Narrative Review of Primary Preventive Interventions against Water-Borne Diseases: Scientific Evidence of Health-EDRM in Contexts with Inadequate Safe Drinking Water
Abstract
1. Introduction
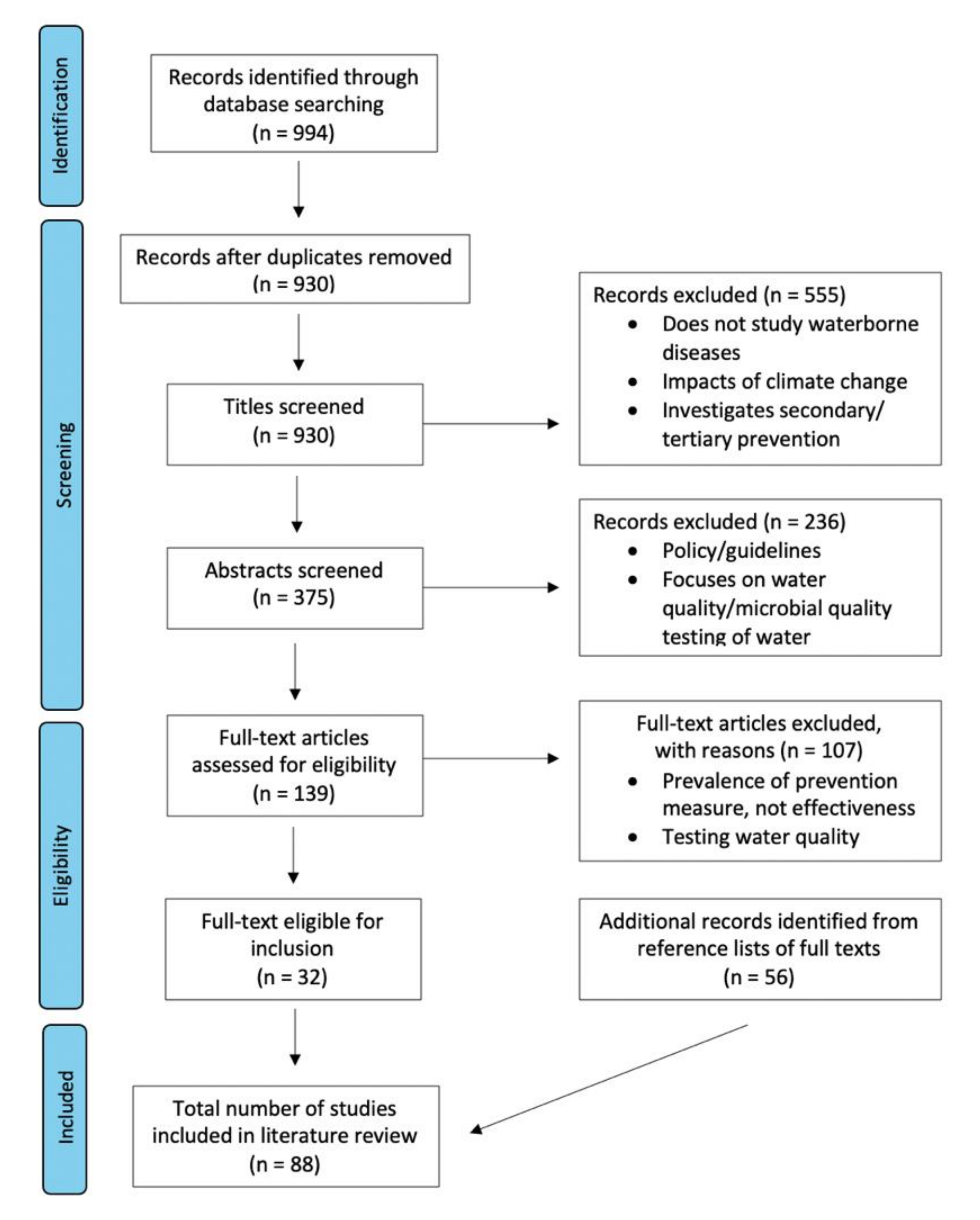
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- English-language based article.
- Published between 1 January 2000 and 24 March 2021.
- Effectiveness of primary prevention methods against waterborne diseases mentioned in the abstract.
- Abstracts that did not mention primary prevention methods against WBD.
- Papers studying only foodborne and/or airborne diseases.
- Papers studying secondary and/or tertiary level prevention.
3. Results
3.1. Strength of Evidence of Identified Studies
3.2. Overview of Studies Included for Analysis
4. Discussion
4.1. Top-Down, Capacity Building, Cultural Relevance and Post-Intervention Monitoring
4.2. Long-Term Sustainability and Long-Term Co-Benefits
4.3. Research Gaps Identified in Current Published Literature
4.4. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Incidence and Severity | Pathogen | Organism | Associated Diseases |
|---|---|---|---|
| High | Burkholderia | Bacteria | Melioidosis |
| Campylobacter | Bacteria | Campylobacteriosis | |
| Escherichia coli | Bacteria | E. Coli | |
| Francisella | Bacteria | Tularemia | |
| Legionella | Bacteria | Legionnaires’ disease | |
| Salmonella | Bacteria | Salmonella | |
| Shigella | Bacteria | Shigella | |
| Vibrio | Bacteria | Cholera | |
| Caliciviridae | Virus | Calciviral infection | |
| Hepeviridae | Virus | Hepatitis | |
| Picornaviridae | Virus | Poliovirus | |
| Reoviridae | Virus | Rotavirus | |
| Acanthamoeba | Protozoa | Acanthamoeba keratitis | |
| Cryptosporidium | Protozoa | Cryptosporidiosis | |
| Cyclospora | Protozoa | Cyclospora infection | |
| Entamoeba | Protozoa | Amebiasis | |
| Giardia | Protozoa | Giardiasis | |
| Naegleria | Protozoa | Naegleria infection | |
| Dracunculus | Helminth | Guinea-worm disease | |
| Moderate | Adenoviridae | Virus | Adenovirus infection |
| Astroviridae | Virus | Astrovirus infection | |
| Low | Mycobacteria | Bacteria | Mycobacteria infection |
References
- World Health Organization. Infectious Diseases. Health in 2015: From MDGs to SDGs. 2015. Available online: https://cdn.who.int/media/docs/default-source/gho-documents/health-in-2015-mdgs-to-sdgs/health-in-2015-from-mdgs-to-sdgs.pdf?sfvrsn=8ba61059_2 (accessed on 6 November 2021).
