Impact of Telehealth Care among Adults Living with Type 2 Diabetes in Primary Care: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
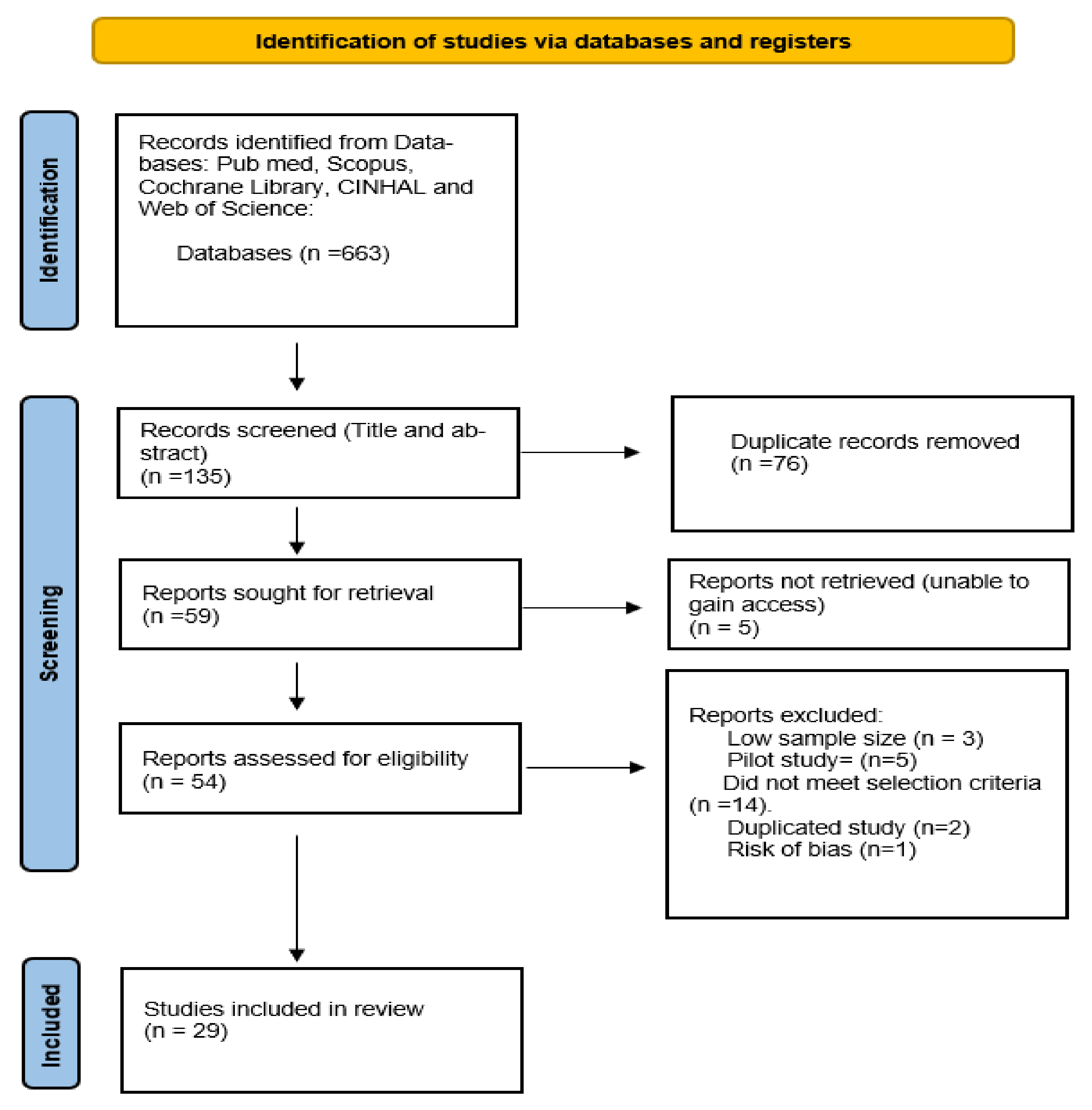
2. Methods
2.1. Search Strategy
| Exclusion Criteria |
| Studies that focus on ‘telerehabilitation’ or ‘telepharmacy’ |
| Multimorbidity studies, subjects with type 1 or gestational diabetes |
| Studies that do not meet RCT checklist criteria |
| Subjects < 18 years of age |
| Pilot studies |
| Low sample size < 50 participants in study |
| HbA1c not listed as a primary or secondary outcome measure |
| Grey literature |
2.2. Risk of Bias Assessment
2.3. Systematic Review Analysis
2.4. Meta-Analysis
3. Results
3.1. Length of Intervention
3.2. Age Range and Average Range of Participants
3.3. Telemonitoring
3.4. mHealth
3.5. Virtual Consultation
3.6. Telephone Communication
3.7. Video Education
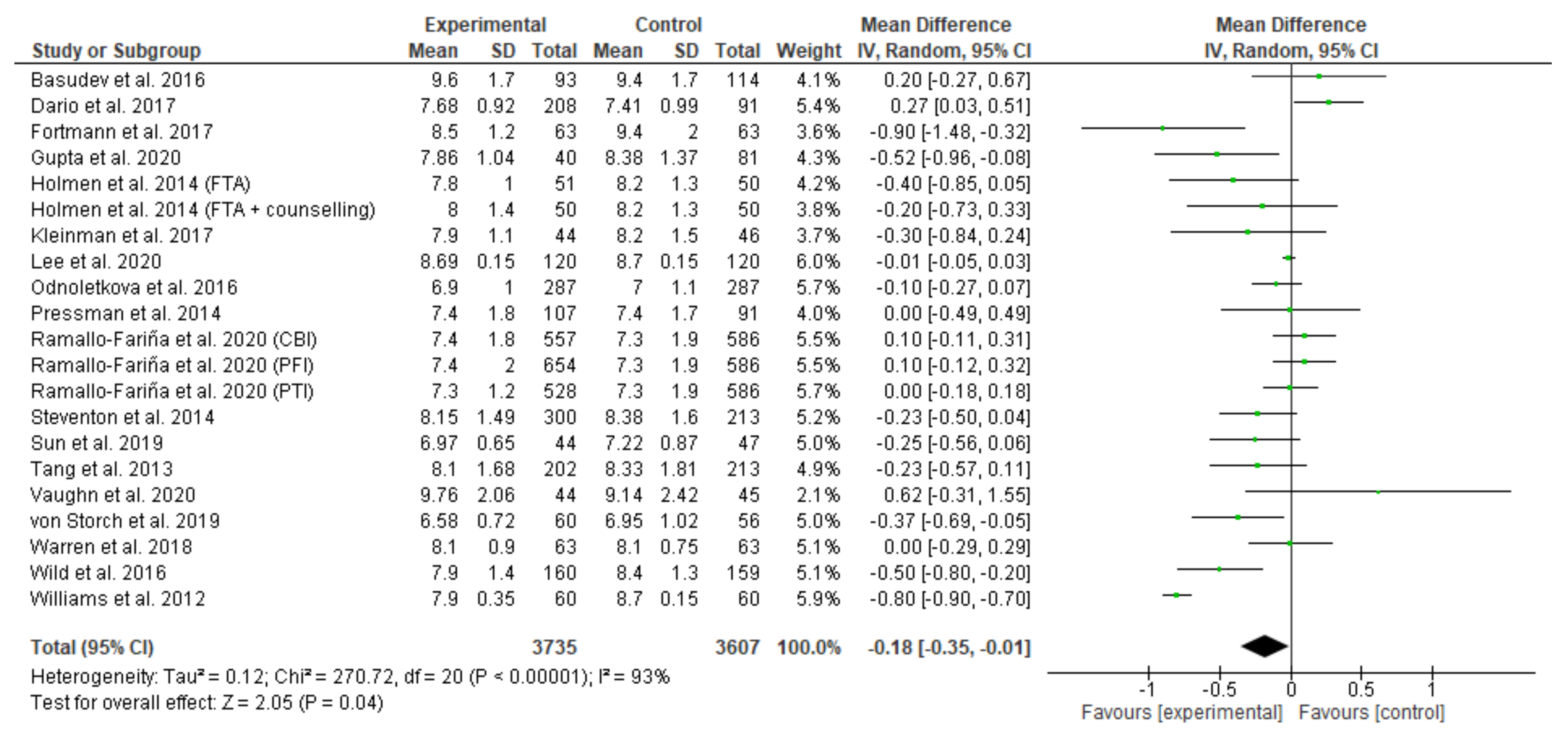
3.8. Meta-Analysis Results
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Cochrane Back Review Group Risk of Bias Assessmen
| Risk of Bias Assessment using the Cochrane Back Review Group Assessment Criteria | Argawal et al., 2019 | Basudev et al., 2016 | Dario et al., 2017 | Egede et al., 2017 | Fortmann et al., 2017 | Gong et al., 2020 | Greenwood et al., 2014 | Gupta et al., 2020 | Hansen et al., 2017 | Holmen et al., 2014 | Kleinman et al., 2017 | Lee et al., 2020 | Lee et al., 2017 | Mcleod et al., 2020 | Odnoletkova et al., 2016 | Parsons et al., 2019 | Pressman et al., 2014 | Ramallo-Fariña et al., 2020 | Shea et al., 2013 | Steventon et al., 2014 | Sun et al., 2019 | Tang et al., 2013 | Trief et al., 2013 | Vaughn et al., 2020 | von Storch et al., 2019 | Warren et al., 2018 | Weinstock et al., 2011 | Wild et al., 2016 | Williams et al., 2012 |
| 1. Was the method of randomisation adequate? | |||||||||||||||||||||||||||||
| 2. Was the treatment allocation concealed? | |||||||||||||||||||||||||||||
| 3. Was the patient blinded to the intervention? | |||||||||||||||||||||||||||||
| 4. Was the care provider blinded to the intervention? | |||||||||||||||||||||||||||||
| 5. Was the outcome assessor blinded to the intervention? | |||||||||||||||||||||||||||||
| 6. Was the dropout rate described and acceptable? | |||||||||||||||||||||||||||||
| 7. Were all randomised participants analysed in the group to which they were allocated? | |||||||||||||||||||||||||||||
| 8. Are reports of the study free of suggestion of selective outcome reporting? | |||||||||||||||||||||||||||||
| 9. Were the groups similar at baseline regarding the most important prognostic indicators? | |||||||||||||||||||||||||||||
| 10. Were co-interventions avoided or similar? | |||||||||||||||||||||||||||||
| 11. Was the compliance acceptable in all groups? | |||||||||||||||||||||||||||||
| 12. Was the timing of the outcome assessment similar in all groups? | |||||||||||||||||||||||||||||
| Key: | Yes | No | Unsure | ||||||||||||||||||||||||||
Appendix B. Meta-Analysis and Subgroup Analysis Results (RevMan 5.4)
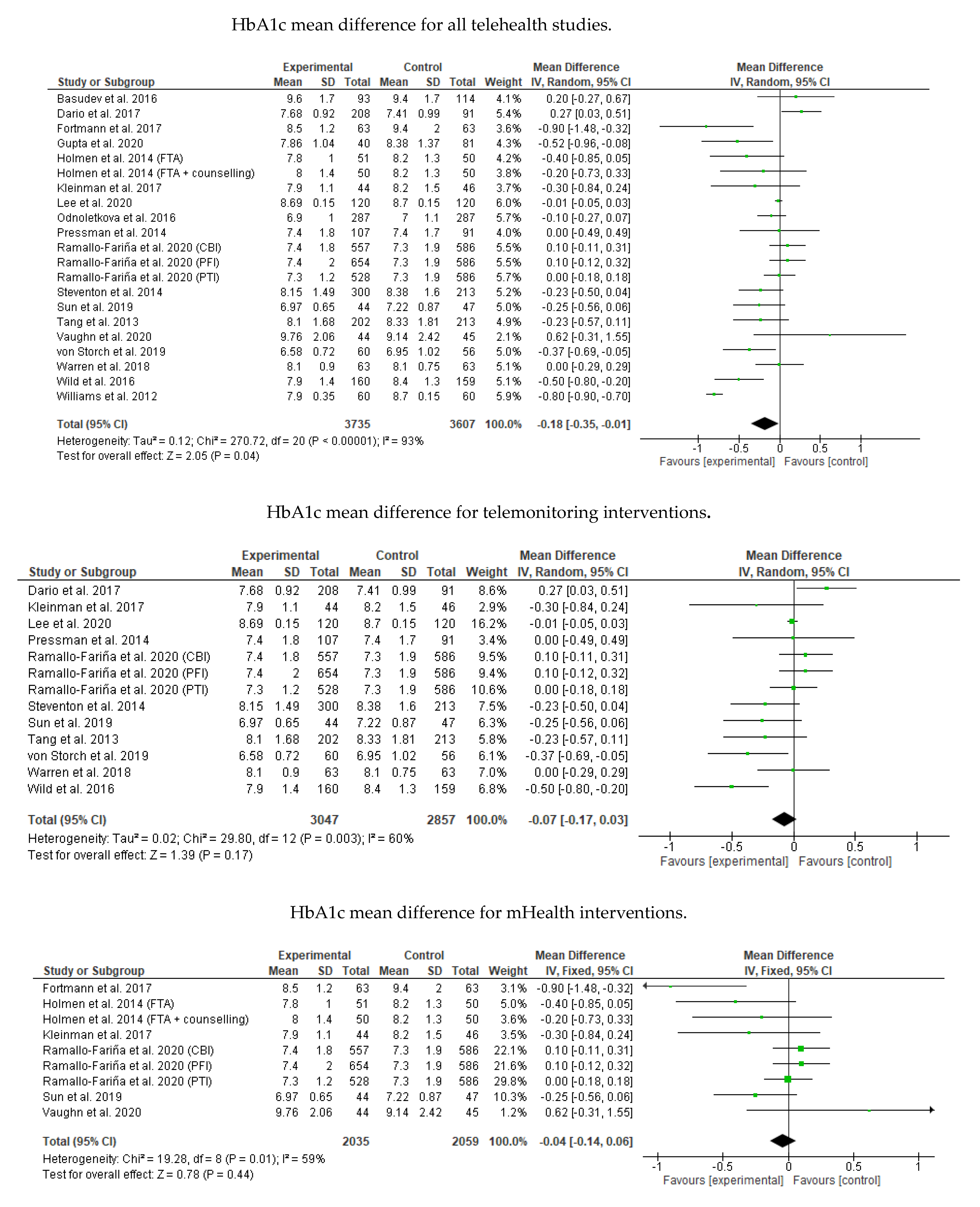
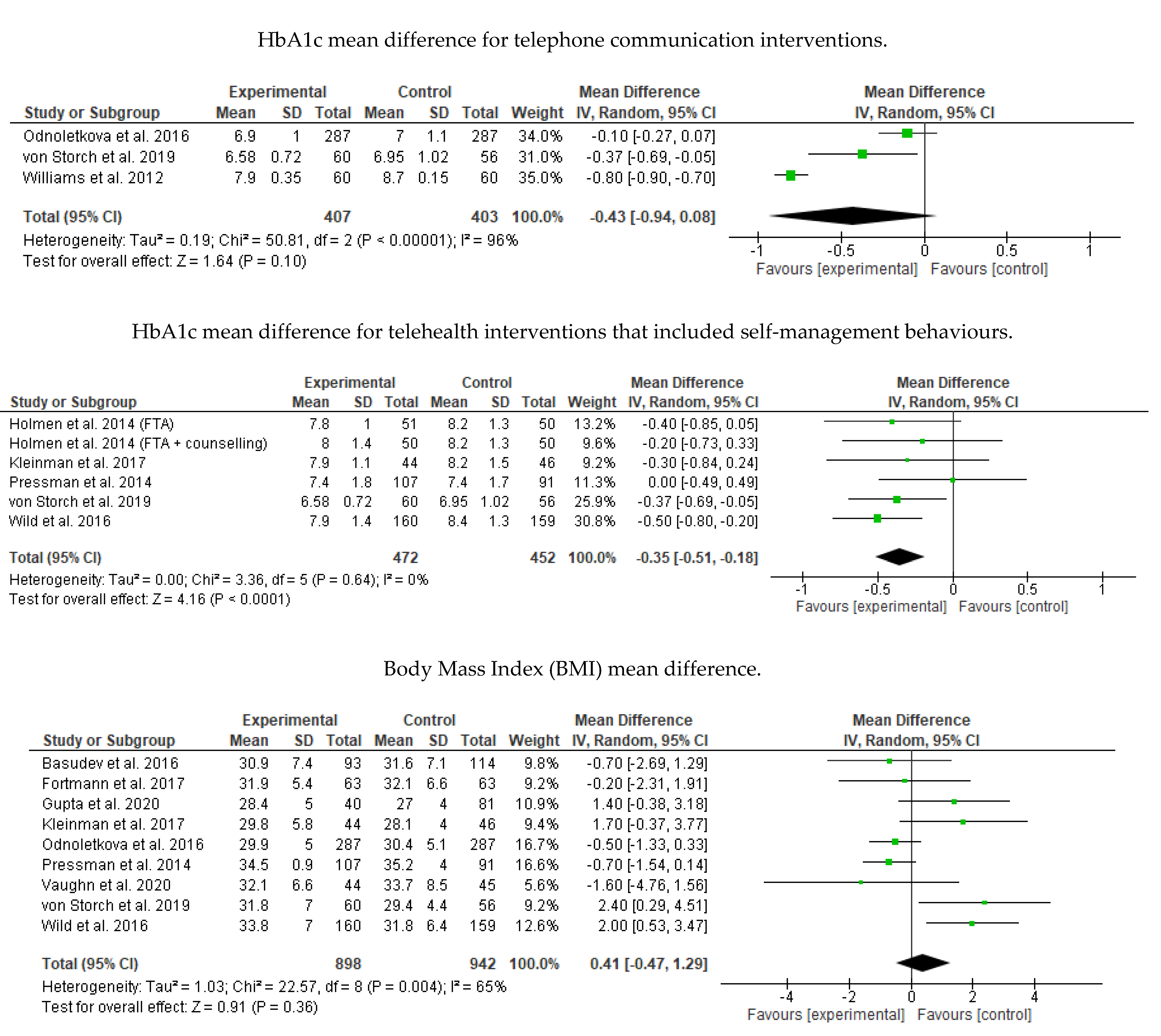
References
- International Organisation for Standardization. Health Informatics—Capacity-Based Ehealth Architecture Roadmap—Part 1: Overview of National Ehealth Initiatives; ISO: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organisation. Telemedicine: Opportunities and Developments in Member States, in Global Observatory for Ehealth Series; WHO: Geneva, Switzerland, 2009; p. 93. [Google Scholar]
- Dinesen, B.; Nonnecke, B.; Lindeman, D.; Toft, E.; Kidholm, K.; Jethwani, K.; Young, H.M.; Spindler, H.; Oestergaard, C.U.; Southard, J.A.; et al. Personalized telehealth in the future: A global research agenda. J. Med. Internet Res. 2016, 18, e53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egede, L.E.; Williams, J.S.; Voronca, D.C.; Knapp, R.G.; Fernandes, J.K. Randomized controlled trial of technology-assisted case management in low income adults with type 2 diabetes. Diabetes Technol. Ther. 2017, 19, 476–482. [Google Scholar] [PubMed]
