Abstract
Uterine myomas or uterine fibroids are the most common benign uterine masses affecting women. The management of large myoma during pregnancy is challenging, and surgical treatment is a possible option. We report nine cases of pregnant women affected by uterine masses larger than 10 cm, who underwent surgical treatment during the second trimester of pregnancy. In all cases, the masses were preconceptionally unknown and diagnosed during the first trimester. In eight cases, no maternal and fetal complications arose during or after surgical treatment and delivery occurred at full term of pregnancy. In one case, spontaneous abortion was recorded. In all cases, histologic diagnosis demonstrated the benign nature. Women affected by large uterine masses diagnosed for the first time in pregnancy could be taken into consideration for surgical treatment in a referral center during the second trimester.
1. Introduction
Uterine myomas or uterine fibroids are the most common benign uterine neoplasms, starting at reproductive age [1,2,3]. In obstetric clinical practice, it is possible to face uterine myomas, but they cannot be easily assessed and the prevalence ranges between 0.1 and 3.9% [4].
Uterine fibroids are symptomatic in about 25% of cases [2,3], and in pregnancy they have been associated with increased risks of spontaneous abortion, preterm delivery, preterm premature rupture of membranes (pPROM), bleeding during pregnancy and postpartum hemorrhage, placental abruption, fetal malpresentation, dystocia and cesarean delivery [5].
Although the management of myomas in non-pregnant women follows clear guidelines outlining different options, which range from medical to surgical treatment [6], not all such treatments are suitable for pregnant women due to the possible risk of fetal and placental damage. Therefore, if conservative management is the preferred therapeutic choice, myomectomy is generally a feasible option in cases such as torsion of pedunculated fibroids, necrosis, severe maternal symptoms or large/rapidly growing myomas located in the lower segment of the uterus deforming the placental site [7].
Diagnosis and follow up of uterine masses are mainly based on ultrasound evaluation [8], and the possibility of leiomyosarcoma should be called into question. When the diagnosis of a large pelvic mass is made during pregnancy, even if compatible with myoma, differential diagnosis should consider malignancy, such as ovarian cancer or seldom leiomyosarcoma [9,10].
We report a case series of pregnant women with large uterine mass(es), which were unknown in the preconceptional period and revealed for the first time in early pregnancy, who underwent surgical treatment during the second trimester of gestation.
2. Materials and Methods
Nine pregnant women with large uterine mass(es) diagnosed for the first time in pregnancy between September 2016 and January 2020 were retrospectively recorded. The inclusion criteria were as follows: an ultrasound diagnosis performed for the first time in pregnancy of at least one mass 10 cm in size or bigger, which we classified as “large uterine mass”; and first spontaneous pregnancy. The exclusion criteria were as follows: patients who denied consent for surgical treatment, previous abdominal surgery, thickness of residual myometrium between the mass and the uterine cavity ≤5 mm and women with uterine mass known for a long time before pregnancy (<10 cm).
To reduce the risk of chorionic membrane trauma during surgical resection, ultrasound evaluation included detailed mapping of the mass (in particular, the site of attachment), assessment of the pedicle (if present) and myometrial thickness. Ultrasound scans (US) were performed transvaginally (GE RIC5-9H 3D-4D Transvaginal Probe) and transabdominally (GE C1-5-D, Convex Probe) by a single physician with specific training in obstetric sonography, using a Voluson E8 system (GE Healthcare Zipf, Tiefenbach, Austria). In all cases, diagnosis was performed during the first trimester, between 9 and 13 weeks of gestational age.
For each case, a detailed briefing was performed by an experienced team of gynecologists to evaluate the risks and benefits of surgical treatment, considering mass(es) volume and site, the unknown nature and the missing evidence of their presence before pregnancy. All patients gave consent to undergo the procedure, which was scheduled during the second trimester of pregnancy, when the risks of spontaneous abortion and preterm contractions are lower, making it the optimal time for elective surgical treatment. Patients were extensively counseled on (a) increased risks of poor pregnancy outcome, especially premature birth, (b) increased risk of maternal organ compression due to mass(es) dimensions and (c) the need of a conclusive histological diagnosis to rule out malignancies (sarcoma).
