The DAMA25 Study: Feasibility of a Lifestyle Intervention Programme for Cancer Risk Reduction in Young Italian Women with Breast Cancer Family History
Abstract
:1. Introduction
2. Materials and Methods
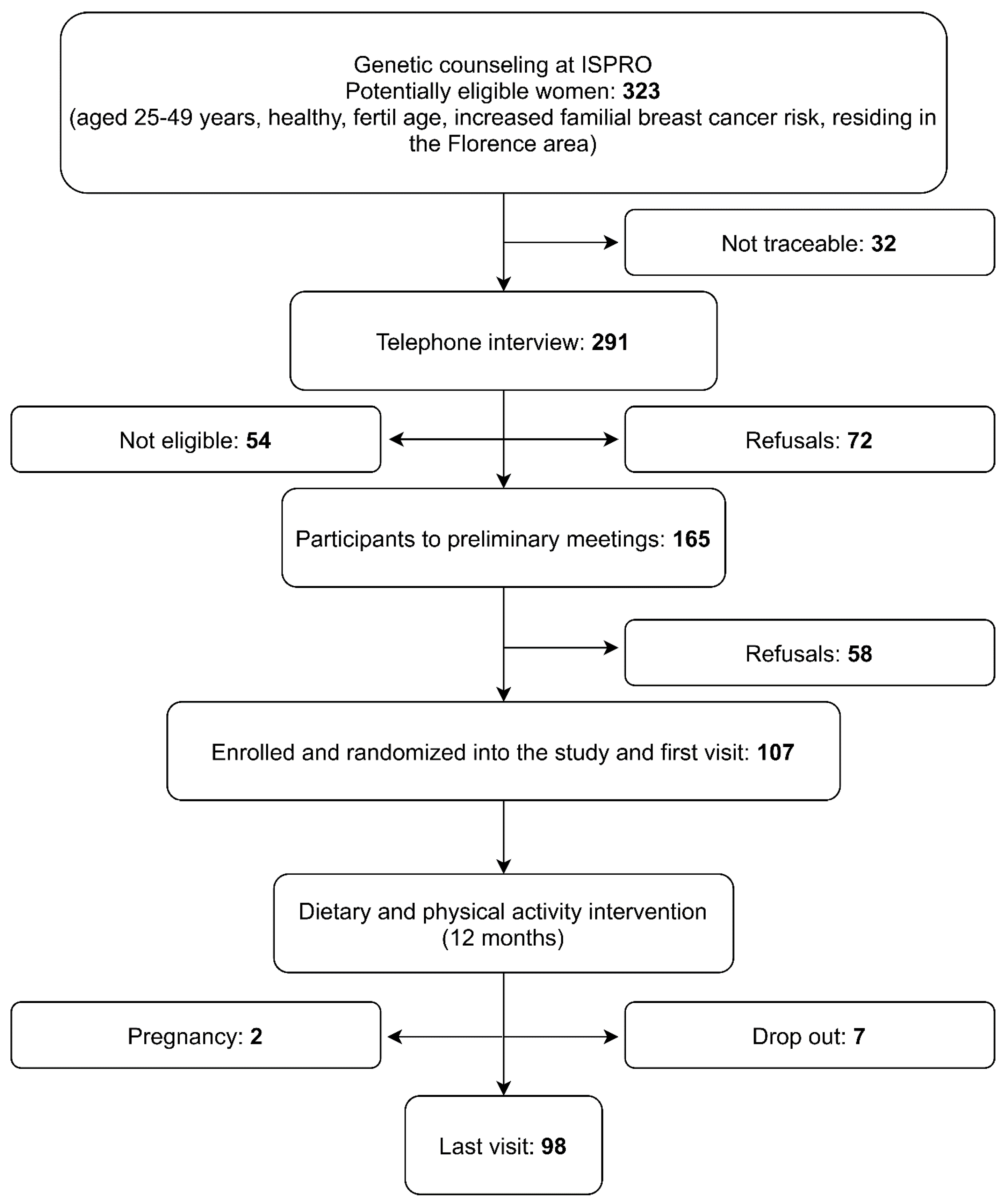
2.1. Selection of Study Participants and Data Collection
2.2. Baseline Visit
2.3. WCRF Score Construction
2.4. Dietary Intervention
- cereals (bread, pasta, and grains): gradual replacement of refined grains with whole grains, in particular whole-wheat bread. Increase in whole grain cereals (such as rice, spelt and barley).