- Sun, C.; Sun, L.; Xi, S.; Zhang, H.; Wang, H.; Feng, Y.; Deng, Y.; Wang, H.; Xiao, X.; Wang, G.; et al. Mobile phone-based telemedicine practice in older chinese patients with type 2 diabetes mellitus: Randomized controlled trial. JMIR Mhealth Uhealth 2019, 7, e10664. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.M.; Hosseinzadeh, H.; Harris, M.; Zwar, N. Self-management experiences among middle-aged population of rural area of Pakistan with type 2 diabetes: A qualitative analysis. Clin. Epidemiol. Glob. Health 2019, 7, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Yadav, U.N.; Lloyd, J.; Hosseinzadeh, H.; Baral, K.P.; Dahal, S.; Bhatta, N.; Harris, M.F. Facilitators and barriers to the self-management of COPD: A qualitative study from rural Nepal. BMJ Open 2020, 10, e035700. [Google Scholar] [CrossRef]
- Greenwood, D.A. Evaluation of a Telehealth Intervention Combining Structured Self-Monitoring of Blood Glucose and Nurse Care Coordination among People with Type 2 Diabetes Noninsulin-Treated; ProQuest LLCAnn: Arbor, MI, USA, 2014; p. 217. [Google Scholar]
- Weinstock, R.S.; Teresi, J.A.; Goland, R.; Izquierdo, R.; Palmas, W.; Eimicke, J.P.; Ebner, S.; Shea, S. Glycemic control and health disparities in older ethnically diverse underserved adults with diabetes: Five-year results from the Informatics for Diabetes Education and Telemedicine (IDEATel) study. Diabetes Care 2011, 34, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Bali, S. Barriers to Development of Telemedicine in Developing Countries; IntechOpen: London, UK, 2019. [Google Scholar]
- Smith, A.C.; Snoswell, C.L.; Mehrotra, A.; Thomas, E.; Haydon, H.; Clemensen, J.; Caffery, L.J. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J. Telemed. Telecare 2020, 26, 5. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Zhou, J.; Kelley, M.S.; Michaud, T.L.; Siahpush, M.; Kim, J.; Wilson, F.; Stimpson, J.P.; Paga´n, J.A. Does telemedicine improve treatment outcomes for diabetes? A meta-analysis of results from 55 randomized controlled trials. Diabetes Res. Clin. Pract. 2016, 116, 12. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2019; p. 168. [Google Scholar]
- WHO Reveals Leading Cause of Death and Disability Worldwide 2000–2019. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 14 July 2021).
- Royal Australian College of General Practitioners. Management of Type 2 Diabetes: A Handbook for General Practice; Royal Australian College of General Practitioners: East Melbourne, VIC, Australia, 2020; p. 198. [Google Scholar]
- Almutairi, N.; Hosseinzadeh, H.; Gopaldasani, V. The effectiveness of patient activation intervention on type 2 diabetes mellitus glycemic control and self-management behaviors: A systematic review of RCTs. Prim. Care Diabetes 2020, 14, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Ansari, R.M.; Harris, M.; Hosseinzadeh, H.; Zwar, N. Healthcare professionals’ perspectives of patients’ experiences of the self-management of type 2 diabetes in the rural areas of pakistan: A qualitative analysis. Int. J. Environ. Res. Public Health 2021, 18, 9869. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Verma, I.; Gopaldasani, V. Patient activation and type 2 diabetes mellitus self-management: A systematic review and meta-analysis. Aust. J. Prim. Health 2020, 26, 431–442. [Google Scholar] [CrossRef]
- Edwards, J.; Hosseinzadeh, H. The impact of structured physical activity on glycaemic control in diabetes prevention programmes: A systematic review. Proc. Singap. Healthc. 2018, 27, 193–204. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Shnaigat, M. Effectiveness of chronic obstructive pulmonary disease self-management interventions in primary care settings: A systematic review. Aust. J. Prim. Health 2019, 25, 195–204. [Google Scholar] [CrossRef]
