1. Introduction
The increasing production of rubber products leads to the rapid growth of rubber industries to meet economic demands. Rubber processing requires a vast quantity of freshwater, and therefore the rubber processing industries are generating a massive amount of industrial effluent [
1,
2]. It is estimated to generate about 20,500 L of rubber effluents during processing 1 tonne of rubber [
2]. The rubber effluents contain a high concentration of various inorganic and organic contaminants. Therefore, the discharge of untreated rubber effluent into a watercourse would raise serious environmental pollution concerns [
3]. Hence, there is an urgency to determine an effective treatment process for the safe discharge of rubber processing effluent to eliminate undesirable substances to preserve the aquatic organisms.
The wastewater treatment mainly allows discharging industrial effluents without posing detrimental effects to the natural environment and human health since the effluent is discharged to a watercourse. However, the disposal of treated or partially treated industrial effluent in a watercourse can affect the aquatic ecosystem by depleting dissolved oxygen in the water [
3,
4]. Malaysian rubber processing industry implements biological wastewater treatment methods, including aerobic and anaerobic digestion, to treat the rubber processing effluent [
4,
5]. However, the biological wastewater treatment methods for industrial effluent treatment have several limitations, including the requirements of large food prints and long hydraulic retention time [
4,
6]. The biological treatment process of industrial effluent cannot minimize the concentration of the residual pollutants below the recommended discharge limits suggested by the Department of Environment (DoE), Malaysia (DoE 2009). Therefore, the biologically treated industrial effluent requires further treatment before discharging in a watercourse to diminish the pollutant concentrations lower the specified discharge limits set by DoE, Malaysia.
Coagulation–flocculation is the utmost effective technique to eliminate the organic and inorganic contaminants from wastewater [
7,
8]. However, the coagulation of industrial effluents is generally carried out by commercially available coagulants, including iron salts, alum, aluminum chloride, and polymer-based coagulants [
2,
8,
9]. However, using these commercial coagulants in industrial effluent treatment is a costly process due to the high price of commercial metals and polymeric-based coagulants [
9]. Furthermore, the aluminum-based coagulant application generates hazardous sludge, which requires costly disposal [
8,
9]. Therefore, scientists and environmentalists are searching for an alternative to commercial coagulants for treating industrial effluents. However, there is an increasing interest in utilizing waste materials to enhance sustainability and minimize environmental pollution [
7,
10]. FeSO
4·7H
2O is an industrial byproduct of the titanium oxide manufacturing industry, generated during extracting the titanium pigment from ilmenite ore in the sulfate method [
7,
11]. The titanium oxide industries are generating a vast amount of FeSO
4·7H
2O waste as a byproduct and storing it at the industry premises because of its high disposal cost. Thus, there is a need for an effective and environmentally friendly disposal method or the sustainable utilization of this massive volume of FeSO
4·7H
2O waste [
11].
Several approaches have been implemented to enhance the sustainable utilization of FeSO
4·7H
2O waste, such as chemical reductant [
12], raw materials for polymeric sulfate synthesis [
13], and sodium–ferrate synthesis [
14]. All these approaches are not sufficient to meet up the challenges of managing the massive volume of FeSO
4·7H
2O waste in the titanium oxide manufacturing industry, urging further implementation of FeSO
4·7H
2O waste to enhance sustainability [
7,
11]. However, FeSO
4·7H
2O waste has the comparable coagulation efficiency of aluminum-based coagulants, and the sludge generated after coagulation is not considered a hazardous element, especially since iron is the essential micronutrient in plant growth and fruits production [
15]. Thus, implementing the FeSO
4·7H
2O wastes as an eco-friendly coagulant in SRPE treatment bears a considerable interest in enhancing sustainable utilization of industrial byproducts and minimizing the hazardous sludge generation from the industrial treatment.