- not starchy vegetables: consumption of one portion of raw and one portion of cooked vegetables at each meal including sauces for pasta based on tomatoes, zucchini, artichokes, broccoli, etc.; vegetable and legume soups, addition of vegetables to meat dishes and sandwiches.
- fish: consumption at least biweekly. Both fresh and frozen, not precooked fish were acceptable choices.
- meat and processed meat: consumption of fresh and processed red meat had to be reduced to less than 1 time per week considering all types. Poultry (chicken/turkey) was suggested as an acceptable alternative (with a maximum of 2 times per week).
- legumes and pulses: their consumption had to be gradually increased to at least three-four portions/week.
- fruit: consumption of 2 to 3 portions of fruit per day including fruit at breakfast or as snacks during the day, as an alternative to cookies and pastries.
- added fat: high-quality extra virgin olive oil had to be used as the only dressing and cooking fat. Its use for cooking dishes had, however, to be reduced, while no restrictions were made for seasoning vegetables, legumes, soups, etc.
- processed foods: to be reduced to a minimum, decreasing the consumption of ready-to-eat dishes, desserts, cookies, processed meat, etc.
- sugar, sweets, desserts: consumption had to be reduced or avoided. Advice was given to replace sweets with fruit.
- milk and dairy products: limited consumption, exclusion of full-fat varieties.
- wine and spirits: consumption of no more than 1 glass of wine per day, at meals, if already used to drink wine. The consumption of other alcoholic beverages and of ready-to-eat dishes was discouraged.
2.5. Physical Activity Intervention
2.6. Final Visit
2.7. Statistical Analysis
3. Results
3.1. Family History
3.2. Baseline Characteristics
3.3. WCRF Adherence
3.4. Compliance with the Proposed Interventions
3.5. Adherence to Protocol and Satisfaction Questionnaire
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- I Numeri del Cancro in Italia 2019. Available online: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operatori-web.pdf (accessed on 12 September 2019).
- Mavaddat, N.; Peock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Evans, D.G.; Izatt, L.; Eeles, R.A.; Adlard, J.; et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013, 105, 812–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavaddat, N.; Pharoah, P.D.; Michailidou, K.; Tyrer, J.; Brook, M.N.; Bolla, M.K.; Wang, Q.; Dennis, J.; Dunning, A.M.; Shah, M.; et al. Prediction of breast cancer risk based on profiling with common genetic variants. J. Natl. Cancer Inst. 2015, 107, djv036. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Lubinski, J.; Salmena, L.; Lynch, H.T.; Kim-Sing, C.; Foulkes, W.D.; Ghadirian, P.; Neuhausen, S.L.; Demsky, R.; Tung, N.; et al. Hereditary Breast Cancer Clinical Study Group Breastfeeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2012, 14, R42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehm, R.D.; Genkinger, J.M.; MacInnis, R.J.; John, E.M.; Phillips, K.A.; Dite, G.S.; Milne, R.L.; Zeinomar, N.; Liao, Y.; Knight, J.A.; et al. Recreational physical activity is associated with reduced breast cancer risk in adult women at high risk for breast cancer: A cohort study of women selected for familial and genetic risk. Cancer Res. 2020, 80, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Pettapiece-Phillips, R.; Narod, S.A.; Kotsopoulos, J. The role of body size and physical activity on the risk of breast cancer in BRCA mutation carriers. Cancer Causes Control 2015, 26, 333–344. [Google Scholar] [CrossRef]
- World Cancer Research Fund (WCFR) Recommendations 2018. Available online: https://www.wcrf.org/diet-and-cancer/cancer-prevention-recommendations/ (accessed on 1 October 2021).