- Niknami, M.; Mirbalouchzehi, A.; Zareban, I.; Kalkalinia, E.; Rikhtgarha, G.; Hosseinzadeh, H. Association of health literacy with type 2 diabetes mellitus self-management and clinical outcomes within the primary care setting of Iran. Aust. J. Prim. Health 2018, 24, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Esmatjes, E.; Jansà, M.; Roca, D.; Pérez-Ferre, N.; Del Valle, L.; Martínez-Hervás, S.; Ruiz De Adana, M.; Linares, F.; Batanero, R.; Vázquez, F.; et al. The efficiency of telemedicine to optimize metabolic control in patients with type 1 diabetes mellitus: Telemed study. Diabetes Technol. 2014, 16, 435–441. [Google Scholar] [CrossRef]
- González-Molero, I.; Domínguez-López, M.; Guerrero, M.; Carreira, M.; Caballero, F.F.; Rubio-Martín, E.; Linares, F.; Cardona, I.; Anarte Ortiz, M.T.; Adana, M.; et al. Use of telemedicine in subjects with type 1 diabetes equipped with an insulin pump and real-time continuous glucose monitoring. J. Telemed. Telecare 2012, 18, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Landau, Z.; Mazor-Aronovitch, K.; Boaz, M.; Blaychfeld-Magnazi, M.; Graph-Barel, C.; Levek-Motola, N.; Pinhas-Hamiel, O. The effectiveness of Internet-based blood glucose monitoring system on improving diabetes control in adolescents with type 1 diabetes. Pediatric Diabetes 2012, 13, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.D.; Pennick, V.; Bombardier, C.; van Tulder, M. 2009 updated method guidelines for systematic reviews in the cochrane back review group. SPINE 2009, 34, 12. [Google Scholar] [CrossRef]
- Corbin, L.J.; Richmond, R.C.; Wade, K.H.; Burgess, S.; Bowden, J.; Davey Smith, G.; Timpson, N.J. BMI as a modifiable risk factor for type 2 diabetes: Refining and understanding causal estimates using mendelian randomization. Diabetes 2016, 65, 3002–3007. [Google Scholar] [CrossRef] [Green Version]
- Ryan, R. Cochrane Consumers and Communication Group reviews: Meta-analysis. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Consumers and Communication: Melbourne, Australia, 2016. [Google Scholar]
- Basudev, N.; Crosby-Nwaobi, R.; Thomas, S.; Chamley, M.; Murrells, T.; Forbes, A. A prospective randomized controlled study of a virtual clinic integrating primary and specialist care for patients with type 2 diabetes mellitus. Diabetes Med. 2016, 33, 768–776. [Google Scholar] [CrossRef]
- Gupta, U.; Gupta, Y.; Jose, D.; Mani, K.; Jyotsna, V.P.; Sharma, G.; Tandon, N. Effectiveness of a video-based lifestyle education program compared to usual care in improving hba1c and other metabolic parameters in individuals with type 2 diabetes: An open-label parallel arm randomized control trial (RCT). Diabetes Ther. 2020, 11, 667–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, P.; Mukerji, G.; Desveaux, L.; Ivers, N.M.; Bhattacharyya, O.; Hensel, J.M.; Shaw, J.; Bouck, Z.; Jamieson, T.; Onabajo, N.; et al. Mobile app for improved self-management of type 2 diabetes: Multicenter pragmatic randomized controlled trial. JMIR Mhealth Uhealth 2019, 7, e10321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinman, N.J.; Shah, A.; Shah, S.; Phatak, S.; Viswanathan, V. Improved medication adherence and frequency of blood glucose self-testing using an m-health platform versus usual care in a multisite randomized clinical trial among people with type 2 diabetes in India. Telemed. J. E Health 2017, 23, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Ramallo-Fariña, Y.; García-Bello, M.A.; García-Pérez, L.; Boronat, M.; Wägner, A.M.; Rodríguez-Rodríguez, L.; de Pablos-Velasco, P.; Llorente Gómez de Segura, I.; González- Pacheco, H.; Carmona Rodríguez, M. Effectiveness of internet-based multicomponent interventions for patients and health care professionals to improve clinical outcomes in type 2 diabetes evaluated through the INDICA study: Multiarm cluster randomized controlled trial. JMIR Mhealth Uhealth 2020, 8, e18922. [Google Scholar] [CrossRef]