- World Health Organization. Diarrhoeal Disease, 2 May 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 5 November 2021).
- Patwari, A.K. Diarrhoea and Malnutrition Interaction. Indian J. Pediatr. 1999, 66, S124–S134. [Google Scholar]
- Sun, R.; An, D.; Lu, W.; Shi, Y.; Wang, L.; Zhang, C.; Zhang, P.; Qi, H.; Wang, Q. Impacts of a Flash Flood on Drinking Water Quality: Case Study of Areas Most Affected by the 2012 Beijing Flood. Heliyon 2016, 2, e00071. [Google Scholar] [CrossRef]
- Li, S.; Elliott, S.J. Facilitators and Barriers to Effective Water and Sanitation Interventions for Characterizing Shigellosis Incidence in Jiangsu, China. Procedia Environ. Sci. 2016, 36, 65–69. [Google Scholar] [CrossRef][Green Version]
- Giordano, M.; Barron, J.; Ünver, O. Chapter 5-Water Scarcity and Challenges for Smallholder Agriculture. In Sustainable Food and Agriculture; Campanhola, C., Pandey, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 75–94. [Google Scholar]
- Macy, J.T.; Quick, R.E. Transmission and Prevention of Water-Related Diseases. Water Health 2010, 1, 102–119. [Google Scholar]
- Walker, D.B.; Baumgartner, D.J.; Gerba, C.P.; Fitzsimmons, K. Chapter 16-Surface Water Pollution. In Environmental and Pollution Science, 3rd ed.; Brusseau, M.L., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 261–292. [Google Scholar]
- United Nations. Clean Water and Sanitation: Why It Matters. Available online: https://www.un.org/sustainabledevelopment/wp-content/uploads/2018/09/Goal-6.pdf (accessed on 16 September 2021).
- Ziv, T.; Heymann, A.D.; Azuri, J.; Leshno, M.; Cohen, D. Assessment of the Underestimation of Childhood Diarrhoeal Disease Burden in Israel. Epidemiol. Infect. 2011, 139, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Emergency and Disaster Risk Management Framework. 2019. Available online: https://www.who.int/hac/techguidance/preparedness/health-emergency-and-disaster-risk-management-framework-eng.pdf (accessed on 16 September 2021).
- World Health Organization. Sendai Framework for Disaster RIsk Reduction 2015–2030. 2015. Available online: https://www.preventionweb.net/files/43291_sendaiframeworkfordrren.pdf (accessed on 16 September 2021).
- Chan, E.Y.Y.; Shaw, R. Public Health and Disasters: Health Emergency and Disaster Risk Management in Asia; Springer: Singapore, 2020. [Google Scholar]
- World Health Organization. Improving Healthcare: Individual Interventions. Global Status Report on Noncommunicable Diseases 2010. 2010. Available online: https://www.who.int/nmh/publications/ncd_report_full_en.pdf (accessed on 16 September 2021).
- Piper, J.D.; Chandna, J.; Allen, E.; Linkman, K.; Cumming, O.; Prendergast, A.J.; Gladstone, M.J. Water, Sanitation and Hygiene (WASH) Interventions: Effects on Child Development in Low- and Middle-Income Countries. Cochrane Database Syst. Rev. 2017, 2017, CD012613. [Google Scholar] [CrossRef]
- United Nations. The Sustainable Development Goals Report. 2020. Available online: https://unstats.un.org/sdgs/report/2020/The-Sustainable-Development-Goals-Report-2020.pdf (accessed on 16 September 2021).
- OCEBM Levels of Evidence Working Group. “The Oxford Levels of Evidence 2”. Oxford Centre for Evidence-Based Medicine. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 16 September 2021).
- Ananga, E.O.; Njoh, A.J.; Pappas, C.; Ananga, G.O. Examining the Relationship Between Community Participation and Water Handling Hygiene Practices in the Informal Neighborhoods of Kisumu, Kenya. Habitat. Int. 2017, 62, 1–10. [Google Scholar] [CrossRef]
- Anthonj, C.; Githinji, S.; Kistemann, T. The Impact of Water on Health and Ill-Health in a Sub-Saharan African Wetland: Exploring Both Sides of the Coin. Sci. Total Environ. 2018, 624, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.K.; O’Reilly, C.E.; Levine, M.M.; Kotloff, K.L.; Nataro, J.P.; Ayers, T.L.; Farag, T.H.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; et al. Sanitation and Hygiene-Specific Risk Factors for Moderate-to-Severe Diarrhea in Young Children in the Global Enteric Multicenter Study, 2007–2011: Case-Control Study. PLoS Med. 2016, 13, e1002010. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.D.; Lowther, S.A.; Chingoli, F.; Chilima, B.; Kabuluzi, S.; Ayers, T.L.; Warne, T.A.; Mintz, E. Assessment of Water, Sanitation and Hygiene Interventions in Response to an Outbreak of Typhoid Fever in Neno District, Malawi. PLoS ONE 2018, 13, e0193348. [Google Scholar] [CrossRef]
- Bitew, B.D.; Gete, Y.K.; Biks, G.A.; Adafrie, T.T. The Effect of SODIS Water Treatment Intervention at the Household Level in Reducing Diarrheal Incidence among Children under 5 Years of Age: A Cluster Randomized Controlled Trial in Dabat District, Northwest Ethiopia. Trials 2018, 19, 412. [Google Scholar] [CrossRef]
- Cha, S.; Kang, D.; Tuffuor, B.; Lee, G.; Cho, J.; Chung, J.; Kim, M.; Lee, H.; Lee, J.; Oh, C. The Effect of Improved Water Supply on Diarrhea Prevalence of Children under Five in the Volta Region of Ghana: A Cluster-Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2015, 12, 12127–12143. [Google Scholar] [CrossRef]
