Association of Occupational Distress and Low Sleep Quality with Syncope, Presyncope, and Falls in Workers
Abstract
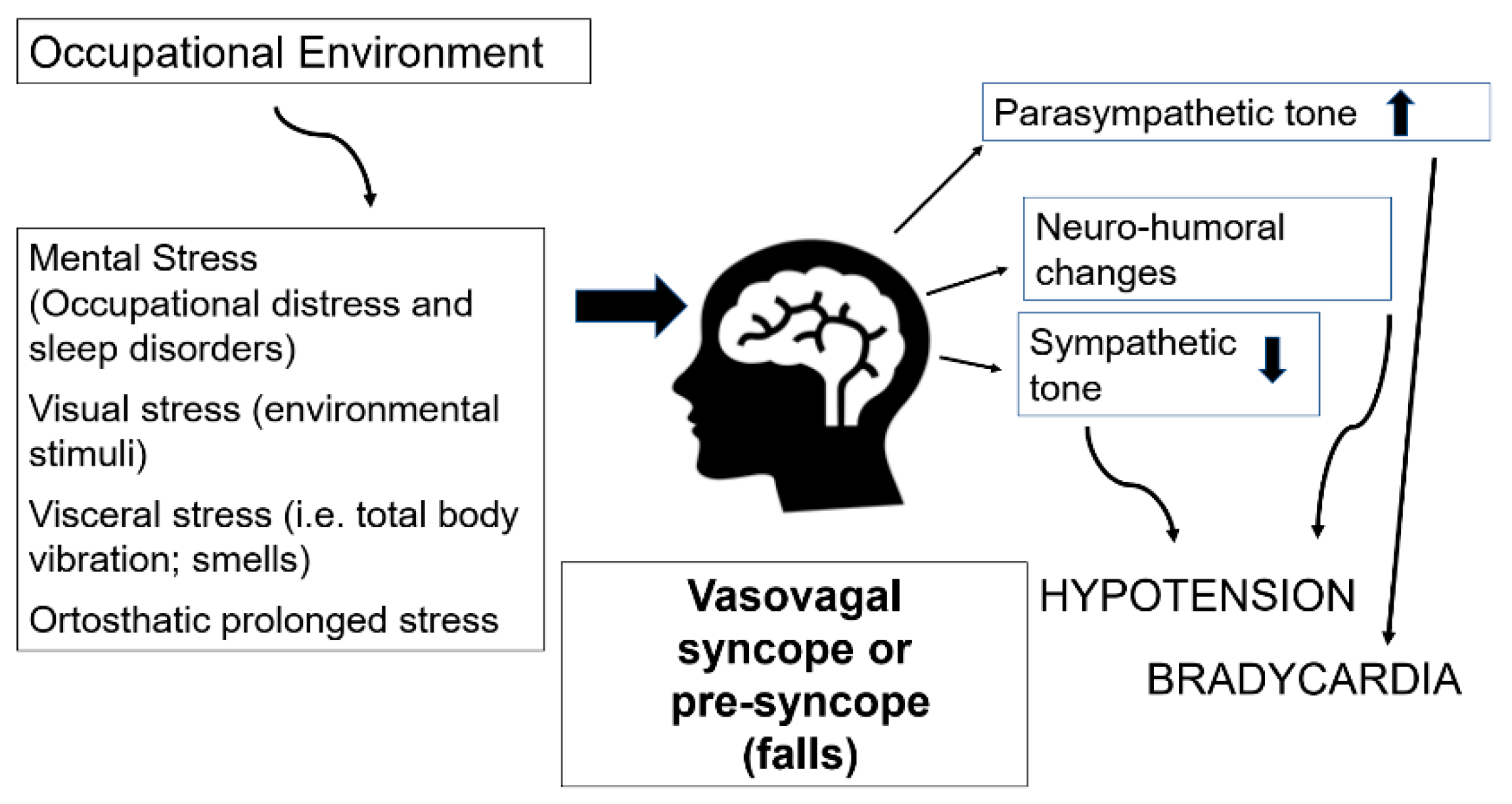
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Questionnaire
2.2.1. Prevalence of Syncope, Presyncope, and Falls
2.2.2. Occupational Stress
2.2.3. Sleep Quality
2.2.4. Mental Health
2.3. Medical Examination and Blood Tests
Metabolic Syndrome Prevalence
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, W.K.; Sheldon, R.S.; Benditt, D.G.; Cohen, M.I.; Forman, D.E.; Goldberger, Z.D.; Grubb, B.P.; Hamdan, M.H.; Krahn, A.D.; Link, M.S.; et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017, 136, e60–e122, Erratum in 2017, 136, e271–e272. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef]
- Mosqueda-Garcia, R.; Furlan, R.; Tank, J.; Fernandez-Violante, R. The elusive pathophysiology of neurally mediated syncope. Circulation 2000, 102, 2898–2906. [Google Scholar] [CrossRef]
- Barbic, F.; Heusser, K.; Minonzio, M.; Shiffer, D.; Cairo, B.; Tank, J.; Jordan, J.; Diedrich, A.; Gauger, P.; Zamuner, R.A.; et al. Effects of Prolonged Head-Down Bed Rest on Cardiac and Vascular Baroreceptor Modulation and Orthostatic Tolerance in Healthy Individuals. Front. Physiol. 2019, 10, 1061. [Google Scholar] [CrossRef]
- Jacob, G.; Barbic, F.; Glago, M.; Dipaola, F.; Porta, A.; Furlan, R. Cardiovascular autonomic profile in women with constitutional hypotension. J. Hypertens. 2018, 36, 2068–2076. [Google Scholar] [CrossRef]
- Goldberger, Z.D.; Petek, B.J.; Brignole, M.; Shen, W.K.; Sheldon, R.S.; Solbiati, M.; Deharo, J.C.; Moya, A.; Hamdan, M.H. ACC/AHA/HRS Versus ESC Guidelines for the Diagnosis and Management of Syncope: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 74, 2410–2423. [Google Scholar] [CrossRef]
- Alciati, A.; Shiffer, D.; Dipaola, F.; Barbic, F.; Furlan, R. Psychogenic Pseudosyncope: Clinical Features; Diagnosis and Management. J. Atr. Fibrillation 2020, 13, 2399. [Google Scholar] [CrossRef]
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya, A.; Sutton, R.; Ammirati, F.; Blanc, J.J.; Brignole, M.; et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur. Heart J. 2009, 30, 2631–2671. [Google Scholar] [CrossRef]
- Bhangu, J.; Hall, P.; Devaney, N.; Bennett, K.; Carroll, L.; Kenny, R.A.; McMahon, C.G. The prevalence of unexplained falls and syncope in older adults presenting to an Irish urban emergency department. Eur. J. Emerg. Med. 2019, 26, 100–104. [Google Scholar] [CrossRef]
- Sun, B.C.; Costantino, G.; Barbic, F.; Bossi, I.; Casazza, G.; Dipaola, F.; McDermott, D.; Quinn, J.; Reed, M.; Sheldon, R.S.; et al. Priorities for emergency department syncope research. Ann. Emerg. Med. 2014, 64, 649–655.e2. [Google Scholar] [CrossRef]