- Steventon, A.; Bardsley, M.; Doll, H.; Tuckey, E.; Newman, S.P. Effect of telehealth on glycaemic control: Analysis of patients with type 2 diabetes in the Whole Systems Demonstrator cluster randomised trial. BMC Health Serv. Res. 2014, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.R.; Perrild, H.; Koefoed, B.G.; Zander, M. Video consultations as add-on to standard care among patients with type 2 diabetes not responding to standard regimens: A randomized controlled trial. Eur. J. Endocrinol. 2017, 176, 727–736. [Google Scholar] [CrossRef]
- Von Storch, K.; Graaf, E.; Wunderlich, M.; Rietz, C.; Polidori, M.C.; Woopen, C. Telemedicine-assisted self-management program for type 2 diabetes patients. Diabetes Technol. Ther. 2019, 21, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.N.; Luzio, S.D.; Harvey, J.N.; Bain, S.C.; Cheung, W.Y.; Watkins, A.; Owens, D.R. Effect of structured self-monitoring of blood glucose, with and without additional TeleCare support, on overall glycaemic control in non-insulin treated Type 2 diabetes: The SMBG Study, a 12-month randomized controlled trial. Diabetes Med. 2019, 36, 578–590. [Google Scholar] [CrossRef] [Green Version]
- Wild, S.H.; Hanley, J.; Lewis, S.C.; McKnight, J.A.; McCloughan, L.B.; Padfield, P.L.; Parker, R.A.; Paterson, M.; Pinnock, H.; Sheikh, A.; et al. Supported telemonitoring and glycemic control in people with type 2 diabetes: The telescot diabetes pragmatic multicenter randomized controlled trial. PLoS Med. 2016, 13, 1–16. [Google Scholar]
- Dario, C.; Toffanin, R.; Calcaterra, F.; Saccavini, C.; Stafylas, P.; Mancin, S.; Vio, E. Telemonitoring of type 2 diabetes mellitus in Italy. Telemed. J. e-Health 2017, 23, 143–152. [Google Scholar] [CrossRef]
- Pressman, A.R.; Kinoshita, L.; Kirk, S.; Barbosa, G.M.; Chou, C.; Minkoff, J. A novel telemonitoring device for improving diabetes control: Protocol and results from a randomized clinical trial. Telemed. J. e-Health 2014, 20, 109–114. [Google Scholar] [CrossRef]
- Tang, P.C.; Overhage, M.J.; Chan, A.S.; Brown, N.L.; Aghighi, B.; Entwistle, M.P.; Hui, S.L.; Hyde, S.M.; Klieman, L.H.; Mitchell, C.J.; et al. Online disease management of diabetes: Engaging and motivating patients online with enhanced resources-diabetes (EMPOWER-D), a randomized controlled trial. J. Am. Med. Inform. Assoc. 2013, 20, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Fortmann, A.L.; Gallo, L.C.; Garcia, M.I.; Taleb, M.; Euyoque, J.A.; Clark, T.; Skidmore, J.; Ruiz, M.; Dharkar-Surber, S.; Schultz, J. Dulce digital: An mhealth sms-based intervention improves glycemic control in hispanics with type 2 diabetes. Diabetes care 2017, 40, 1349–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, E.M.; Hyman, D.J.; Naik, A.D.; Samson, S.L.; Razjouyan, J.; Foreyt, J.P. A telehealth-supported, integrated care with CHWs, and MEdication-access (TIME) program for diabetes improves HbA1c: A randomized clinical trial. J. Gen. Intern. Med. 2020, 36, 455–463. [Google Scholar] [CrossRef]
- Gong, E.; Baptista, S.; Russell, A.; Scuffham, P.; Riddell, M.; Speight, J.; Bird, D.; Williams, E.; Lotfaliany, M.; Oldenburg, B. My diabetes coach, a mobile app based interactive conversational agent to support type 2 diabetes self-management: Randomized effectiveness-implementation trial. J. Med. Internet Res. 2020, 22, e20322. [Google Scholar] [CrossRef] [PubMed]
- Holmen, H.; Torbjørnsen, A.; Wahl, A.K.; Jenum, A.K.; Småstuen, M.C.; Årsand, E.; Ribu, L. A mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: One-year results from the norwegian randomized controlled trial RENEWING HEALTH. JMIR mHealth uHealth 2014, 2, e57. [Google Scholar] [CrossRef] [PubMed]
- Odnoletkova, I.; Goderis, G.; Nobels, F.; Fieuws, S.; Aertgeerts, B.; Annemans, L.; Ramaekers, D. Optimizing diabetes control in people with Type 2 diabetes through nurse-led telecoaching. Diabetes Med. 2016, 33, 777–785. [Google Scholar] [CrossRef]