- Clasen, T.F.; Bostoen, K.; Schmidt, W.P.; Boisson, S.; Fung, I.C.; Jenkins, M.W.; Scott, B.; Sugden, S.; Cairncross, S. Interventions to Improve Disposal of Human Excreta for Preventing Diarrhoea. Cochrane Database Syst. Rev. 2010, 2010, Cd007180. [Google Scholar] [CrossRef]
- Clasen, T.F.; Alexander, K.T.; Sinclair, D.; Boisson, S.; Peletz, R.; Chang, H.H.; Majorin, F.; Cairncross, S. Interventions to Improve Water Quality for Preventing Diarrhoea. Cochrane Database Syst. Rev. 2015, 2015, Cd004794. [Google Scholar] [CrossRef]
- De Ver Dye, T.; Apondi, R.; Lugada, E.; Kahn, J.G.; Sandiford-Day, M.A.; Dasbanerjee, T. A Qualitative Assessment of Beliefs, Attitudes, and Behaviors Related to Diarrhea and Water Filtration in Rural Kenya. Am. J. Public Health 2011, 101, 1515–1520. [Google Scholar] [CrossRef]
- Demberere, T.; Muyambo, M.; Mutengu, S.; Ncozana, T.; Manyeruke, N. An Analysis of the Effectiveness of WASH Interventions in Relation to Diarrhoeal Diseases in Chipinge District, Zimbabwe. Phys. Chem. Earth 2014, 76–78, 98–103. [Google Scholar] [CrossRef]
- Dey, N.C.; Parvez, M.; Islam, M.R.; Mistry, S.K.; Levine, D.I. Effectiveness of a Community-Based Water, Sanitation, and Hygiene (WASH) Intervention in Reduction of Diarrhoea among Under-Five Children: Evidence from a Repeated Cross-Sectional Study (2007–2015) in Rural Bangladesh. Int. J. Hyg. Environ. Health 2019, 222, 1098–1108. [Google Scholar] [CrossRef]
- Fagerli, K.; Trivedi, K.K.; Sodha, S.V.; Blanton, E.; Ati, A.; Nguyen, T.; Delea, K.C.; Ainslie, R.; Figueroa, M.E.; Kim, S.; et al. Comparison of Boiling and Chlorination on the Quality of Stored Drinking Water and Childhood Diarrhoea in Indonesian Households. Epidemiol. Infect. 2017, 145, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Garrett, V.; Ogutu, P.; Mabonga, P.; Ombeki, S.; Mwaki, A.; Aluoch, G.; Phelan, M.; Quick, R.E. Diarrhoea Prevention in a High-Risk Rural Kenyan Population through Point-of-use Chlorination, Safe Water Storage, Sanitation, and Rainwater Harvesting. Epidemiol. Infect. 2008, 136, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Clasen, T.F. Household Water Treatment and Safe Storage in Low-Income Countries. In Water and Sanitation-Related Diseases and the Changing Environment: Challenges, Interventions, and Preventive Measures, 2nd ed.; Selendy, J.M.H., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 197–211. [Google Scholar]
- Gungoren, B.; Latipov, R.; Regallet, G.; Musabaev, E. Effect of Hygiene Promotion on the Risk of Reinfection Rate of Intestinal Parasites in Children in Rural Uzbekistan. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R.; Ramírez Toro, G.I.; Minnigh, H.A. Impact on Diarrhoeal Illness of a Community Educational Intervention to Improve Drinking Water Quality in Rural Communities in Puerto Rico. BMC Public Health 2010, 10, 219. [Google Scholar] [CrossRef]
- Kamara, J.K.; Galukande, M.; Maeda, F.; Luboga, S.; Renzaho, A.M.N. Understanding the Challenges of Improving Sanitation and Hygiene Outcomes in a Community Based Intervention: A Cross-Sectional Study in Rural Tanzania. Int. J. Environ. Res. Public Health 2017, 14, 602. [Google Scholar] [CrossRef] [PubMed]
- La Con, G.; Schilling, K.; Harris, J.; Person, B.; Owuor, M.; Ogange, L.; Faith, S.; Quick, R. Evaluation of Student Handwashing Practices During a School-Based Hygiene Program in Rural Western Kenya, 2007. Int. Q. Community Health Educ. 2017, 37, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Marcynuk, P.B.; Flint, J.A.; Sargeant, J.M.; Jones-Bitton, A.; Brito, A.M.; Luna, C.F.; Szilassy, E.; Thomas, M.K.; Lapa, T.M.; Perez, E.; et al. Comparison of the Burden of Diarrhoeal Illness among Individuals with and without Household Cisterns in Northeast Brazil. BMC Infect. Dis. 2013, 13, 65. [Google Scholar] [CrossRef]
- Mbakaya, B.C.; Kalembo, F.W.; Zgambo, M. Community-Based Interventions for Preventing Diarrhoea in People Living with HIV in Sub-Sahara Africa: A Systematic Review. Malawi Med. J. 2019, 31, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Mbakaya, B.C.; Kalembo, F.W.; Zgambo, M. Use, Adoption, and Effectiveness of Tippy-Tap Handwashing Station in Promoting Hand Hygiene Practices in Resource-Limited Settings: A Systematic Review. BMC Public Health 2020, 20, 1005. [Google Scholar] [CrossRef]
- McDonald, E.; Bailie, R.; Brewster, D.; Morris, P. Are Hygiene and Public Health Interventions Likely to Improve Outcomes for Australian Aboriginal Children Living in Remote Communities? A Systematic Review of the Literature. BMC Public Health 2008, 8, 153. [Google Scholar] [CrossRef]
- Moropeng, R.C.; Budeli, P.; Mpenyana-Monyatsi, L.; Momba, M.N.B. Dramatic Reduction in Diarrhoeal Diseases through Implementation of Cost-Effective Household Drinking Water Treatment Systems in Makwane Village, Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 410. [Google Scholar] [CrossRef]
- Peletz, R.; Simunyama, M.; Sarenje, K.; Baisley, K.; Filteau, S.; Kelly, P.; Clasen, T. Assessing Water Filtration and Safe Storage in Households with Young Children of HIV-Positive Mothers: A Randomized, Controlled Trial in Zambia. PLoS ONE 2012, 7, e46548. [Google Scholar] [CrossRef]