- Barbic, F.; Casazza, G.; Zamunér, A.R.; Costantino, G.; Orlandi, M.; Dipaola, F.; Capitanio, C.; Achenza, S.; Sheldon, R.; Furlan, R. Driving and working with syncope. Auton. Neurosci. 2014, 184, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Barbic, F.; Dipaola, F.; Casazza, G.; Borella, M.; Minonzio, M.; Solbiati, M.; Raj, S.R.; Sheldon, R.; Quinn, J.; Costantino, G.; et al. Syncope in a Working-Age Population: Recurrence Risk and Related Risk Factors. J. Clin. Med. 2019, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Numé, A.K.; Kragholm, K.; Carlson, N.; Kristensen, S.L.; Bøggild, H.; Hlatky, M.A.; Torp-Pedersen, C.; Gislason, G.; Ruwald, M.H. Syncope and Its Impact on Occupational Accidents and Employment: A Danish Nationwide Retrospective Cohort Study. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003202. [Google Scholar] [CrossRef] [PubMed]
- Armed Forces Health Surveillance Center (AFHSC). Syncope, active and reserve components, U.S. Armed Forces, 1998–2012. MSMR 2013, 20, 5–9. [Google Scholar]
- Rudnicki, J.; Zyśko, D.; Gajek, J.; Kuliczkowski, W.; Rosińczuk-Tonderys, J.; Zielińska, D.; Terpiłowski, Ł.; Agrawal, A.K. The risk for syncope and presyncope during surgery in surgeons and nurses. Pacing Clin. Electrophysiol. 2011, 34, 1486–1491. [Google Scholar] [CrossRef]
- Soteriades, E.S.; Evans, J.C.; Larson, M.G.; Chen, M.H.; Chen, L.; Benjamin, E.J.; Levy, D. Incidence and prognosis of syncope. N. Engl. J. Med. 2002, 347, 878–885. [Google Scholar] [CrossRef]
- Ganzeboom, K.S.; Mairuhu, G.; Reitsma, J.B.; Linzer, M.; Wieling, W.; van Dijk, N. Lifetime cumulative incidence of syncope in the general population: A study of 549 Dutch subjects aged 35-60 years. J. Cardiovasc. Electrophysiol. 2006, 17, 1172–1176. [Google Scholar] [CrossRef]
- Parsons, I.T.; Cox, A.T.; Mollan, I.A.; Boos, C.J. Managing the military patient with syncope. J. R Army Med. Corps. 2015, 161, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Barbic, F.; Borella, M.; Perego, F.; Dipaola, F.; Costantino, G.; Galli, A.; Mantovani, C.; Seghizzi, P.; Malliani, A.; Furlan, R. La sincope in età lavorativa. Studio multicentrico prospettico STePS [Syncope and work. STePS study (Short Term Prognosis of Syncope)]. G. Ital. Med. Lav. Ergon. 2005, 27, 272–274. [Google Scholar]
- Gaggioli, G.; Laffi, M.; Montemanni, M.; Mocini, A.; Rubartelli, P.; Brignole, M. Risk of syncope during work. Europace 2014, 16, 289–292. [Google Scholar] [CrossRef]
- Furlan, R.; Alboni, P.; Mosqueda-Garcia, R. Pathophysiology of vasovagal syncope: Conclusive remarks. In Vasovagal Syncope, 1st ed.; Alboni, P., Furlan, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 95–102. [Google Scholar] [CrossRef]