- Williams, E.D.; Bird, D.; Forbes, A.W.; Russell, A.; Ash, S.; Friedman, R.; Scuffham, P.A.; Oldenburg, B. Randomised controlled trial of an automated, interactive telephone intervention (TLC Diabetes) to improve type 2 diabetes management: Baseline findings and six-month outcomes. BMC Public Health 2012, 12, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.A.; Greenfield, G.; Pappas, Y. The impact of telehealth remote patient monitoring on glycemic control in type 2 diabetes: A systematic review and meta-analysis of systematic reviews of randomised controlled trials. BMC Health Serv. Res. 2018, 18, 495. [Google Scholar] [CrossRef] [PubMed]
- Faruque, L.I.; Wiebe, N.; Ehteshami-Afshar, A.; Liu, Y.; Dianati-Maleki, N.; Hemmelgarn, B.R.; Manns, B.J.; Tonelli, M. Effect of telemedicine on glycated hemoglobin in diabetes: A systematic review and meta-analysis of randomized trials. CMAJ 2017, 189, E341–E364. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, M.M.; Kum, H.C.; Gonzalez Coronado, K.; Foster, M.J.; Ortega, P.; Lawley, M.A. Barriers to remote health interventions for type 2 diabetes: A systematic review and proposed classification scheme. J. Med. Internet Res. 2017, 19, e28. [Google Scholar] [CrossRef] [PubMed]
- Tchero, H.; Kangambega, P.; Briatte, C.; Brunet-Houdard, S.; Retali, G.R.; Rusch, E. Clinical effectiveness of telemedicine in diabetes mellitus: A meta-analysis of 42 randomized controlled trials. Telemed. e-Health 2019, 25, 569–583. [Google Scholar] [CrossRef]
- McLeod, M.S.; Signal, J.; Stairmand, V.; Thompson, J.; Henderson, D.; Davies, K.; Krebs, C.; Dowell, J.; Grainger, A.R. Impact of a comprehensive digital health programme on HbA1c and weight after 12 months for people with diabetes and prediabetes: A randomised controlled trial. Diabetology 2020, 63, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Trief, P.M.; Izquierdo, R.; Eimicke, J.P.; Teresi, J.A.; Goland, R.; Palmas, W.; Shea, S.; Weinstock, R.S. Adherence to diabetes self care for white, African-American and Hispanic American telemedicine participants: 5 year results from the IDEATel project. Ethn. Health 2013, 18, 83–96. [Google Scholar] [CrossRef]
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014, 67, 11. [Google Scholar]
- Oussedik, E.; Foy, C.G.; Masicampo, E.J.; Kammrath, L.K.; Anderson, R.E.; Feldman, S.R. Accountability: A missing construct in models of adherence behavior and in clinical practice. Patient Prefer. Adherence 2017, 11, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Shea, S.; Kothari, D.; Teresi, J.A.; Kong, J.; Eimicke, J.P.; Lantigua, R.A.; Palmas, W.; Weinstock, R.S. Social impact analysis of the effects of a telemedicine intervention to improve diabetes outcomes in an ethnically diverse, medically underserved population: Findings from the IDEATel Study. Am. J. Public Health 2013, 103, 1888–1894. [Google Scholar] [CrossRef]
- Stringhini, S.; Batty, G.D.; Bovet, P.; Shipley, M.J.; Marmot, M.G.; Kumari, M.; Tabak, A.G.; Kivimaki, M. Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: The Whitehall II prospective cohort study. PLoS Med. 2013, 10, e1001479. [Google Scholar] [CrossRef]
- Darrat, I.; Tam, S.; Boulis, M.; Williams, A.M. Socioeconomic disparities in patient use of telehealth during the coronavirus disease 2019 surge. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 287–295. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Xie, B.; Berkley, A.; Kim, M. Barriers and facilitators for sustainability of tele-homecare programs: A systematic review. Health Serv. Res. 2016, 51, 48–75. [Google Scholar] [CrossRef] [Green Version]
| Author/Year Country | Sample Size (n) Participant Characteristics (% Male) | Age (Years) | Description of Intervention | Length of Intervention | p value (95% CI) Mean HbA1c% |
|---|---|---|---|---|---|
| Argawal et al., 2019 Canada | n = 223 Intervention (110) 55% (1 participant gender not specified) Control: (113) 49% | Intervention 51.5 ± 10.6 Control 52.1 ± 10.7 | Telemonitoring + mHealth Use of mobile app to enter data baseline health, blood glucose readings, exercise activity and food intake. App provided tailored messages to participants targeting motivation, education and behaviour change. | 6 months | p = 0.19 CI (−1.05, 0.21) |