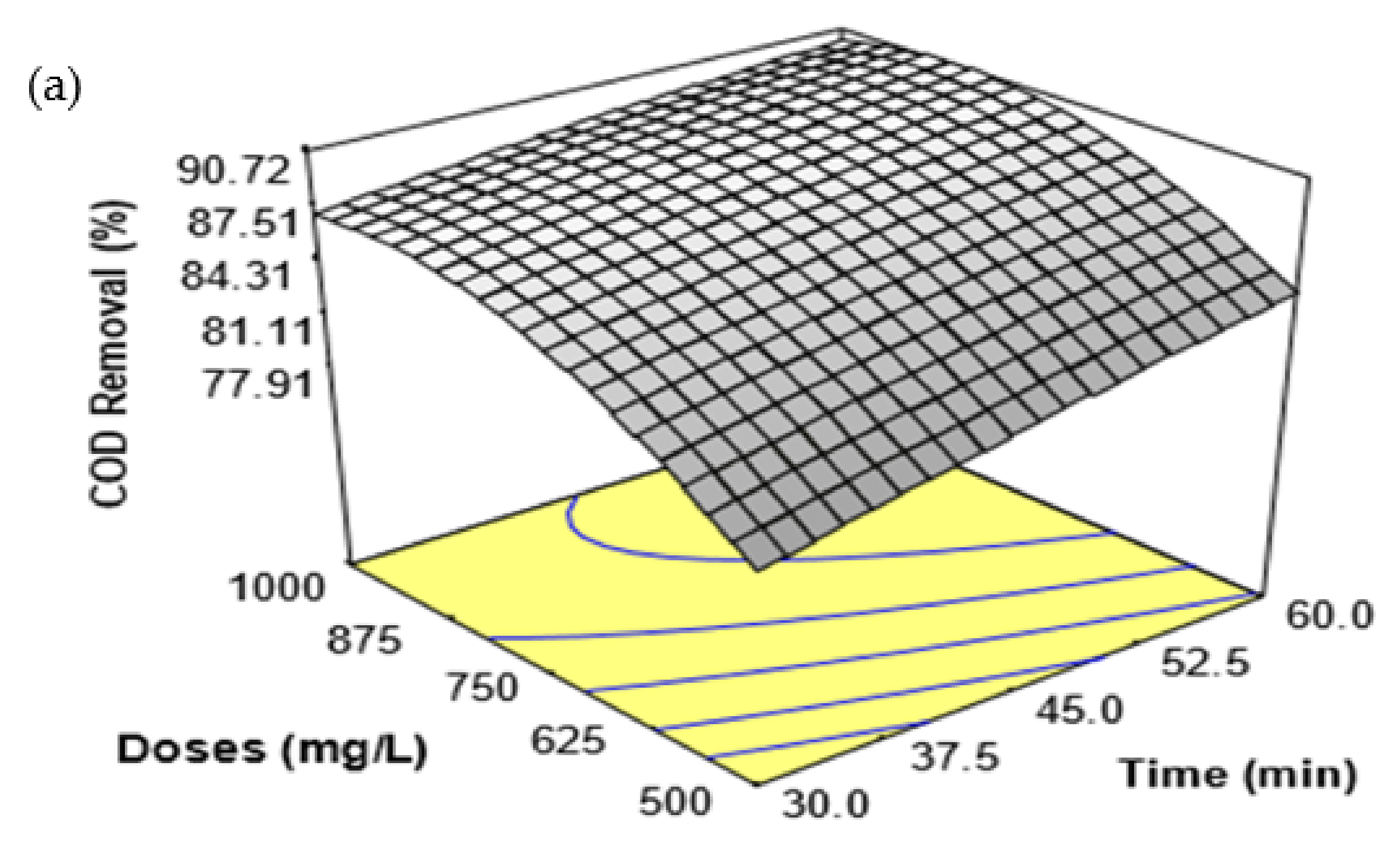
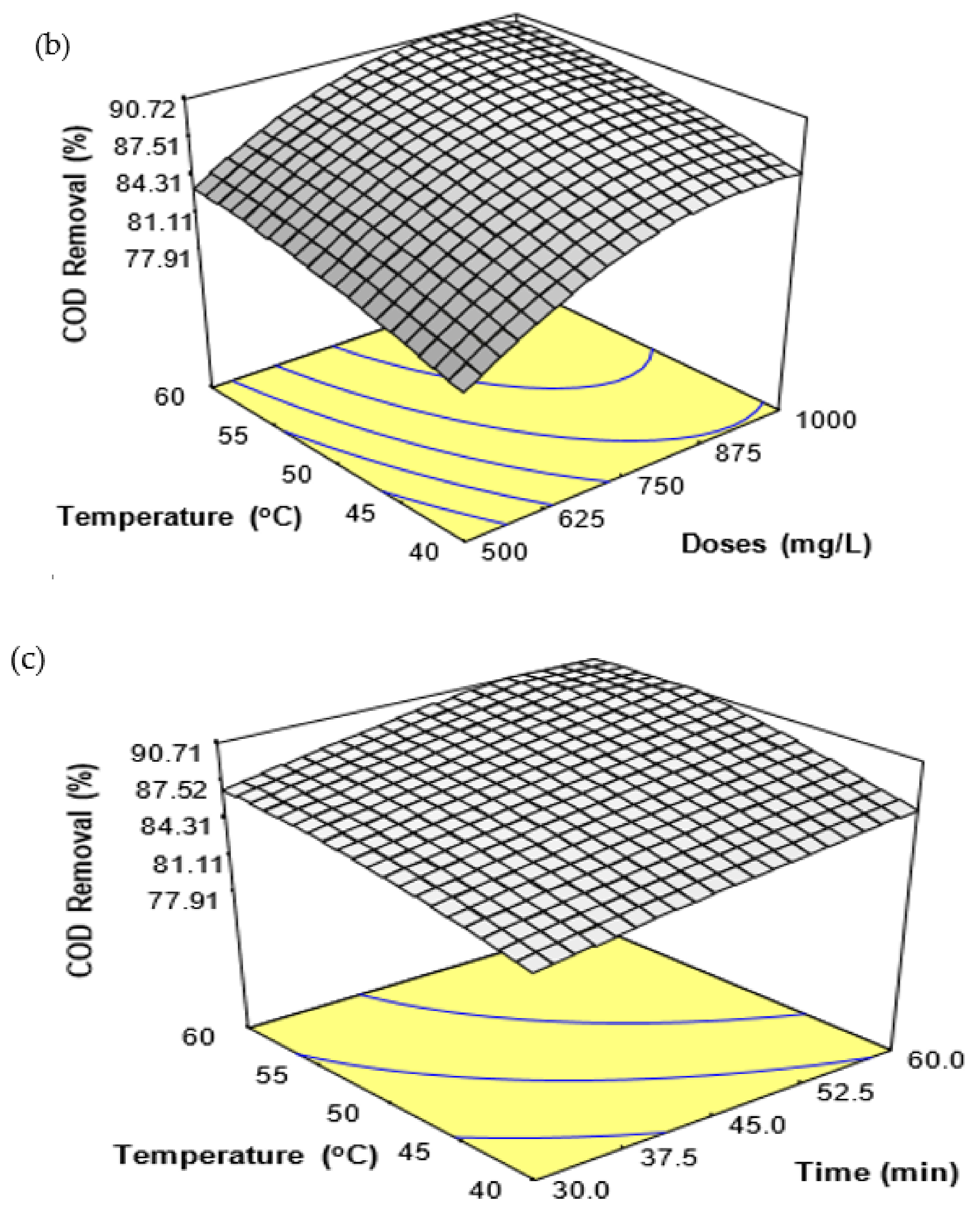
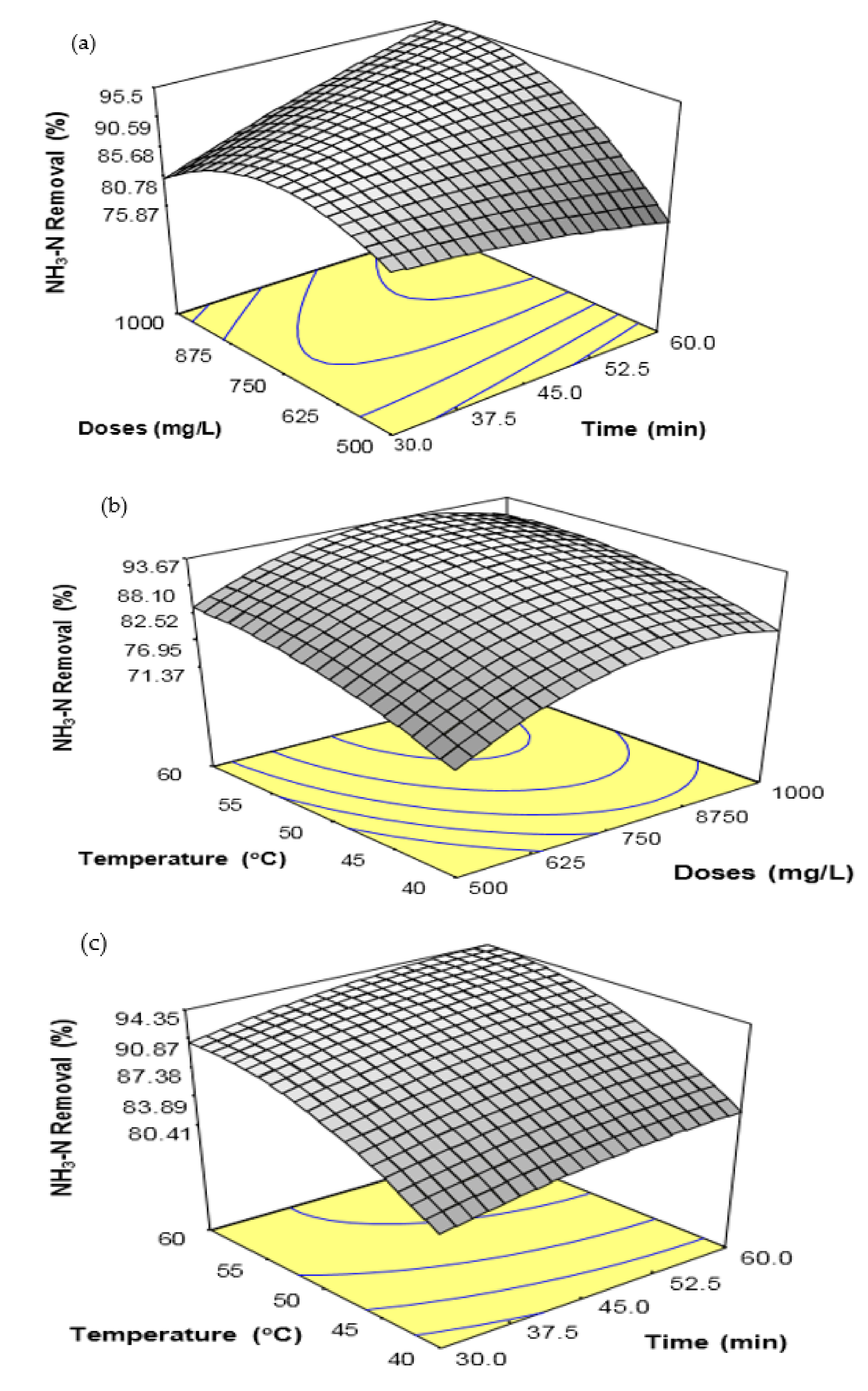
Many parameters, such as coagulant doses, treatment time, temperature, and pH, play an important role in enhancing or maximizing coagulation efficiency. Thus, the FeSO
4·7H
2O waste coagulation process variables urge a quantitative assessment to improve the NH
3-N and COD removal from SRPE. Further, the evaluation of coagulation appliances is a convenient tool to determine the suitability and coagulant potential for a coagulation process [
16]. The conventional experimental method implies that one parameter at a time could avoid some critical features of the experiments, such as the quadratic effect of the parameters and the interaction effect between or among the parameters [
16,