- Ramesh, A.; Blanchet, K.; Ensink, J.H.; Roberts, B. Evidence on the Effectiveness of Water, Sanitation, and Hygiene (WASH) Interventions on Health Outcomes in Humanitarian Crises: A Systematic Review. PLoS ONE 2015, 10, e0124688. [Google Scholar]
- Roberts, L.; Chartier, Y.; Chartier, O.; Malenga, G.; Toole, M.; Rodka, H. Keeping Clean Water Clean in a Malawi Refugee Camp: A Randomized Intervention Trial. Bull. World Health Organ. 2001, 79, 280–287. [Google Scholar] [PubMed]
- Solomon, E.T.; Robele, S.; Kloos, H.; Mengistie, B. Effect of Household Water Treatment with Chlorine on Diarrhea among Children under the Age of Five Years in Rural Areas of Dire Dawa, Eastern Ethiopia: A Cluster Randomized Controlled Trial. Infect. Dis. Poverty 2020, 9, 64. [Google Scholar] [CrossRef]
- Sugar, N.R.; Schilling, K.A.; Kim, S.; Ahmed, A.; Ngui Muyanga, D.; Sivapalasingam, S.; Quick, R. Integrating Household Water Treatment, Hand Washing, and Insecticide-Treated Bed Nets Into Pediatric HIV Care in Mombasa, Kenya: Impact on Diarrhea and Malaria Risk. J. Acquir. Immune Defic. Syndr. 2017, 76, 266–272. [Google Scholar] [CrossRef]
- Uwimpuhwe, M.; Reddy, P.; Barratt, G.; Bux, F. The Impact of Hygiene and Localised Treatment on the Quality of Drinking Water in Masaka, Rwanda. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2014, 49, 434–440. [Google Scholar] [CrossRef]
- Shrestha, A.; Six, J.; Dahal, D.; Marks, S.; Meierhofer, R. Association of Nutrition, Water, Sanitation and Hygiene Practices with Children’s Nutritional Status, Intestinal Parasitic Infections and Diarrhoea in Rural Nepal: A Cross-Sectional Study. BMC Public Health 2020, 20, 1241. [Google Scholar] [CrossRef] [PubMed]
- Tintle, N.; Van De Griend, K.; Ulrich, R.; Wade, R.D.; Baar, T.M.; Boven, E.; Cooper, C.E.A.; Couch, O.; Eekhoff, L.; Fry, B.; et al. Diarrhea Prevalence in a Randomized, Controlled Prospective Trial of Point-Of-Use Water Filters in Homes and Schools in the Dominican Republic. Trop. Med. Health 2021, 49, 1. [Google Scholar] [CrossRef]
- Pawestri, A.R.; Thima, K.; Leetachewa, S.; Maneekan, P.; Deesitthivech, O.; Pinna, C.; Yingtaweesak, T.; Moonsom, S. Seasonal Prevalence, Risk Factors, and One Health Intervention for Prevention of Intestinal Parasitic Infection in Underprivileged Communities on the Thai-Myanmar Border. Int. J. Infect. Dis. 2021, 105, 152–160. [Google Scholar] [CrossRef]
- Lindquist, E.D.; George, C.M.; Perin, J.; Neiswender de Calani, K.J.; Norman, W.R.; Davis, T.P.; Perry, H. A Cluster Randomized Controlled Trial to Reduce Childhood Diarrhea Using Hollow Fiber Water Filter and/or Hygiene-Sanitation Educational Interventions. Am. J. Trop. Med. Hyg. 2014, 91, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Reller, M.E.; Mendoza, C.E.; Lopez, M.B.; Alvarez, M.; Hoekstra, R.M.; Olson, C.A.; Baier, K.G.; Keswick, B.H.; Luby, S.P. A Randomized Controlled Trial of Household-Based Flocculant-Disinfectant Drinking Water Treatment for Diarrhea Prevention in Rural Guatemala. Am. J. Trop. Med. Hyg. 2003, 69, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Stauber, C.E.; Ortiz, G.M.; Loomis, D.P.; Sobsey, M.D. A Randomized Controlled Trial of the Concrete Biosand Filter and Its Impact on Diarrheal Disease in Bonao, Dominican Republic. Am. J. Trop. Med. Hyg. 2009, 80, 286–293. [Google Scholar] [CrossRef]
- Fabiszewski de Aceituno, A.M.; Stauber, C.E.; Walters, A.R.; Meza Sanchez, R.E.; Sobsey, M.D. A Randomized Controlled Trial of the Plastic-Housing Biosand Filter and Its Impact on Diarrheal Disease in Copan, Honduras. Am. J. Trop. Med. Hyg. 2012, 86, 913–921. [Google Scholar] [CrossRef]
- McGuinness, S.L.; O’Toole, J.; Forbes, A.B.; Boving, T.B.; Patil, K.; D’Souza, F.; Gaonkar, C.A.; Giriyan, A.; Barker, S.F.; Cheng, A.C.; et al. A Stepped Wedge Cluster-Randomized Trial Assessing the Impact of a Riverbank Filtration Intervention to Improve Access to Safe Water on Health in Rural India. Am. J. Trop. Med. Hyg. 2020, 102, 497–506. [Google Scholar] [CrossRef]
- Gruber, J.S.; Reygadas, F.; Arnold, B.F.; Ray, I.; Nelson, K.; Colford, J.M. A Stepped Wedge, Cluster-Randomized Trial of a Household UV-Disinfection and Safe Storage Drinking Water Intervention in Rural Baja California Sur, Mexico. Am. J. Trop. Med. Hyg. 2013, 89, 238–245. [Google Scholar] [CrossRef]
- Habib, M.A.; Soofi, S.; Sadiq, K.; Samejo, T.; Hussain, M.; Mirani, M.; Rehmatullah, A.; Ahmed, I.; Bhutta, Z.A. A Study to Evaluate the Acceptability, Feasibility and Impact of Packaged Interventions (“Diarrhea Pack”) for Prevention and Treatment of Childhood Diarrhea in Rural Pakistan. BMC Public Health 2013, 13, 922. [Google Scholar] [CrossRef]