- Premenopausal Breast Cancer Collaborative Group; Schoemaker, M.J.; Nichols, H.B.; Wright, L.B.; Brook, M.N.; Jones, M.E.; O’Brien, K.M.; Adami, H.O.; Baglietto, L.; Bernstein, L.; et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018, 4, e181771. [Google Scholar] [CrossRef]
- Hopper, J.L.; Dite, G.S.; MacInnis, R.J.; Liao, Y.; Zeinomar, N.; Knight, J.A.; Southey, M.C.; Milne, R.L.; Chung, W.K.; Giles, G.G.; et al. Age-specific breast cancer risk by body mass index and familial risk: Prospective family study cohort (ProF-SC). Breast Cancer Res. 2018, 20, 132. [Google Scholar] [CrossRef] [Green Version]
- Cloud, A.J.; Thai, A.; Lyao, Y.; Terry, M.B. The impact of cancer prevention guideline adherence on overall mortality in a high-risk cohort of women from the New York site of the Breast Cancer Family Registry. Breast Cancer Res. Treat. 2015, 149, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, S.; Lux, M.P.; Fasching, P.A.; Strissl, P.; Renner, S.P.; Poehls, U.; Bender, H.G.; Beckmann, M.W. Acceptance for Preventive Genetic Testing and Prophylactic Surgery in Women With a Family History of Breast and Gynaecological Cancers. Eur. J. Cancer Prev. 2006, 15, 474–479. [Google Scholar] [CrossRef]
- Quillin, J.M. Lifestyle Risk Factors Among People Who Have Had Cancer Genetic Testing. J. Genet Couns. 2016, 25, 957–964. [Google Scholar] [CrossRef]
- Palli, D.; Berrino, F.; Vineis, P.; Tumino, R.; Panico, S.; Masala, G.; Saieva, C.; Salvini, S.; Ceroti, M.; Pala, V.; et al. EPIC-Italy: A molecular epidemiology project on diet and cancer: The EPIC-Italy Prospective Study. Design and baseline characteristics of participants. Tumori 2003, 89, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian EPIC Cohorts: Presentation of Data and Methodological Issues. Tumori 2003, 89, 594–607. [Google Scholar] [CrossRef] [PubMed]
- InterAct Consortium; Peters, T.; Brage, S.; Westgate, K.; Franks, P.W.; Gradmark, A.; Tormo Diaz, M.J.; Huerta, J.M.; Bendinelli, B.; Vigl, M.; et al. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur. J. Epidemiol. 2012, 27, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romaguera, D.; Vergnaud, A.C.; Peeters, P.H.; Van Gils, C.H.; Chan, D.S.; Ferrari, P.; Romieu, I.; Jenab, M.; Slimani, N.; Clavel-Chapelon, F.; et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am. J. Clin. Nutr. 2012, 96, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Brockton, N.T.; Mitrou, P.; Romaguera, D.; Brown, S.; Bender, A.; Kahle, L.L.; Reedy, J. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: A Standardized Scoring System. Nutrients 2019, 11, 1572. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef] [Green Version]
- National Institute for health and Care Excellence (NICE) Clinical Guidelines 2017. Available online: https://www.nice.org.uk/guidance/cg164/resources (accessed on 1 October 2021).
- Linos, E.; Willett, W.C.; Cho, E.; Colditz, G.; Frazier, L.A. Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2146–2151. [Google Scholar] [CrossRef] [Green Version]
- Farvid, M.S.; Cho, E.; Chen, W.Y.; Eliassen, A.H.; Willett, W.C. Adolescent meat intake and breast cancer risk. Int. J. Cancer 2015, 136, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.D.; Agudo, A.; Sánchez, M.J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef]
