degradation of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Groundwater: Kinetic Effects of Matrix Inorganics. Environ. Sci. Technol. 2010, 44, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drees, C.W. Sonochemical Degradation of Perfluorooctane Sulfonate (PFOS). Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2005. [Google Scholar]
- Murugananthan, M.; Yoshihara, S.; Rakuma, T.; Uehara, N.; Shirakashi, T. Electrochemical degradation of 17β-estradiol (E2) at boron-doped diamond (Si/BDD) thin film electrode. Electrochim. Acta 2007, 52, 3242–3249. [Google Scholar] [CrossRef]
- Cañizares, P.; Lobato, J.; Paz, R.; Rodrigo, M.A.; Sáez, C. Electrochemical oxidation of phenolic wastes with boron-doped diamond anodes. Water Res. 2005, 39, 2687–2703. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Yue, P.L. Anodic oxidation of dyes at novel Ti/B-diamond electrodes. Chem. Eng. Sci. 2003, 58, 995–1001. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design, 3rd ed.; Crittenden, J.C., Montgomery Watson Harza (Firm), Eds.; ETH Zurich: Zürich, Switzerland, 2012; ISBN 978-0-470-40539-0. [Google Scholar]
- Wang, L.; Batchelor, B.; Pillai, S.D.; Botlaguduru, V.S.V. Electron beam treatment for potable water reuse: Removal of bromate and perfluorooctanoic acid. Chem. Eng. J. 2016, 302, 58–68. [Google Scholar] [CrossRef]
- Ma, S.-H.; Wu, M.-H.; Tang, L.; Sun, R.; Zang, C.; Xiang, J.-J.; Yang, X.-X.; Li, X.; Xu, G. EB degradation of perfluorooctanoic acid and perfluorooctane sulfonate in aqueous solution. Nucl. Sci. Tech. 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Yu, G.; Zhang, Q.; Deng, S.; Wang, B. Destruction of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) by Ball Milling. Environ. Sci. Technol. 2013, 47, 6471–6477. [Google Scholar] [CrossRef]
- Javahrrian, M. Bench-Scale VEG Research & Development Study: Implementation Memorandum for Ex-Situ Thermal Desorption of Perfluoroalkyl Compounds (PFCs) in Soils; Endpoint Consulting Pty Ltd.: Sydney, Australia, 2016. [Google Scholar]
- Dickson, M. AusPat Patent Search Homepage. Available online: https://pericles.ipaustralia.gov.au/ols/auspat/applicationDetails.do?applicationNo=2012289835 (accessed on 5 October 2021).
- Dickson, M. Patent Application Publication. Available online: https://patentimages.sthttps://patentimages.storage.googleapis.com/a4/4f/92/6864036f22203d/US20140190896A1.pdf (accessed on 5 October 2021).
- Cao, M.H.; Wang, B.B.; Yu, H.S.; Wang, L.L.; Yuan, S.H.; Chen, J. Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation. J. Hazard. Mater. 2010, 179, 1143–1146. [Google Scholar] [CrossRef]
- Marrone, P.A.; Hodes, M.; Smith, K.A.; Tester, J.W. Salt precipitation and scale control in supercritical water oxidation—part B: Commercial/full-scale applications. J. Supercrit. Fluids 2004, 29, 289–312. [Google Scholar] [CrossRef]
- Mitton, D.B.; Eliaz, N.; Cline, J.A.; Latanision, R.M. An Overview of the Current Understanding of Corrosion in SCWO Systems for the Destruction of Hazardous Waste Products. Mater. Technol. 2001, 16, 44–53. [Google Scholar] [CrossRef]
- Jama, Y.; Lindholst, S.; Andreasen, R.R.; Andersen, H.R.; Kokkoli, A.; Svendsen, T.; Cai, Z.; Kragelund, C. PFAS removal from percolate by super critical water oxidation (SCWO). In Proceedings of the 14th DWF Water Research Conference-Abstracts, University of Copenhagen, Frederiksberg, Denmark, 30 January 2020; p. 48. [Google Scholar]
- Zhang, W.; Zhang, D.; Zagorevski, D.V.; Liang, Y. Exposure of Juncus effusus to seven perfluoroalkyl acids: Uptake, accumulation and phytotoxicity. Chemosphere 2019, 233, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Gobelius, L.; Lewis, J.; Ahrens, L. Plant Uptake of Per- and Polyfluoroalkyl Substances at a Contaminated Fire Training Facility to Evaluate the Phytoremediation Potential of Various Plant Species. Environ. Sci. Technol. 2017, 51, 12602–12610. [Google Scholar] [CrossRef]
- Huff, D.K.; Morris, L.A.; Sutter, L.; Costanza, J.; Pennell, K.D. Accumulation of six PFAS compounds by woody and herbaceous plants: Potential for phytoextraction. Int. J. Phytoremediation 2020, 22, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Tseng, N.; Wang, N.; Szostek, B.; Mahendra, S. Biotransformation of 6:2 Fluorotelomer Alcohol (6:2 FTOH) by a Wood-Rotting Fungus. Environ. Sci. Technol. 2014, 48, 4012–4020. [Google Scholar] [CrossRef]
- OECD Glossary of Statistical Terms—Biodegradation Definition. Available online: https://stats.oecd.org/glossary/detail.asp?ID=%20203 (accessed on 5 October 2021).