| Basudev et al., 2016 United Kingdom | n = 208 Intervention (93) 55% Control (114) 60% | Intervention 60.5 ± 12.3 Control 59.3 ± 12.0 | Virtual clinic Virtual clinic model where participants have a virtual consultation with practice team for care planning related to type 2 diabetes management. Participants reviewed every 3 months. | 12 months | p = 0.4 CI-not available |
| Dario et al., 2017 Italy | n = 299 Intervention (208) 57% Control (91) 54% | Intervention 73.05 ± 5.79 Control 73.04 ± 5.28 | Telemonitoring with feedback Participants uploaded blood glucose readings to an online e-Health Centre. Clinicians monitor participant data through a Home Care portal with feedback provided to next of kin if indicated. | 12 months | p = 0.76 CI (−0.2, 0.2) |
| Egede et al., 2017 USA | n = 113 Intervention (59) 17% Control (54) 19% | Intervention 55.1 ± 11.4 Control 53.4 ± 10.5 | Telemonitoring with feedback Telemonitoring + case management via FORA 2 in 1 blood glucose and blood pressure monitoring system. Nurse case manager provides weekly medication adjustments based on daily participant data. | 6 months | p = 0.024 CI (−1.86, −0.13) |
| Fortmann et al., 2017 USA | n = 126 Intervention (63) 27% Control (63) 24% | Intervention 47.8 ± 9.0 Control 49.1 ± 10.6 | mHealth mHealth intervention involving text messages with motivational, educational and call to action messages 2–3 x day over a 6-month period. Participants encouraged to send blood glucose readings after text prompt. | 6 months | p = 0.03 CI-not available |
| Gong et al., 2020 Australia | n = 187 Intervention (93) 53% Control (94) 64% | Intervention 55.4 ± 9.7 Control 58.4 ± 10.5 | mHealth mHealth intervention involving mobile app which provides support, monitoring and motivational coaching via an embodied conversation agent. | 12 months | p = 0.84 CI (−0.45, 0.36) |
| Greenwood et al., 2014 USA | n = 90 Intervention (45) 49% Control (45) 58% | Intervention 53.9 ± 10.4 Control 57.5 ± 10.6 | Telemonitoring with feedback Telemonitoring with feedback which included a daily health session with an audible prompt for participant to take blood glucose readings with diabetes related education provided. Participants can access a diabetes clinical educator who is available via phone call or text to discuss diabetes care. | 6 months | p < 0.001 CI-not available |
| Gupta et al., 2020 India | n = 81 Intervention (40) 45% Control (41) 59% | Intervention 50.1 ± 9.4 Control 50.2 ± 8.6 | Video based education program Video based lifestyle education program involving 12 modules over a 4-month period focused on type 2 diabetes health topics such as self-monitoring, diet, meal planning, exercise, etc. | 4 months | p = 0.013 CI (0.14,1.14) p = 0.021 adjusted CI (0.10, 1.12) |
| Hansen et al., 2017 Denmark | n = 165 Intervention (83) 64% Control (82) 65% | Intervention 57.8 ± 9.4 Control 58.3 ± 9.3 | Video consultation + telemonitoring Video consultation + telemonitoring involving 2 x monthly video conferences with a nurse via tablet computer for 32 weeks. Participants provided blood glucose readings, blood pressure readings and body weight readings to nurse. | 8 months | p = 0.023 CI-not available |
| Holmen et al., 2014 Norway | n = 151 Intervention app (51) 67% Intervention app + coaching (50) 50% Control (50) 40% | Intervention (app) 58.6 ± 11.8 Intervention (app + coaching) 57.4 ± 12.1 Control 55.9 ± 12.2 | mHealth mHealth intervention with or without telephone counselling from diabetes specialist nurse for the first 4 months of the intervention. Mobile app was utilised for wireless transfer of blood glucose data, manual entry of diet, physical activity and personal goals related to type 2 diabetes management. | 12 months | p = 0.42 (app) CI (−0.75, 0.32) p = 0.97(app + coaching) CI (–0.52, 0.54) |