- Gundry, S.; Wright, J.; Conroy, R. A Systematic Review of the Health Outcomes Related to Household Water Quality in Developing Countries. J. Water Health 2004, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Prüss-Ustün, A.; Cumming, O.; Bartram, J.; Bonjour, S.; Cairncross, S.; Clasen, T.; Colford, J.M., Jr.; Curtis, V.; De France, J.; et al. Assessing the Impact of Drinking Water and Sanitation on Diarrhoeal Disease in Low- and Middle-Income Settings: Systematic Review and Meta-Regression. Trop. Med. Int. Health 2014, 19, 928–942. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Handzel, T.; Venczel, L. Chlorination and Safe Storage of Household Drinking Water in Developing Countries to Reduce Waterborne Disease. Water Sci. Technol. 2003, 47, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Luby, S.P.; Agboatwalla, M.; Painter, J.; Altaf, A.; Billhimer, W.; Keswick, B.; Hoekstra, R.M. Combining Drinking Water Treatment and Hand Washing for Diarrhoea Prevention, A Cluster Randomised Controlled Trial. Trop. Med. Int. Health 2006, 11, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Luby, S.P.; Agboatwalla, M.; Hoekstra, R.M.; Rahbar, M.H.; Billhimer, W.; Keswick, B.H. Delayed Effectiveness of Home-Based Interventions in Reducing Childhood Diarrhea, Karachi, Pakistan. Am. J. Trop. Med. Hyg. 2004, 71, 420–427. [Google Scholar] [CrossRef]
- Migele, J.; Ombeki, S.; Ayalo, M.; Biggerstaff, M.; Quick, R. Diarrhea Prevention in a Kenyan School through the Use of a Simple Safe Water and Hygiene Intervention. Am. J. Trop. Med. Hyg. 2007, 76, 351–353. [Google Scholar] [CrossRef]
- Barzilay, E.J.; Aghoghovbia, T.S.; Blanton, E.M.; Akinpelumi, A.A.; Coldiron, M.E.; Akinfolayan, O.; Adeleye, O.A.; LaTrielle, A.; Hoekstra, R.M.; Gilpin, U.; et al. Diarrhea Prevention in People Living with HIV: An Evaluation of a Point-Of-Use Water Quality Intervention in Lagos, Nigeria. AIDS Care 2011, 23, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Quick, R.E.; Kimura, A.; Thevos, A.; Tembo, M.; Shamputa, I.; Hutwagner, L.; Mintz, E. Diarrhea Prevention through Household-Level Water Disinfection and Safe Storage in Zambia. Am. J. Trop. Med. Hyg. 2002, 66, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Greene, S.K.; Thomas, T.K.; Ndivo, R.; Okanda, J.; Masaba, R.; Nyangau, I.; Thigpen, M.C.; Hoekstra, R.M.; Quick, R.E. Effect of a Point-Of-Use Water Treatment and Safe Water Storage Intervention on Diarrhea in Infants of HIV-Infected Mothers. J. Infect. Dis. 2009, 200, 1186–1193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jensen, P.K.; Ensink, J.H.; Jayasinghe, G.; van der Hoek, W.; Cairncross, S.; Dalsgaard, A. Effect of Chlorination of Drinking-Water on Water Quality and Childhood Diarrhoea in a Village in Pakistan. J. Health Popul. Nutr. 2003, 21, 26–31. [Google Scholar]
- Barreto, M.L.; Genser, B.; Strina, A.; Teixeira, M.G.; Assis, A.M.O.; Rego, R.F.; Teles, C.A.; Prado, M.S.; Matos, S.M.A.; Santos, D.N.; et al. Effect of City-Wide Sanitation Programme on Reduction in Rate of Childhood Diarrhoea in Northeast Brazil: Assessment by Two Cohort Studies. Lancet 2007, 370, 1622–1628. [Google Scholar] [CrossRef]
- Luby, S.P.; Agboatwalla, M.; Feikin, D.R.; Painter, J.; Billhimer, W.; Altaf, A.; Hoekstra, R.M. Effect of Handwashing on Child Health: A Randomised Controlled Trial. Lancet 2005, 366, 225–233. [Google Scholar] [CrossRef]
- Lule, J.R.; Mermin, J.; Ekwaru, J.P.; Malamba, S.; Downing, R.; Ransom, R.; Nakanjako, D.; Wafula, W.; Hughes, P.; Bunnell, R.; et al. Effect of Home-Based Water Chlorination and Safe Storage on Diarrhea among Persons with Human Immunodeficiency Virus in Uganda. Am. J. Trop. Med. Hyg. 2005, 73, 926–933. [Google Scholar] [CrossRef]
- Boisson, S.; Stevenson, M.; Shapiro, L.; Kumar, V.; Singh, L.P.; Ward, D.; Clasen, T. Effect of Household-Based Drinking Water Chlorination on Diarrhoea among Children under Five in Orissa, India: A Double-Blind Randomised Placebo-Controlled Trial. PLoS Med. 2013, 10, e1001497. [Google Scholar] [CrossRef]
- Pickering, A.J.; Crider, Y.; Sultana, S.; Swarthout, J.; Goddard, F.G.B.; Anjerul Islam, S.; Sen, S.; Ayyagari, R.; Luby, S.P. Effect of In-Line Drinking Water Chlorination at the Point of Collection on Child Diarrhoea in Urban Bangladesh: A Double-Blind, Cluster-Randomised Controlled Trial. Lancet Glob. Health 2019, 7, e1247–e1256. [Google Scholar] [CrossRef]
- Luby, S.P.; Agboatwalla, M.; Painter, J.; Altaf, A.; Billhimer, W.L.; Hoekstra, R.M. Effect of Intensive Handwashing Promotion on Childhood Diarrhea in High-Risk Communities in Pakistan: A Randomized Controlled Trial. JAMA 2004, 291, 2547–2554. [Google Scholar] [CrossRef]
- Curtis, V.; Cairncross, S. Effect of Washing Hands with Soap on Diarrhoea Risk in the Community: A Systematic Review. Lancet Infect. Dis. 2003, 3, 275–281. [Google Scholar] [CrossRef]
- Kirby, M.A.; Nagel, C.L.; Rosa, G.; Zambrano, L.D.; Musafiri, S.; Ngirabega, J.D.; Thomas, E.A.; Clasen, T. Effects of a Large-Scale Distribution of Water Filters and Natural Draft Rocket-Style Cookstoves on Diarrhea and Acute Respiratory Infection: A Cluster-Randomized Controlled Trial in Western Province, Rwanda. PLoS Med. 2019, 16, e1002812. [Google Scholar] [CrossRef]
- Harshfield, E.; Lantagne, D.; Turbes, A.; Null, C. Evaluating the Sustained Health Impact of Household Chlorination of Drinking Water in Rural Haiti. Am. J. Trop. Med. Hyg. 2012, 87, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.; Arana, B.; Mäusezahl, D.; Hubbard, A.; Colford, J.M., Jr. Evaluation of a Pre-Existing, 3-Year Household Water Treatment and Handwashing Intervention in Rural Guatemala. Int. J. Epidemiol. 2009, 38, 1651–1661. [Google Scholar] [CrossRef]