17]. Response surface methodology (RSM) is a widely applied statistical and mathematical method for analyzing and modeling a process behavior to assess the influences of several parameters and simultaneously optimize their responses [
18]. In a recent study, Ngteni et al. [
2] employed the FeSO
4·7H
2O waste to eliminate biological oxygen demand(BOD), chemical oxygen demand (COD), suspended solids (SS), and NH
3-N from SPRE with varying pH, coagulant doses, treatment time, and temperature. It was found that pH, coagulant doses, temperature, treatment time, and sedimentation time have potentially influenced the elimination of NH
3-N and COD from SPRE. The NH
3-N and COD removal increased with increasing coagulant doses, temperature, and treatment time. However, the NH
3-N and COD removal increased with increasing pH up to pH 5.0, and the removal was negligible over 60 min of sedimentation time. Although various parameters influenced the FeSO
4·7H
2O coagulation efficiency, the residual NH
3-N and COD were not reduced below the suggested discharge limits for the residual NH
3-N and COD concentration in treated rubber effluent set by DoE, Malaysia.
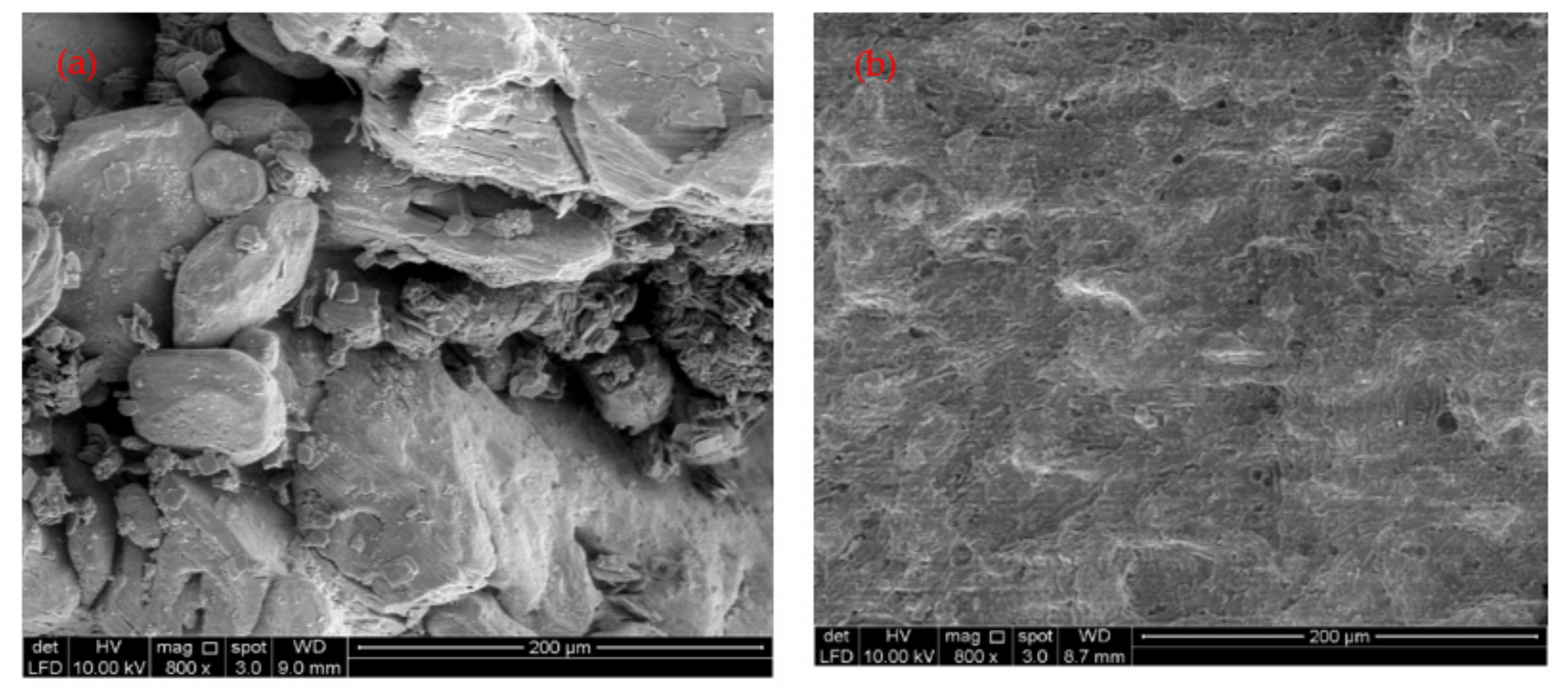
The aim of this study is to promote the sustainable utilization of FeSO4·7H2O waste from the titanium manufacturing industry as an environmental remediation potential coagulant for wastewater treatment and to zero waste generation from industrial effluent treatment. Therefore, in the present study, the FeSO4·7H2O waste, obtained as a byproduct of the titanium oxide manufacturing industry, was employed as a coagulant