| Kleinman et al., 2017 India | n = 91 Intervention (44) 82% Control (46) 59% | Intervention 48.8 ± 9.0 Control 48.0 ± 9.5 | mHealth mHealth intervention involving mobile app reminding participants to complete blood glucose readings with access to a health coach. Out of range blood glucose levels prompted questions for participants and generated alerts to a health care team. | 6 months | p = 0.02 CI (0.10, 1.37) |
| Lee et al., 2020 Malaysia | n = 240 Intervention (120) 44% Control (120) 46% | Intervention 56.1 ± 9.2 Control 56.3 ± 8.6 | Telemonitoring + case management Telemonitoring + team-based management. Blood glucose levels uploaded to system, and care team adjusts medication accordingly. Healthy lifestyle education and advice also provided. Participants advised to report 6 glucose readings/week (3 prandial and 3 post-prandial). Feedback provided from care team if indicated. | 12 months | p = 0.226 CI (−0.07, 0.02) |
| Lee et al., 2017 Malaysia | n = 85 Intervention (45) 53% Control (40) 40% | Intervention 53.24 ± 7.29 Control 53.77 ± 8.03 | Telemonitoring + feedback Blood glucose readings uploaded to an online portal via mobile device which is viewed by researcher. Reminders sent to participants to measure blood glucose levels. Case manager contacts participant to provide advice re: medication and lifestyle education. | 3 months | p < 0.01 CI-not available |
| McLeod et al., 2020 New Zealand | n = 429 Intervention (215) 50% Control (214) 48% | Intervention 61.8 ± 9.5 Control 62.4 ± 8.7 | mHealth mHealth intervention involving mobile device and web-based program with 4 components, health coaching, evidence-based resources, peer support and goal tracking. Health coaching for the first 4 months of intervention via text or fortnightly video meetings. | 12 months | p = 0.990 CI (−0.1, 0.1) |
| Odnolekova et al., 2016 Belgium | n = 287 Intervention (287) 60% Control (287) 63% | Intervention 63.8 ±8.7 Control 62.4 ± 8.9 | Telephone communication “telecoaching” Tele-coaching via telephone. Five nurse led telephone sessions averaging 30 mins every 3–8 weeks utilising motivational interviewing techniques. | 6 months | p = 0.003 CI (−0.3, −0.1) |
| Parsons et al., 2019 United Kingdom | n = 323 Intervention (self-monitoring + feedback) (148) 59% Intervention (self-monitoring) (147) 56% Control (151) 58% | Intervention (self-monitoring + telecare) 61.6 ±9.82 Intervention (self-monitoring) 62.9 ± 9.34 Control 60.7 ± 10.98 | Telemonitoring + feedback Telemonitoring via diabetes management software, with or without telecare support via monthly phone call from diabetes nurse. | 12 months | Intervention (SM + telecare) p < 0.0001 CI (−1.40, −0.94) Intervention (SM only) p < 0.0001 CI (–1.29, –0.81) |
| Pressman et al., 2014 USA | n = 225 Intervention (107) 63% Control (91) 60% | Intervention 54.8 ± 9.8 Control 56.4 ± 8.7 | Telemonitoring + feedback Telemonitoring of blood glucose levels along with weekly communication with diabetes nurse. | 6 months | p = 0.310 CI-not available |
| Ramallo-Fariña et al., 2020 Canary Islands | n = 2334 Intervention 1 PTI (587) 53% Intervention 2 PFI (654) 56% Intervention 3 CBI (557) 47% Control (586) 49% | Intervention (1) 55.9 ± 7.0 Intervention (2) 56.2 ± 7.0 Intervention (3) 55.5 ± 7.1 Control 55.2 ± 7.3 | Telemonitoring via web platform with SMS (mHealth) Telemonitoring via web-based platform with automated SMS. Participants required to log blood glucose levels for feedback. Intervention also involved health care provider training regarding telehealth intervention. | 24 months | p = 0.3 (PTI) CI (−0.48, −0.04) p = 0.9 (PFI) CI (−0.41, 0.03) p = 0.3 (CBI) CI (−0.47, −0.03) |
| Shea et al., 2013 | Data obtained from Weinstock et al., 2011 | ||||
| Steventon et al., 2014 United Kingdom | n = 513 Intervention (300) 53% Control (213) 64% | Intervention 63.9 ± 13.0 Control 66.2 ± 11.9 | Telemonitoring + automated SMS feedback Telemonitoring with feedback when indicated. Participants encouraged to upload blood glucose data with automated educational messages provided. | 12 months | p = 0.125 unadjusted CI (−0.60, 0.07) p = 0.0009 adjusted CI (−0.52, −0.07) |