- Nanan, D.; White, F.; Azam, I.; Afsar, H.; Hozhabri, S. Evaluation of a Water, Sanitation, and Hygiene Education Intervention on Diarrhoea in Northern Pakistan. Bull. World Health Organ. 2003, 81, 160–165. [Google Scholar] [PubMed]
- Mbakaya, B.C.; Lee, P.H.; Lee, R.L. Hand Hygiene Intervention Strategies to Reduce Diarrhoea and Respiratory Infections among Schoolchildren in Developing Countries: A Systematic Review. Int. J. Environ Res Public Health 2017, 14, 317. [Google Scholar] [CrossRef]
- Ejemot, R.I.; Ehiri, J.E.; Meremikwu, M.M.; Critchley, J.A. Hand Washing for Preventing Diarrhoea. Cochrane Database Syst. Rev. 2008, Cd004265. [Google Scholar] [CrossRef]
- Hashi, A.; Kumie, A.; Gasana, J. Hand Washing with Soap and WASH Educational Intervention Reduces Under-Five Childhood Diarrhoea Incidence in Jigjiga District, Eastern Ethiopia: A Community-Based Cluster Randomized Controlled Trial. Prev. Med. Rep. 2017, 6, 361–368. [Google Scholar] [CrossRef]
- Graf, J.; Zebaze Togouet, S.; Kemka, N.; Niyitegeka, D.; Meierhofer, R.; Gangoue Pieboji, J. Health Gains from Solar Water Disinfection (SODIS): Evaluation of a Water Quality Intervention in Yaoundé, Cameroon. J. Water Health 2010, 8, 779–796. [Google Scholar] [CrossRef]
- McGuigan, K.G.; Samaiyar, P.; du Preez, M.; Conroy, R.M. High Compliance Randomized Controlled Field Trial of Solar Disinfection of Drinking Water and Its Impact on Childhood Diarrhea in Rural Cambodia. Environ. Sci. Technol. 2011, 45, 7862–7867. [Google Scholar] [CrossRef]
- Crump, J.A.; Otieno, P.O.; Slutsker, L.; Keswick, B.H.; Rosen, D.H.; Hoekstra, R.M.; Vulule, J.M.; Luby, S.P. Household Based Treatment of Drinking Water with Flocculant-Disinfectant for Preventing Diarrhoea in Areas with Turbid Source Water in Rural Western Kenya: Cluster Randomised Controlled Trial. BMJ 2005, 331, 478. [Google Scholar] [CrossRef] [PubMed]
- Mengistie, B.; Berhane, Y.; Worku, A. Household Water Chlorination Reduces Incidence of Diarrhea among Under-Five Children in Rural Ethiopia: A Cluster Randomized Controlled Trial. PLoS ONE 2013, 8, e77887. [Google Scholar] [CrossRef] [PubMed]
- Clasen, T.; Garcia Parra, G.; Boisson, S.; Collin, S. Household-Based Ceramic Water Filters for the Prevention of Diarrhea: A Randomized, Controlled Trial of a Pilot Program in Colombia. Am. J. Trop. Med. Hyg. 2005, 73, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.S.K.; Schmidt, W.P.; Darby, J.; Kariuki, Z.G.; Jenkins, M.W. Intermittent Slow Sand Filtration for Preventing Diarrhoea among Children in Kenyan Households Using Unimproved Water Sources: Randomized Controlled Trial. Trop. Med. Int. Health 2009, 14, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Clasen, T.; Schmidt, W.P.; Rabie, T.; Roberts, I.; Cairncross, S. Interventions to Improve Water Quality for Preventing Diarrhoea: Systematic Review and Meta-Analysis. BMJ 2007, 334, 782. [Google Scholar] [CrossRef]
- Brown, J.; Sobsey, M.D.; Loomis, D. Local Drinking Water Filters Reduce Diarrheal Disease in Cambodia: A Randomized, Controlled Trial of the Ceramic Water Purifier. Am. J. Trop. Med. Hyg. 2008, 79, 394–400. [Google Scholar] [CrossRef]
- Doocy, S.; Burnham, G. Point-Of-Use Water Treatment and Diarrhoea Reduction in the Emergency Context: An Effectiveness Trial in Liberia. Trop. Med. Int. Health 2006, 11, 1542–1552. [Google Scholar] [CrossRef]
- Clasen, T.F.; Brown, J.; Collin, S.M. Preventing Diarrhoea with Household Ceramic Water Filters: Assessment of a Pilot Project in Bolivia. Int. J. Environ. Health Res. 2006, 16, 231–239. [Google Scholar] [CrossRef]
- Islam, M.S.; Mahmud, Z.H.; Uddin, M.H.; Islam, K.; Yunus, M.; Islam, M.S.; Nair, G.B.; Endtz, H.P.; Sack, D.A. Purification of Household Water using a Novel Mixture Reduces Diarrhoeal Disease in Matlab, Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 341–345. [Google Scholar] [CrossRef]
- Du Preez, M.; Conroy, R.M.; Ligondo, S.; Hennessy, J.; Elmore-Meegan, M.; Soita, A.; McGuigan, K.G. Randomized Intervention Study of Solar Disinfection of Drinking Water in the Prevention of Dysentery in Kenyan Children Aged under 5 Years. Environ. Sci. Technol. 2011, 45, 9315–9323. [Google Scholar] [CrossRef]
- Chiller, T.M.; Mendoza, C.E.; Lopez, M.B.; Alvarez, M.; Hoekstra, R.M.; Keswick, B.H.; Luby, S.P. Reducing Diarrhoea in Guatemalan Children: Randomized Controlled Trial of Flocculant-Disinfectant for Drinking-Water. Bull. World Health Organ. 2006, 84, 28–35. [Google Scholar] [CrossRef]
- Clasen, T.F.; Brown, J.; Collin, S.; Suntura, O.; Cairncross, S. Reducing Diarrhea through the Use of Household-Based Ceramic Water Filters: A Randomized, Controlled Trial in Rural Bolivia. Am. J. Trop. Med. Hyg. 2004, 70, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Sima, L.C.; Desai, M.M.; McCarty, K.M.; Elimelech, M. Relationship between Use of Water from Community-Scale Water Treatment Refill Kiosks and Childhood Diarrhea in Jakarta. Am. J. Trop. Med. Hyg. 2012, 87, 979–984. [Google Scholar] [CrossRef]