- Mosqueda-Garcia, R. Role of Autonomic Nervous System in Vasovagal Syncope. In Vasovagal Syncope, 1st ed.; Alboni, P., Furlan, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 53–65. [Google Scholar] [CrossRef]
- Solbiati, M.; Casazza, G.; Dipaola, F.; Rusconi, A.M.; Cernuschi, G.; Barbic, F.; Montano, N.; Sheldon, R.S.; Furlan, R.; Costantino, G. Syncope recurrence and mortality: A systematic review. Europace 2015, 17, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N. Two tools for health surveillance of job stress: The Karasek Job Content Questionnaire and the Siegrist Effort Reward Imbalance Questionnaire. G. Ital. Med. Lav. Ergon. 2007, 29, 667–670. [Google Scholar] [PubMed]
- Magnavita, N.; Garbarino, S.; Siegrist, J. The use of parsimonious questionnaires in occupational health surveillance. Psychometric properties of the short Italian version of the Effort/Reward Imbalance questionnaire. TSWJ Sci. World J. 2012, 2012, 372852. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siegrist, J. Adverse health effects of high-effort/low-reward conditions. J. Occup. Health Psychol. 1996, 1, 27–41. [Google Scholar] [CrossRef]
- Siegrist, J.; Wege, N.; Puhlhofer, F.; Wahrendorf, M. A short generic measure of work stress in the era of globalization: Effort-reward imbalance. Int. Arch. Occup. Environ. Health 2009, 82, 1005–1013. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian version of the Pittsburgh Sleep Quality Index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric research and practice. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Magnavita, N. Lavoro Umano. Il Benessere nei Luoghi di Lavoro; EDUCatt: Milano, Italy, 2009; ISBN 978-88-8311-722-0. [Google Scholar]
- Piccinelli, M.; Bisoffi, G.; Bon, M.G.; Cunico, L.; Tansella, M. Validity and test-retest reliability of the Italian version of the 12-item GHQ in general practice: A comparison between three scoring methods. Compr. Psychiatry 1993, 34, 198–205. [Google Scholar] [CrossRef]
- Goldberg, D. The Detection of Psychiatric Illness by Questionnaire; Oxford University Press: London, UK, 1972. [Google Scholar]
- Goldberg, D.; Hillier, V.F. A scaled version of the General Health Questionnaire. Psychol. Med. 1979, 9, 139–145. [Google Scholar] [CrossRef]
- Banks, M.H.; Clegg, C.W.; Jackson, P.R.; Kemp, N.J.; Stafford, E.M.; Wall, T.D. The use of the General Health Questionnaire as an indicator of mental health in occupational studies. J. Occup. Psychol. 1980, 53, 187–194. [Google Scholar] [CrossRef]
- The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 13 September 2021).
- Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Einhorn, D.; Reaven, G.M.; Cobin, R.H.; Ford, E.; Ganda, O.P.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Kendall, D.; Krauss, R.M.; et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003, 9, 237–252. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Poscia, A.; Moscato, U.; La Milia, D.I.; Milovanovic, S.; Stojanovic, J.; Borghini, A.; Collamati, A.; Ricciardi, W.; Magnavita, N. Workplace Health Promotion for Older Workers: A Systematic Literature Review. BMC Health Serv. Res. 2016, 16 (Suppl. 5), 329. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.M. Syncope: Epidemiology, etiology, and prognosis. Front. Physiol. 2014, 5, 471. [Google Scholar] [CrossRef]
- Serletis, A.; Rose, S.; Sheldon, A.G.; Sheldon, R.S. Vasovagal syncope in medical students and their first-degree relatives. Eur. Heart J. 2006, 27, 1965–1970. [Google Scholar] [CrossRef]
- Lamb, L.E. Incidence of loss of consciousness in 1980 Air Force personnel. Aerosp. Med. 1960, 31, 973–988. [Google Scholar] [PubMed]
- Ganzeboom, K.S.; Colman, N.; Reitsma, J.B.; Shen, W.K.; Wieling, W. Prevalence and triggers of syncope in medical students. Am. J. Cardiol. 2003, 91, A8. [Google Scholar] [CrossRef]
- Ruwald, M.H.; Hansen, M.L.; Lamberts, M.; Hansen, C.M.; Højgaard, M.V.; Køber, L.; Torp-Pedersen, C.; Hansen, J.; Gislason, G.H. The relation between age, sex, comorbidity, and pharmacotherapy and the risk of syncope: A Danish nationwide study. Europace 2012, 14, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Shen, W.K.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J. Prevalence of syncope in a population aged more than 45 years. Am. J. Med. 2006, 119, 1088.e1–1088.e7. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.P.; Sheldon, R.; Maxey, C.; Ritchie, D.; Doucette, S.; Parkash, R. Sex Differences in Vasovagal Syncope: A Post Hoc Analysis of the Prevention of Syncope Trials (POST) I and II. Can. J. Cardiol. 2020, 36, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L. Transient loss of consciousness and syncope. Handb. Clin. Neurol. 2014, 119, 169–191. [Google Scholar] [CrossRef]
- Kenny, R.A.; McNicholas, T. The management of vasovagal syncope. QJM 2016, 109, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Gao, L.; Yuan, Y. Advance in the understanding of vasovagal syncope in children and adolescents. World J. Pediatr. 2021, 17, 58–62. [Google Scholar] [CrossRef]
- Zimmermann, T.; du Fay de Lavallaz, J.; Nestelberger, T.; Gualandro, D.M.; Strebel, I.; Badertscher, P.; Lopez-Ayala, P.; Widmer, V.; Freese, M.; Miró, Ò.; et al. Incidence, characteristics, determinants, and prognostic impact of recurrent syncope. Europace 2020, 22, 1885–1895. [Google Scholar] [CrossRef]