to eliminate NH3-N and COD from the secondary rubber processing effluent (SRPE). The coagulation process was optimized using RSM based on the maximum elimination of NH3-N and COD from SRPE. Furthermore, elemental compositions and mineral compositions in generated sludge were determined using scanning electron microscopy with energy dispersive X-Ray Analysis (SEM-EDX), and inductively coupled plasma optical emission spectrometry (ICP-OES) was employed to determine the possible utilization of the generated sludge as an organic fertilizer. The findings of the present study will be helpful in determining alternatives to commercial inorganic coagulants in treating industrial effluent without generating toxic sludge.
4. Conclusions
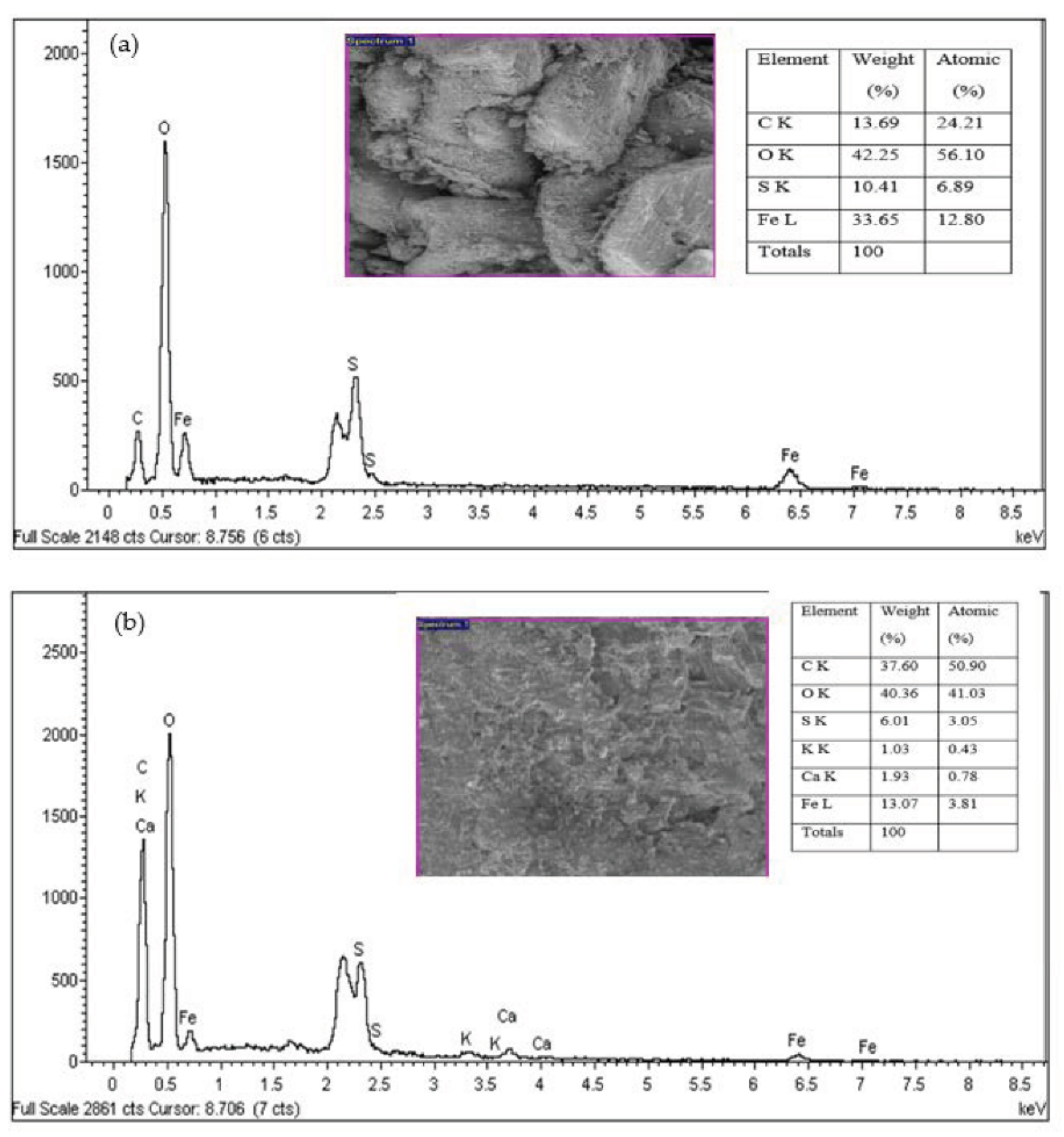