- Conroy, R.M.; Meegan, M.E.; Joyce, T.; McGuigan, K.; Barnes, J. Solar Disinfection of Drinking Water Protects against Cholera in Children under 6 Years of Age. Arch. Dis. Child. 2001, 85, 293–295. [Google Scholar] [CrossRef]
- Rose, A.; Roy, S.; Abraham, V.; Holmgren, G.; George, K.; Balraj, V.; Abraham, S.; Muliyil, J.; Joseph, A.; Kang, G. Solar Disinfection of Water for Diarrhoeal Prevention in Southern India. Arch Dis. Child. 2006, 91, 139–141. [Google Scholar] [CrossRef]
- Mäusezahl, D.; Christen, A.; Pacheco, G.D.; Tellez, F.A.; Iriarte, M.; Zapata, M.E.; Cevallos, M.; Hattendorf, J.; Cattaneo, M.D.; Arnold, B.; et al. Solar Drinking Water Disinfection (SODIS) to Reduce Childhood Diarrhoea in Rural Bolivia: A Cluster-Randomized, Controlled Trial. PLoS Med. 2009, 6, e1000125. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.C.; Clasen, T.; Dreibelbis, R.; Saboori, S.; Greene, L.E.; Brumback, B.; Muga, R.; Rheingans, R. The Impact of a School-Based Water Supply and Treatment, Hygiene, and Sanitation Programme on Pupil Diarrhoea: A Cluster-Randomized Trial. Epidemiol. Infect. 2014, 142, 340–351. [Google Scholar] [CrossRef]
- Arnold, B.F.; Colford, J.M., Jr. Treating Water with Chlorine at Point-Of-Use to Improve Water Quality and Reduce Child Diarrhea in Developing Countries: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2007, 76, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, M.; Conroy, R.M.; Wright, J.A.; Moyo, S.; Potgieter, N.; Gundry, S.W. Use of Ceramic Water Filtration in the Prevention of Diarrheal Disease: A Randomized Controlled Trial in Rural South Africa and Zimbabwe. Am. J. Trop. Med. Hyg. 2008, 79, 696–701. [Google Scholar] [CrossRef]
- Opryszko, M.C.; Majeed, S.W.; Hansen, P.M.; Myers, J.A.; Baba, D.; Thompson, R.E.; Burnham, G. Water and Hygiene Interventions to Reduce Diarrhoea in Rural Afghanistan: A Randomized Controlled Study. J. Water Health 2010, 8, 687–702. [Google Scholar] [CrossRef]
- Pavlinac, P.B.; Naulikha, J.M.; Chaba, L.; Kimani, N.; Sangaré, L.R.; Yuhas, K.; Singa, B.O.; John-Stewart, G.; Walson, J.L. Water Filter Provision and Home-Based Filter Reinforcement Reduce Diarrhea in Kenyan HIV-Infected Adults and their Household Members. Am. J. Trop. Med. Hyg. 2014, 91, 273–280. [Google Scholar] [CrossRef][Green Version]
- Cairncross, S.; Hunt, C.; Boisson, S.; Bostoen, K.; Curtis, V.; Fung, I.C.H.; Schmidt, W.P. Water, Sanitation and Hygiene for the Prevention of Diarrhoea. Int. J. Epidemiol. 2010, 39 (Suppl. 1), i193–i205. [Google Scholar] [CrossRef]
- Fewtrell, L.; Kaufmann, R.B.; Kay, D.; Enanoria, W.; Haller, L.; Colford, J.M., Jr. Water, Sanitation, and Hygiene Interventions to Reduce Diarrhoea in Less Developed Countries: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2005, 5, 42–52. [Google Scholar] [CrossRef]
- Carter, M.J. Enterically Infecting Viruses: Pathogenicity, Transmission and Significance for Food and Waterborne Infection. J. Appl. Microbiol. 2005, 98, 1354–1380. [Google Scholar] [CrossRef] [PubMed]
- Jumaa, P.A. Hand Hygiene: Simple and Complex. Int. J. Infect. Dis. 2005, 9, 3–14. [Google Scholar] [CrossRef]
- Kwong, L.H.; Ercumen, A.; Pickering, A.J.; Arsenault, J.E.; Islam, M.; Parvez, S.M.; Unicomb, L.; Rahman, M.; Davis, J.; Luby, S.P. Ingestion of Fecal Bacteria along Multiple Pathways by Young Children in Rural Bangladesh Participating in a Cluster-Randomized Trial of Water, Sanitation, and Hygiene Interventions (WASH Benefits). Environ. Sci. Technol. 2020, 54, 13828–13838. [Google Scholar] [CrossRef] [PubMed]
- Guelinckx, I.; Tavoularis, G.; König, J.; Morin, C.; Gharbi, H.; Gandy, J. Contribution of Water from Food and Fluids to Total Water Intake: Analysis of a French and UK Population Surveys. Nutrients 2016, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, Y.; Mota, N.J.A.; Jagadish, K.S.; Bredenoord, J.; Vardon, P.J.; van Loosdrecht, M.C.M.; Jonkers, H.M. The Potential and Current Status of Earthen Material for Low-Cost Housing in Rural India. Constr. Build. Mater. 2020, 247, 118615. [Google Scholar] [CrossRef]
- Barreiro, C.; Albano, H.; Silva, J.; Teixeira, P. Role of Flies as Vectors of Foodborne Pathogens in Rural Areas. ISRN Microbiol. 2013, 2013, 718780. [Google Scholar] [CrossRef]
- Chan, E.Y.Y.; Sham, T.S.T.; Shahzada, T.S.; Dubois, C.; Huang, Z.; Liu, S.; Hung, K.K.C.; Tse, S.L.A.; Kwok, K.O.; Chung, P.-H.; et al. Narrative Review on Health-EDRM Primary Prevention Measures for Vector-Borne Diseases. Int. J. Environ. Res. Public Health 2020, 17, 5981. [Google Scholar] [CrossRef]
- Chan, E.Y.Y.; Shahzada, T.S.; Sham, T.S.T.; Dubois, C.; Huang, Z.; Liu, S.; Ho, J.Y.; Hung, K.K.C.; Kwok, K.O.; Shaw, R. Narrative Review of Non-Pharmaceutical Behavioural Measures for the Prevention of COVID-19 (SARS-CoV-2) Based on the Health-EDRM Framework. Br. Med. Bull. 2020, 136, 46–87. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. Progress on Drinking Water and Sanitation: 2012 Update. World Health Organization. 2012. Available online: https://apps.who.int/iris/handle/10665/44842 (accessed on 16 September 2021).