- Butzen, M.L.; Wilkinson, J.T.; McGuinness, S.R.; Amezquita, S.; Peaslee, G.F.; Fein, J.B. Sorption and desorption behavior of PFOS and PFOA onto a Gram-positive and a Gram-negative bacterial species measured using particle-induced gamma-ray emission (PIGE) spectroscopy. Chem. Geol. 2020, 552, 119778. [Google Scholar] [CrossRef]
- Brown, P. Race, Class, and Environmental Health: A Review and Systematization of the Literature. Environ. Res. 1995, 69, 15–30. [Google Scholar] [CrossRef]
- Ringquist, E.J. Assessing evidence of environmental inequities: A meta-analysis. J. Policy Anal. Manag. 2005, 24, 223–247. [Google Scholar] [CrossRef]
- Nelson, J.W.; Scammell, M.K.; Hatch, E.E.; Webster, T.F. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003-2006. Environ. Health 2012, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Park, S.K.; Peng, Q.; Ding, N.; Mukherjee, B.; Harlow, S.D. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ. Res. 2019, 175, 186–199. [Google Scholar] [CrossRef]
- The PFAS Project Lab. Available online: https://pfasproject.com/conference-presentations/ (accessed on 5 October 2021).
- Streimikiene, D. Environmental indicators for the assessment of quality of life. Intellect. Econ. 2015, 9, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Buekers, J.; Colles, A.; Cornelis, C.; Morrens, B.; Govarts, E.; Schoeters, G. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. Int. J. Environ. Res. Public Health 2018, 15, 2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, N.; Harlow, S.D.; Batterman, S.; Mukherjee, B.; Park, S.K. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: The Study of Women′s Health Across the Nation. Environ. Int. 2020, 135, 105381. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.W.; Kantrowitz, E. Socioeconomic Status and Health: The Potential Role of Environmental Risk Exposure. Annu. Rev. Public Health 2002, 23, 303–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohai, P.; Pellow, D.; Roberts, J.T. Environmental Justice. Annu. Rev. Environ. Resour. 2009, 34, 405–430. [Google Scholar] [CrossRef]
- Gochfeld, M.; Burger, J. Disproportionate Exposures in Environmental Justice and Other Populations: The Importance of Outliers. Am. J. Public Health 2011, 101, S53–S63. [Google Scholar] [CrossRef]
- Fernandez-Bou, A.S.; Ortiz-Partida, J.P.; Dobbin, K.B.; Flores-Landeros, H.; Bernacchi, L.A.; Medellín-Azuara, J. Underrepresented, understudied, underserved: Gaps and opportunities for advancing justice in disadvantaged communities. Environ. Sci. Policy 2021, 122, 92–100. [Google Scholar] [CrossRef]
- Banzhaf, S.; Ma, L.; Timmins, C. Environmental Justice: The Economics of Race, Place, and Pollution. J. Econ. Perspect. 2019, 33, 185–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbin, K.; Fencl, A. Institutional Diversity and Safe Drinking Water Provision in the United States. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
| Industry/Source | PFAS Compound(s) | Uses | Reference |
|---|---|---|---|
| Textile, electrical, metal, laundry and cleaning industries | PFOA, PFOS, PFBA and other PFAS | Industrial, commercial and consumer products | [13,14] |
| Aqueous Film Forming Foams (AFFFs) | PFOA, PFOS, PFBA and other PFAS | Fire training facilities/airports, military bases | [15,16,17] |
| Landfill leachate/waste disposal | PFOA, PFOS, PFBA, PFHxS and other PFAS | Reservoir for products containing PFAS chemicals that undergo decomposition, disposal of waste during primary and secondary manufacturing process using PFAS | [15,18,19] |
| Printing/paper product production | PFOA, PFOS, PFBA and other PFAS | Surface coatings to repel grease and moisture | [13,20] |
| Wastewater treatment plants/biosolids, recycled water | PFOA, PFOS, PFBA and other PFAS | Application of treated wastewater especially in agricultural lands water from manufacturing, industrial and household wastewater which are sources of PFOA and PFOS | [15,17,21,22,23,24] |