- Jorge, J.G.; Pournazari, P.; Raj, S.R.; Maxey, C.; Sheldon, R.S. Frequency of injuries associated with syncope in the prevention of syncope trials. Europace 2020, 22, 1896–1903. [Google Scholar] [CrossRef]
- Zysko, D.; Melander, O.; Fedorowski, A. Vasovagal syncope related to emotional stress predicts coronary events in later life. Pacing Clin. Electrophysiol. 2013, 36, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Flint, B.; Baker, C.; Freeston, M.; Newton, J.L. Level of psychosocial impairment predicts early response to treatment in vasovagal syncope. Europace 2009, 11, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.C.; Jones, H.; Ainslie, P.N.; Thompson, A.; Marrin, K.; Atkinson, G. Influence of nocturnal and daytime sleep on initial orthostatic hypotension. Eur. J. Appl. Physiol. 2015, 115, 269–276. [Google Scholar] [CrossRef]
- Puel, V.; Godard, I.; Papaioannou, G.; Gosse, P.; Pepin, J.L.; Thoin, F.; Deharo, J.C.; Roche, F.; Zarqane, N.; Gagnadoux, F.; et al. Management of sleep apnoea syndrome (SAS) in patients with vasovagal syncope (VVS): A protocol for the VVS-SAS cohort study. BMJ Open 2020, 10, e038791. [Google Scholar] [CrossRef]
- Garbarino, S.; Magnavita, N. Sleep problems are a strong predictor of stress-related metabolic changes in police officers. A prospective study. PLoS ONE 2019, 14, e0224259. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Tripepi, G.; Di Prinzio, R.R. Symptoms in Health Care Workers during the COVID-19 Epidemic. A Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2020, 17, 5218. [Google Scholar] [CrossRef] [PubMed]
- Giada, F.; Silvestri, I.; Rossillo, A.; Nicotera, P.G.; Manzillo, G.F.; Raviele, A. Psychiatric profile quality of life and risk of syncopal recurrence in patients with tilt-induced vasovagal syncope. Europace 2005, 7, 465–471. [Google Scholar] [CrossRef]
- D’Antono, B.; Dupuis, G.; St-Jean, K.; Lévesque, K.; Nadeau, R.; Guerra, P.; Thibault, B.; Kus, T. Prospective evaluation of psychological distress and psychiatric morbidity in recurrent vasovagal and unexplained syncope. J. Psychosom. Res. 2009, 67, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Picavet, H.S.; Hoeymans, N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann. Rheum. Dis. 2004, 63, 723–729. [Google Scholar] [CrossRef]
- Anderson, J.B.; Czosek, R.J.; Knilans, T.K.; Marino, B.S. The effect of paediatric syncope on health-related quality of life. Cardiol. Young. 2012, 22, 583–588. [Google Scholar] [CrossRef]
- Rose, M.S.; Koshman, M.L.; Ritchie, D.; Sheldon, R. The development and preliminary validation of a scale measuring the impact of syncope on quality of life. Europace 2009, 11, 1369–1374. [Google Scholar] [CrossRef]