- Chokshi, D.A.; Kesselheim, A.S. Rethinking Global Access to Vaccines. BMJ 2008, 336, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Kivuti-Bitok, L.W.; Chepchirchir, A.; Waithaka, P.; Ngune, I. Dry Taps? A Synthesis of Alternative “Wash” Methods in the Absence of Water and Sanitizers in the Prevention of Coronavirus in Low-Resource Settings. J. Prim. Care Community Health 2020, 11, 2150132720936858. [Google Scholar] [CrossRef] [PubMed]
- United Nations. ‘Water-Related Diseases Responsible for 80 Per Cent of All Illnesses, Deaths in Developing World’, Says Secretary-General in Environment Day Message, 16 May 2003. Available online: https://www.un.org/press/en/2003/sgsm8707.doc.htm (accessed on 16 September 2021).
- World Health Organization. Chapter 6-Vaccine Preventable Diseases and Vaccines. International Travel and Health. 2019. Available online: https://www.who.int/ith/CHAPTER_6_For_Publication.pdf?ua=1 (accessed on 16 September 2021).
- World Health Organization. Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum. World Health Organization. 2017. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 5 November 2021).
| Level | Therapy/Prevention, Etiology/Harm |
|---|---|
| 1A | Systematic Review (SR) (with homogeneity of randomized controlled trials (RCTs) |
| 1B | Individual RCT (with narrow confidence interval) |
| 1C | All or None |
| 2A | SR (with homogeneity) of cohort studies |
| 2B | Individual cohort study (including low quality RCT; e.g., <80% follow-up) |
| 2C | “Outcomes” research; ecological studies |
| 3A | SR (with homogeneity) of case control studies |
| 3B | Individual case control study |
| 4 | Case series (and poor-quality cohort and case control studies) |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” |
| Category | Primary Preventive Interventions | Number of Referenced Articles Per OCEBM Categorization Level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 2a | 2b | 2c | 3a | 3b | 4 | 5 | Total | ||
| Personal Interventions | Handwashing | 4 | 4 | 1 | 1 | 3 | 1 | 0 | 1 | 4 | 1 | 20 |
| Prophylactic Supplements | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Household Interventions | Water treatment | 5 | 34 | 0 | 4 | 8 | 4 | 0 | 0 | 8 | 2 | 65 |
| Household safe water storage | 1 | 8 | 0 | 2 | 3 | 1 | 0 | 0 | 3 | 2 | 20 | |
| Household Cleanliness | 4 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 3 | 0 | 11 | |
| Household Waste Disposal | 4 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 3 | 0 | 10 | |
| Community Interventions | Community Infrastructure | 1 | 3 | 0 | 0 | 3 | 1 | 0 | 1 | 3 | 1 | 13 |
| Community Education | 2 | 7 | 0 | 1 | 5 | 2 | 0 | 1 | 4 | 0 | 22 | |
| Total | 21 | 57 | 1 | 9 | 25 | 10 | 0 | 5 | 28 | 6 | 162 1 | |
Key: Number of referenced articles reviewed per category, per intervention. | ||||||||||||
| Parameters | Regular Handwashing | Prophylactic Supplements |
|---|---|---|
| Risk |
|
|
| Behavioral Change |
| |
| Co-benefits | ||
| Enabling Factors |
| |
| Limiting Factors |
|
|
| Alternatives for resource-poor settings |
|
|
| Strength of evidence |
|
|
| Parameters | Household Water Treatment | Household Water Storage |
|---|---|---|
| Risk |
| |
| Behavioral Change |
| |
| Co-benefits |
|
|
| Enabling Factors |
|
|
| Limiting Factors |
|
|
| Alternatives for resource-poor settings |
|
|
| Strength of evidence |
| Parameters | Household Cleanliness | Household Waste Management |
|---|---|---|
| Risk |
|
|
| Behavioral Change |
|
|
| Co-benefits |
| |
| Enabling Factors |
| |
| Limiting Factors |
|
|
| Alternatives for resource-poor settings |
| |
| Strength of evidence |
|
| Parameters | Community Infrastructure | Community Education |
|---|---|---|
| Risk |
| |
| Behavioral Change | ||
| Co-benefits |
| |
| Enabling Factors | ||
| Limiting Factors |
|
|
| Alternatives for resource-poor settings | ||
| Strength of evidence |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, E.Y.Y.; Tong, K.H.Y.; Dubois, C.; Mc Donnell, K.; Kim, J.H.; Hung, K.K.C.; Kwok, K.O. Narrative Review of Primary Preventive Interventions against Water-Borne Diseases: Scientific Evidence of Health-EDRM in Contexts with Inadequate Safe Drinking Water. Int. J. Environ. Res. Public Health 2021, 18, 12268. https://doi.org/10.3390/ijerph182312268
Chan EYY, Tong KHY, Dubois C, Mc Donnell K, Kim JH, Hung KKC, Kwok KO. Narrative Review of Primary Preventive Interventions against Water-Borne Diseases: Scientific Evidence of Health-EDRM in Contexts with Inadequate Safe Drinking Water. International Journal of Environmental Research and Public Health. 2021; 18(23):12268. https://doi.org/10.3390/ijerph182312268
Chicago/Turabian StyleChan, Emily Ying Yang, Kimberley Hor Yee Tong, Caroline Dubois, Kiara Mc Donnell, Jean H. Kim, Kevin Kei Ching Hung, and Kin On Kwok. 2021. "Narrative Review of Primary Preventive Interventions against Water-Borne Diseases: Scientific Evidence of Health-EDRM in Contexts with Inadequate Safe Drinking Water" International Journal of Environmental Research and Public Health 18, no. 23: 12268. https://doi.org/10.3390/ijerph182312268
APA StyleChan, E. Y. Y., Tong, K. H. Y., Dubois, C., Mc Donnell, K., Kim, J. H., Hung, K. K. C., & Kwok, K. O. (2021). Narrative Review of Primary Preventive Interventions against Water-Borne Diseases: Scientific Evidence of Health-EDRM in Contexts with Inadequate Safe Drinking Water. International Journal of Environmental Research and Public Health, 18(23), 12268. https://doi.org/10.3390/ijerph182312268