- Neilson, H.K.; Farris, M.S.; Stone, C.R.; Vaska, M.M.; Brenner, D.R.; Friedenreich, C.M. Moderate-vigorous Recreational Physical Activity and Breast Cancer Risk, Stratified by Menopause Status: A Systematic Review and Meta-Analysis. Menopause 2017, 24, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Massetti, G.M.; Dietz, W.H.; Richardson, L.C. Excessive weight gain, obesity, and cancer: Opportunities for clinical intervention. JAMA 2017, 318, 1975–1976. [Google Scholar] [CrossRef]
- Anderson, A.S.; Dunlop, J.; Gallant, S.; Macleod, M.; Miedzybrodzka, Z.; Mutrie, N.; O’Carroll, R.E.; Stead, M.; Steele, R.J.C.; Taylor, R.S.; et al. Feasibility study to assess the impact of a lifestyle intervention (’LivingWELL’) in people having an assessment of their family history of colorectal or breast cancer. BMJ Open 2017, 8, e019410. [Google Scholar] [CrossRef]
- Hewitt, R.M.; Pegington, M.; Harvie, M.; French, D.P. How acceptable is a weight maintenance programme for healthy weight young women who are at increased risk of breast cancer? Psychol. Health 2019, 20, 1–18. [Google Scholar] [CrossRef]
- Albada, A.; Vernooij, M.; van Osch, L.; Pijpe, A.; van Dulmen, S.; Ausems, M. Does and should breast cancer genetic counselling include lifestyle advice? Fam Cancer 2014, 13, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiechle, M.; Dukatz, R.; Yahiaoui-Doktor, M.; Berling, A.; Basrai, M.; Staiger, V.; Niederberger, U.; Marter, N.; Lammert, J.; Grill, S.; et al. Feasibility of structured endurance training and Mediterranean diet in BRCA1 and BRCA2 mutation carriers - an interventional randomized controlled multicenter trial (LIBRE-1). BMC Cancer 2017, 17, 752. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Williams, N.I.; Kontos, D.; Kurzer, M.S.; Schnall, M.; Domchek, S.; Stopfer, J.; Galantino, M.L.; Hwang, W.T.; Morales, K.; et al. Women In Steady Exercise Research (WISER) Sister: Study Design and Methods. Contemp. Clin. Trials 2015, 41, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, M.; Engel, C.; Berling, A.; Hebestreit, K.; Bischoff, S.C.; Dukatz, R.; Siniatchkin, M.; Pfeifer, K.; Grill, S.; Yahiaoui-Doktor, M.; et al. Effects of lifestyle intervention in BRCA1/2 mutation carriers on nutrition, BMI, and physical fitness (LIBRE study): Study protocol for a randomized controlled trial. Trials 2016, 17, 368. [Google Scholar] [CrossRef] [Green Version]
| n (%) | |
|---|---|
| Age (years) | |
| <40 | 31 (31.6) |
| ≥40 | 67 (68.4) |
| BMI (kg/m2) | |
| <25 | 67 (68.4) |
| ≥25 | 31 (31.6) |
| Living with a partner | |
| No 1 | 34 (34.7) |
| yes | 64 (65.3) |
| Number of children | |
| 0 | 31 (31.6) |
| 1 | 30 (30.6) |
| ≥2 | 37 (37.8) |
| Breastfeeding 2 | |
| no | 6 (9.0) |
| yes | 61 (91.0) |
| Education | |
| Primary school | 3 (3.1) |
| High school | 45 (45.9) |
| University | 50 (51.0) |
| Paid work | |
| no | 7 (7.2) |
| part-time | 31 (31.6) |
| full time | 60 (61.2) |
| Physical activity at work | |
| sedentary | 66 (72.5) |
| standing | 15 (16.5) |
| manual | 10 (11.0) |
| Smoking history | |
| Current | 22 (22.5) |
| Former | 21 (21.4) |
| Never | 55 (56.1) |
| Contraceptive pill use | |
| Current | 14 (14.3) |
| Ever | 71 (72.4) |
| Never | 13 (13.3) |
| Total | 98 |