In the present study, FeSO4·7H2O waste from the titanium oxide manufacturing industry was employed as a coagulant for the NH3-N and COD removal from SRPE. It was found that the second-order polynomial equation adequately fitted experimental data. The coagulation time, FeSO4·7H2O waste doses, and temperature were statistically significant for the COD removal from SRPE, while FeSO4·7H2O waste doses and temperature were statistically significant for the NH3-N removal from SRPE. The optimal experimental conditions were determined to be the coagulation time of 70 min, doses 900 mg/L, and the temperature 62 °C. The maximum COD and NH3-N removal obtained were 93.86% and 98.19%, respectively, at the optimized FeSO4·7H2O coagulation experimental conditions. The residual COD and NH3-N in treated SPRE were 57 ± 4 mg/L and 16 ± 2 mg/L, respectively, below the stipulated industrial effluent discharge limits set by DoE, Malaysia. Hence, the optimized conditions found in the present study show a satisfactory outcome and can be applied to remove COD and NH3-N in treating SRPE generated by the rubber processing industry. The sludge generated after coagulation SRPE contains essential plant nutrients, including iron (Fe), phosphorous (P), potassium (K), calcium (Ca), magnesium (Mg), and boron (B). Thus, the sludge generated from the SRPE treatment using FeSO4·7H2O waste as a coagulant could be utilized as an organic fertilizer for plant growth. Therefore, it can be postulated that using FeSO4·7H2O waste as a coagulant would enhance the sustainable utilization of a waste product and promote zero waste generation from industrial effluent treatment.