| Sun et al., 2019 China | n = 91 Intervention (44) 43% Control (47) 38% | Intervention 67.9 ± 2.5 Control 68.04 ± 3.0 | Telemonitoring + mHealth Telemonitoring combined with mHealth. Daily wireless transmission of blood glucose data through glucometer along with feedback via telemedicine system regarding management of type 2 diabetes. | 6 months | p = 0.02 CI-not available |
| Tang et al., 2013 USA | n = 415 Intervention (202) 59% Control (213) 61% | Intervention 54.0 ± 10.7 Control 53.5 ± 10.2 | Telemonitoring + feedback Telemonitoring + personalised feedback as well as personalised text and video educational content. | 12 months | p = 0.133 CI-not available |
| Trief et al., 2013 | Data obtained from Weinstock et al., 2011 | ||||
| Vaughan et al., 2020 USA | n = 89 Intervention (44) 23% Control (45) 33% | Intervention 55.99 ± 7.12 Control 53.86 ± 9.07 | mHealth mHealth intervention, regular communication call or SMS with health care practitioner, diabetes group visits with community health worker. | 6 months | unadjusted p = 0.007 CI-not available Adjusted p = 0.002 CI-not available |
| Von Storch et al., 2019 Germany | n = 115 Intervention (60) 78% Control (56) 84% | Intervention 59.4 ± 6.3 Control 58.4 ± 7.3 | Telemonitoring + telephone communication Telemonitoring of blood glucose levels along with feedback if indicated utilising tablet computer, glucometer and step counter with monthly telephone coaching. | 12 months (results reported at 3 months) | p = 0.038 CI-not available |
| Warren et al., 2018 Australia | n = 126 Intervention (63) 60% Control (63) 48% | Intervention 61.3 ± 10.8 Control 61.3 ± 11.4 | Telemonitoring + feedback Telemonitoring of blood glucose levels along with feedback. | 12 months | p = 0.004 CI-not available |
| Weinstock et al., 2011 USA | n = 1665 Intervention (844) 36% Control (821) 38% | Intervention 70.8 ± 6.5 Control 70.9 ± 6.8 | Telemonitoring + video conferencing Telemonitoring of blood glucose levels + video consultation with health care provider. | 5 years | p = 0.001 CI-not available |
| Wild et al., 2016 United Kingdom | n = 321 Intervention (160) 66% Control (159) 67% | Intervention 60.5 ± 9.8 Control 61.4± 9.8 | Telemonitoring + feedback Telemonitoring of blood glucose le levels + once weekly feedback/review with diabetes educator. | 9 months | p = 0.007 CI (0.22, 0.81) |
| Williams et al., 2012 Australia | n = 120 Intervention (60) 62% Control (60) 63% | Intervention 58.4 ± 8.2 Control 56.4 ± 8.3 | Telephone communication Automated telephone management intervention, with 1 × weekly phone calls lasting 5–20 minutes and education provided on a variety of diabetes related topics. | 6 months | p = 0.002 CI (0.86–0.93) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robson, N.; Hosseinzadeh, H. Impact of Telehealth Care among Adults Living with Type 2 Diabetes in Primary Care: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 12171. https://doi.org/10.3390/ijerph182212171
Robson N, Hosseinzadeh H. Impact of Telehealth Care among Adults Living with Type 2 Diabetes in Primary Care: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. International Journal of Environmental Research and Public Health. 2021; 18(22):12171. https://doi.org/10.3390/ijerph182212171
Chicago/Turabian StyleRobson, Natalie, and Hassan Hosseinzadeh. 2021. "Impact of Telehealth Care among Adults Living with Type 2 Diabetes in Primary Care: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" International Journal of Environmental Research and Public Health 18, no. 22: 12171. https://doi.org/10.3390/ijerph182212171
APA StyleRobson, N., & Hosseinzadeh, H. (2021). Impact of Telehealth Care among Adults Living with Type 2 Diabetes in Primary Care: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. International Journal of Environmental Research and Public Health, 18(22), 12171. https://doi.org/10.3390/ijerph182212171