| Commercial and industrial products | PFOA, PFOS, PFBA and other PFAS | Products that repel water and oil in the textiles, paper industries (paper and packaging, clothing, carpets, nonstick cookware, pharmaceutical and personal care products (cosmetics, toothpaste), agricultural products (pesticides, herbicides), industrial (wire coating and insulation, corrosion prevention, surfactant, fluoroplastics, fluoropolymers, rubber) | [14,16,25] |
| Component | Description | PFOS | PFOA | Source |
|---|---|---|---|---|
| Soil | Includes agricultural, background and secondary-source contaminated soils | 3–5,500,000 ng/kg | 10–2,531,000 ng/kg | [38,91] |
| Water | Rainwater and groundwater used for irrigation and or livestock | 0.073–113 ng/L | 23–2752 ng/L | [89,91,92] |
| Milk | Includes raw, retail and full-cream milk | n.d.–9060 ng/L | n.d.–151.8 ng/L | [88,89,93,94,95] |
| Meat | Beef Muscle Beef Liver | 21–2700 ng/kg 24–91,000 ng/kg | 7–500 ng/kg 9–114,000 ng/kg | [89,96] [89,96,97] |
| Crops | Cereal grains (silage, wheat, barely, maize) | 3.9–860 ng/kg | 8.3–39,300 ng/kg | [89,91] |
| Matrix | Method Name | Developed by | Technology or Instrumentation | Sampling and Storage Methods | Remarks |
|---|---|---|---|---|---|
| Soil | ASTM D7968 17a | ASTM International | Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) |
| This method is applicable to determine 21 PFAS compounds |
| Non-potable water | SW-846 Method 8327 | USEPA | Multiple Reaction Monitoring (MRM) Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) |
| This method measures 24 PFAS compounds. Matrix-Groundwater, surface water and wastewater |
| SW-846 Method | USEPA | Isotope Dilution Method | Research underway | Collaborative efforts of USEPA and Department of Defense to analyze non-drinking water, biosolids and sediments | |
| Potable or drinking water | Method 537.1 | USEPA | Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) |
| This method is applicable to determine 18 PFAS compounds |
| Milk | C-010.01 | USFDA | Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS); modified QuEChERS extraction technique |
| This method is applicable to determine 16 PFAS compounds and can be used for milk, bread, lettuce and fish as matrices. |
| Meat (beef) | USDA | Methanolic Extraction analyzed by Liquid Chromatography/Tandem Mass Spectrometry |
| It is applicable to bovine muscle and plasma and can be analysed for 16 PFAS compounds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, G.; Kankarla, V.; McLennon, E.; Pal, S.; Sihi, D.; Dari, B.; Diaz, D.; Nocco, M. Per- and Polyfluoroalkyl Substances (PFAS) in Integrated Crop–Livestock Systems: Environmental Exposure and Human Health Risks. Int. J. Environ. Res. Public Health 2021, 18, 12550. https://doi.org/10.3390/ijerph182312550
Jha G, Kankarla V, McLennon E, Pal S, Sihi D, Dari B, Diaz D, Nocco M. Per- and Polyfluoroalkyl Substances (PFAS) in Integrated Crop–Livestock Systems: Environmental Exposure and Human Health Risks. International Journal of Environmental Research and Public Health. 2021; 18(23):12550. https://doi.org/10.3390/ijerph182312550
Chicago/Turabian StyleJha, Gaurav, Vanaja Kankarla, Everald McLennon, Suman Pal, Debjani Sihi, Biswanath Dari, Dawson Diaz, and Mallika Nocco. 2021. "Per- and Polyfluoroalkyl Substances (PFAS) in Integrated Crop–Livestock Systems: Environmental Exposure and Human Health Risks" International Journal of Environmental Research and Public Health 18, no. 23: 12550. https://doi.org/10.3390/ijerph182312550
APA StyleJha, G., Kankarla, V., McLennon, E., Pal, S., Sihi, D., Dari, B., Diaz, D., & Nocco, M. (2021). Per- and Polyfluoroalkyl Substances (PFAS) in Integrated Crop–Livestock Systems: Environmental Exposure and Human Health Risks. International Journal of Environmental Research and Public Health, 18(23), 12550. https://doi.org/10.3390/ijerph182312550