| 2018 WCRF/AIRC Adherence Score | Absolute Frequency (N) | Relative Frequency (%) |
|---|---|---|
| 1 Category 1 | 25 | 23.36 |
| 1 Category 2 | 68 | 63.55 |
| 1 Category 3 | 14 | 13.09 |
| 2018 WCRF/AIRC Recommendations | Operationalisation of Recommendations | Points | Absolute Frequency (N) | Relative Frequency (%) |
|---|---|---|---|---|
| 1. Be a healthy weight | BMI (kg/m2): | |||
| <18.5 or ≥30 | 0 | 15 | 14.02 | |
| 25–29.9 | 0.25 | 19 | 17.76 | |
| 18.5–24.9 | 0.5 | 73 | 68.22 | |
| Waist circumference (cm(in)): | ||||
| ≥88 (≥35) | 0 | 20 | 18.69 | |
| 80–<8 (31.5–<35) | 0.25 | 16 | 14.95 | |
| <80 (31.5) | 0.5 | 71 | 66.36 | |
| 2. Be physically active | Total moderate-vigorous physical activity (min/wk): | |||
| <75 | 0 | 52 | 48.60 | |
| 75–<150 | 0.5 | 17 | 15.89 | |
| ≥150 | 1 | 38 | 35.51 | |
| 3. Eat a diet rich in wholegrains, vegetables, fruit and beans | Fruits and vegetables (g/day): | |||
| <200 | 0 | 9 | 8.41 | |
| 200–<400 | 0.25 | 36 | 33.64 | |
| ≥400 | 0.5 | 62 | 57.94 | |
| Total fiber (g/day): | ||||
| <15 | 0 | 19 | 17.76 | |
| 15–<30 | 0.25 | 77 | 71.96 | |
| ≥30 | 0.5 | 11 | 10.28 | |
| 4. Limit consumption of “fast food” and other processed food high in fat, starches or sugars | Percent of total Kcal from ultra- processed foods (aUPFs): | |||
| Tertile 1 | 0 | 36 | 33.64 | |
| Tertile 2 | 0.5 | 36 | 33.64 | |
| Tertile 3 | 1 | 35 | 32.71 | |
| 5. Limit consumption of red and processed meat | Total red meat (g/wk) and processed meat (g/wk): | |||
| Red meat >500 or processed meat ≥100 | 0 | 63 | 58.88 | |
| Red meat <500 and processed meat 21–<100 | 0.5 | 34 | 31.78 | |
| Red meat <500 and processed meat <21 | 1 | 10 | 9.35 | |
| 6. Limit consumption of sugar-sweetened drinks | Total sugar-sweetened drinks (g/day): | |||
| >250 | 0 | 1 | 0.93 | |
| >0–≤250 | 0.5 | 81 | 75.70 | |
| >250 | 1 | 25 | 23.36 | |
| 7. Limit alcohol consumption | Total ethanol (g/day): | |||
| >14 (1 drink) | 0 | 12 | 11.21 | |
| ≤14 (1 drink) | 0.5 | 85 | 79.44 | |
| 0 | 1 | 10 | 9.35 |
| Food Groups (g/day) | Baseline Mean Median (10–90°) | Follow up Mean Median (10–90°) | Difference 1 (%) | p Value 2 |
|---|---|---|---|---|
| Total vegetables and soups | 230.0 215.7 (115.2–373.6) | 256.8 225.3 (141.7–413.3) | 26.8 (11.7) | 0.06 |
| - leafy vegetables | 40.4 35.4 (10.6–79.3) | 48.0 38.7 (17.3–85.2) | 7.6 (18.8) | 0.01 |
| Legumes | 21.1 17.2 (4.3–44.9) | 34.9 32.0 (9.2–66.4) | 13.8 (65.4) | 0.39 |
| Fresh fruit | 262.8 230.2 (104.9–474.9) | 273.2 243.6 (136.3–434.6) | 10.4 (4.0) | <0.0001 |
| Nuts, seeds and dried fruit | 2.8 1.4 (0.2–7.3) | 4.8 3.7 (0.2–10.5) | 2.0 (71.4) | 0.02 |
| Bread | 114.2 101.7 (19.0–150.4) | 97.2 80.3 (18.9–203.4) | −17.0 (14.9) | <0.0001 |
| White bread | 42.3 24.3 (0.0–100.00) | 12.5 3.6 (0.0–44.4) | −29.8 (70.5) | <0.0001 |
| Whole grain bread | 34.5 15.9 (0.0–100.0) | 65.1 43.6 (5.1–177.8) | 30.6 (88.7) | <0.0001 |
| Red and processed meat | 61.7 57.2 (16.3–116.1) | 29.9 27.0 (4.9–61.2) | −31.8 (51.5) | <0.0001 |
| Poultry | 33.3 29.4 (2.8–76.7) | 21.3 16.3 (0.8–44.6) | −12.0 (36.0) | <0.0001 |
| Fish | 41.2 39.8 (7.9–71.7) | 59.7 60.1 (19.7–102.6) | 18.5 (44.9) | 0.16 |
| Cakes and cookies | 97.0 89.5 (35.4–174.9) | 60.8 51.8 (26.4–99.8) | −36.2 (37.3) | <0.0001 |
| Olive oil | 27.0 27.4 (13.8–43.2) | 29.4 25.9 (16.8–45.8) | 2.4 (8.9) | 0.04 |