- van Dijk, N.; Sprangers, M.A.; Colman, N.; Boer, K.R.; Wieling, W.; Linzer, M. Clinical factors associated with quality of life in patients with transient loss of consciousness. J. Cardiovasc. Electrophysiol. 2006, 17, 998–1003. [Google Scholar] [CrossRef]
| Type of Problem | Total Number (%) 741 (100) | Male Number (%) 262 (35.4) | Female Number (%) 479 (64.6) | Chi-Square p | Younger 4 Number (%) 526 (71.0) | Older 5 Number (%) 215 (29.0) | Chi-Square p |
|---|---|---|---|---|---|---|---|
| Syncope | 103 (13.9) | 70 (7.6) | 83 (17.3) | 0.000 | 84 (16.0) | 19 (8.8) | 0.011 |
| Presyncope | 200 (27.0) | 48 (18.3) | 152 (31.7/ | 0.000 | 151 (28.7) | 49 (22.8) | 0.100 |
| Fall | 272 (36.7) | 92(35.1) | 180 (37.6) | 0.506 | 185 (35.2) | 87 (40.5) | 0.175 |
| Fall unknown origin | 76 (10.3) | 22 (8.4) | 54 (11.3) | 0.217 | 53 (10.1) | 23 (10.7) | 0.800 |
| Distressed 1 | 278 (38.6) | 110 (42.5) | 168 (36.4) | 0.106 | 182 (35.6) | 96 (45.7) | 0.011 |
| Bad sleeper 2 | 360 (48.6) | 120 (45.8) | 240 (50.1) | 0.263 | 234 (44.5) | 126 (58.6) | 0.001 |
| Low mental health 3 | 152 (20.5) | 51 (19.5) | 101 (21.1) | 0.592 | 97 (18.5) | 55 (25.6) | 0.030 |
| Metabolic syndrome | 91 (12.3) | 46 (17.6) | 45 (9.4) | 0.001 | 43 (8.2) | 48 (22.3) | 0.001 |
| Type of Problem | Stress (ERI) | Sleep Quality (PSQI) | Mental Health (GHQ-12) |
|---|---|---|---|
| Syncope | 1.05 ± 0.46 vs. 0.90 ± 0.43 *** | 6.62 ± 4.32 vs. 4.81 ± 3.23 *** | 2.48 ± 3.32 vs. 1.33 ± 2.32 *** |
| Recurrent syncope | 1.15 ± 0.51 vs. 0.91 ± 0.42 *** | 7.72 ± 4.54 vs. 4.87 ± 3.28 *** | 3.34 ± 3.89 vs. 1.36 ± 2.33 *** |
| Recent syncope | 1.17 ± 0.47 vs. 0.92 ± 0.43 * | 7.56 ± 5.01 vs. 5.00 ± 3.39 * | 2.67 ± 2.97 vs. 1.46 ± 2.49 *** |
| Presyncope | 1.06 ± 0.46 vs. 0.88 ± 0.41 *** | 6.88 ± 3.97 vs. 4.39 ± 2.98 *** | 2.40 ± 3.21 vs. 1.16 ± 2.10 *** |
| Fall of unknown cause | 0.97 ± 0.46 vs. 0.92 ± 0.43 | 6.22 ± 4.28 vs. 4.93 ± 3.33 ** | 2.33 ± 3.57 vs. 1.40 ± 2.34 |
| Type of Problem | Systolic Blood Pressure | Diastolic Blood Pressure | HDL Cholesterol |
|---|---|---|---|
| Syncope | 122.35 ± 16.40 vs. 122.28 ± 16.29 | 79.09 ± 13.97 vs. 79.00 ± 11.96 | 63.86 ± 15.48 vs. 61.96 ± 16.22 |
| Recurrent syncope | 122.00 ± 16.74 vs. 122.31 ± 16.27 | 77.77 ± 12.26 vs. 79.11 ± 12.24 | 65.96 ± 15.64 vs. 61.89 ± 16.13 |
| Presyncope | 121.66 ± 16.63 vs. 122.51 ± 16.19 | 79.34 ± 13.20 vs. 79.90 ± 11.91 | 65.96 ± 15.64 vs. 61.89 ± 16.13 |
| Fall of unknown cause | 119.59 ± 18.17 vs. 122.58 ± 16.07 | 77.84 ± 13.92 vs. 79.13 ± 12.06 | 59.06 ± 16.56 vs. 62.63 ± 16.04 |
| Type of Problem | Triglycerides | Blood Glucose | BMI |
| Syncope | 93.02 ± 54.72 vs. 102.67 ± 54.26 | 87.41 ± 16.43 vs. 91.14 ± 14.23 | 23.25 ± 3.32 vs. 25.03 ± 4.38 *** |