| Seed oil | 0.4 0.3 (0.0–0.4) | 0.3 0.3 (0.0–0.4) | −0.1 (25.0) | <0.0001 |
| Butter | 1.3 0.4 (0.0–2.9) | 0.6 0.4 (0.1–1.1) | 0.7 (53.8) | <0.0001 |
| Milk | 87.6 34.3 (0.0–224.0) | 64.2 14.6 (0.0–164.0) | −23.4 (26.7) | <0.0001 |
| Cheese | 48.9 43.2 (11.0–99.8) | 30.2 24.8 (10.6–55.0) | −18.7 (38.2) | <0.0001 |
| Wine 3 | 49.1 17.9 (2.1–125.0) | 42.7 17.9 (4.2–125.0) | −6.4 (13.0) | <0.0002 |
| Leisure Time Physical Activity (Hours- Week) | Baseline Mean Median (10–90°) | Follow up Mean Median (10–90°) | Absolute Difference 1 (%) | p Value 2 |
|---|---|---|---|---|
| Overall leisure time physical activity | 16.52 10.8 (3.9–24.6) | 20.13 14.4 (5.5–31.3) | 3.61 (21.9) | 0.0002 |
| - Household physical activity | 10.57 10.6 (3.5–24.8) | 11.45 10.8 (3.9–24.6) | 0.88 (8.3) | 0.004 |
| - Recreational physical activity | 5.95 4.6 (1.3–10.8) | 8.67 8.3 (3.3–15.0) | 2.72 (45.7) | <0.0001 |
| Walking | 3.37 2.5 (0.5–7.5) | 5.13 4.5 (1.5–9.5) | 1.76 (52.2) | <0.0001 |
| Fitness | 1.75 0.8 (0.0–5.5) | 2.55 1.5 (0.0–5.5) | 0.80 (45.7) | <0.0001 |
| Baseline Mean Median (10–90°) | Follow up Mean Median (10–90°) | Absolute Difference 1 (%) | p Value 2 | |
|---|---|---|---|---|
| Weight (kg) | 65.86 62.6 (51.0–81.6) | 64.42 62.3 (50.3–80.1) | −1.44 (2.2) | 0.005 |
| Waist (cm) | 79.20 76.0 (67.6–3.50) | 75.60 73.0 (65.0–88.0) | −3.60 (4.5) | <0.001 |
| Fat mass 3 (kg) | 20.00 17.7 (10.7–31.6) | 18.80 17.2 (10.3–30.7) | −1.17 (5.9) | 0.015 |
| Lean mass 3 (kg) | 45.79 44.7 (39.9–53.5) | 45.54 45.3 (39.2–52.6) | −0.19 (0.4) | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masala, G.; Palli, D.; Ermini, I.; Occhini, D.; Facchini, L.; Sequi, L.; Castaldo, M.; Caini, S.; Bendinelli, B.; Saieva, C.; et al. The DAMA25 Study: Feasibility of a Lifestyle Intervention Programme for Cancer Risk Reduction in Young Italian Women with Breast Cancer Family History. Int. J. Environ. Res. Public Health 2021, 18, 12287. https://doi.org/10.3390/ijerph182312287
Masala G, Palli D, Ermini I, Occhini D, Facchini L, Sequi L, Castaldo M, Caini S, Bendinelli B, Saieva C, et al. The DAMA25 Study: Feasibility of a Lifestyle Intervention Programme for Cancer Risk Reduction in Young Italian Women with Breast Cancer Family History. International Journal of Environmental Research and Public Health. 2021; 18(23):12287. https://doi.org/10.3390/ijerph182312287
Chicago/Turabian StyleMasala, Giovanna, Domenico Palli, Ilaria Ermini, Daniela Occhini, Luigi Facchini, Lisa Sequi, Maria Castaldo, Saverio Caini, Benedetta Bendinelli, Calogero Saieva, and et al. 2021. "The DAMA25 Study: Feasibility of a Lifestyle Intervention Programme for Cancer Risk Reduction in Young Italian Women with Breast Cancer Family History" International Journal of Environmental Research and Public Health 18, no. 23: 12287. https://doi.org/10.3390/ijerph182312287
APA StyleMasala, G., Palli, D., Ermini, I., Occhini, D., Facchini, L., Sequi, L., Castaldo, M., Caini, S., Bendinelli, B., Saieva, C., Assedi, M., & Zanna, I. (2021). The DAMA25 Study: Feasibility of a Lifestyle Intervention Programme for Cancer Risk Reduction in Young Italian Women with Breast Cancer Family History. International Journal of Environmental Research and Public Health, 18(23), 12287. https://doi.org/10.3390/ijerph182312287