| Recurrent syncope | 86.85 ± 60.70 vs. 102.59 ± 53.65 | 86.84 ± 21.42 vs. 90.92 ± 13.86 | 24.10 ± 4.03 vs. 24.82 ± 4.30 |
| Presyncope | 98.26 ± 52.51 vs. 102.41 ± 55.09 | 88.53 ± 14.53 vs. 91.36 ± 14.59 | 24.55 ± 4.57 vs. 24.85 ± 4.17 |
| Fall of unknown cause | 114.85 ± 50.77 vs. 99.74 ± 54.64 | 88.97 ± 15.39 vs. 90.79 ± 14.52 | 25.87 ± 5.04 vs. 24.65 ± 4.18 * |
| Type of Problem | Distress OR (CI95%) | Bad Sleep OR (CI95%) | Low Mental Health OR (CI95%) | MetS OR (CI95%) |
|---|---|---|---|---|
| Syncope | 1.62 (1.05; 2.52) * | 1.79 (1.16; 2.77) *** | 2.43 (1.52; 3.87) *** | 0.61 (0.27; 1.39) |
| Recurrent syncope | 2.11 (1.15; 3.88) * | 2.19 (1.18; 4.04) * | 3.88 (2.12; 7.08) *** | 1.02 (0.40; 2.75) |
| Recent syncope | 1.89 (0.71; 5.04) | 1.68 (0.63; 4.45) | 2.55 (0.96; 6.78) | 1.81 (0.48; 6.78) |
| Presyncope | 1.77 (1.25; 2.49) *** | 2.95 (2.08; 4.18) *** | 2.61 (1.78; 3.84) *** | 1.21 (0.71; 2.04) |
| Fall unknown cause | 1.00 (0.61; 1.66) | 1.49 (0.91; 2.42) | 1.69 (0.99; 2.87) | 1.33 (0.67; 2.65) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnavita, N.; Di Prinzio, R.R.; Arnesano, G.; Cerrina, A.; Gabriele, M.; Garbarino, S.; Gasbarri, M.; Iuliano, A.; Labella, M.; Matera, C.; et al. Association of Occupational Distress and Low Sleep Quality with Syncope, Presyncope, and Falls in Workers. Int. J. Environ. Res. Public Health 2021, 18, 12283. https://doi.org/10.3390/ijerph182312283
Magnavita N, Di Prinzio RR, Arnesano G, Cerrina A, Gabriele M, Garbarino S, Gasbarri M, Iuliano A, Labella M, Matera C, et al. Association of Occupational Distress and Low Sleep Quality with Syncope, Presyncope, and Falls in Workers. International Journal of Environmental Research and Public Health. 2021; 18(23):12283. https://doi.org/10.3390/ijerph182312283
Chicago/Turabian StyleMagnavita, Nicola, Reparata Rosa Di Prinzio, Gabriele Arnesano, Anna Cerrina, Maddalena Gabriele, Sergio Garbarino, Martina Gasbarri, Angela Iuliano, Marcella Labella, Carmela Matera, and et al. 2021. "Association of Occupational Distress and Low Sleep Quality with Syncope, Presyncope, and Falls in Workers" International Journal of Environmental Research and Public Health 18, no. 23: 12283. https://doi.org/10.3390/ijerph182312283
APA StyleMagnavita, N., Di Prinzio, R. R., Arnesano, G., Cerrina, A., Gabriele, M., Garbarino, S., Gasbarri, M., Iuliano, A., Labella, M., Matera, C., Mauro, I., & Barbic, F. (2021). Association of Occupational Distress and Low Sleep Quality with Syncope, Presyncope, and Falls in Workers. International Journal of Environmental Research and Public Health, 18(23), 12283. https://doi.org/10.3390/ijerph182312283