Before surgery, an intraoperative ultrasound was performed to assess both the fetal heart rate (Figure 1a) and the position of the larger mass and its pedicle (Figure 1b). Surgery was performed in all cases under general anesthesia, using a longitudinal incision to achieve the best control of the surgical field; this determined the maximum surgical efficacy with as minimal as possible manipulation of the uterus. A careful exploration of the surgical field, defining the number, the site and the position of all masses, enabled the surgeon to further confirm the surgical strategy. Exteriorization of the large mass was subsequently performed to clarify the dimensions of its pedicle and to start the surgical resection along its surface to save enough tissue to perform hysterorrhaphy, thus reducing the stitching on the “functional myometrium” (Figure 2a–d). In case of a large pedicle, the simultaneous forcipressure of the resected tissue ensured good control of the hemostasis, reducing the number of required stitches (Figure 2c). At the end of the surgery, fetal vitality was checked and confirmed by the presence of normal fetal cardiac activity, regular amniotic fluid and normal placenta adhesion.
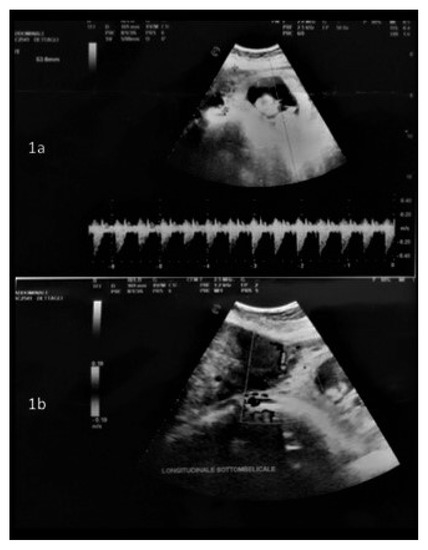
Figure 1.
(a) Doppler ultrasound to check fetal heartbeat; (b) Assessment of the position of larger myoma and its pedicle.
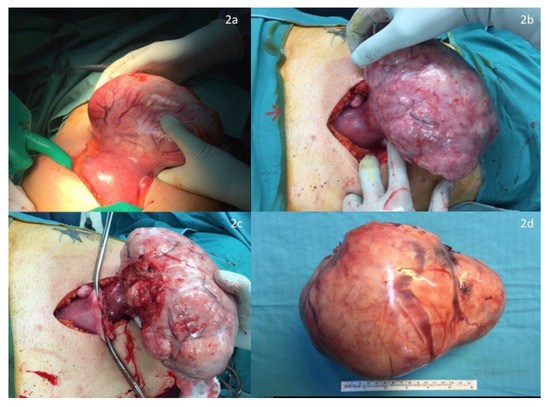
Figure 2.
(a,b) Exteriorization of large mass after longitudinal laparotomy. (c,d) Surgical resection along the surface.
Pharmacologic therapy with antibiotics and progesterone was administered. All patients underwent a monthly follow-up until their delivery.
3. Results
From September 2016 to January 2020, nine pregnant women with large myomas were surgically treated during the second trimester of pregnancy, and at least one large uterine mass, >10 cm, was removed. Patients’ characteristics are reported in Table 1. In seven cases, no severe symptoms were reported despite the mass occupying the pelvis and abdomen up to the subdiaphragmatic site. Pelvic pain and vaginal bleeding were reported in the remaining cases.

Table 1.
Characteristics of patients.
Large masses were all subserous and in six cases they were localized in the right part of the uterine fundus, extending up to the lower surface of the liver. US characteristics of masses are reported in Table 2.

Table 2.
Preoperative, intraoperative and postoperative characteristics of masses (SS: subserous; IM: intramural; EBL: estimated blood loss).
Out of three patients, only one mass was excised, while in the remaining six cases, two or more masses were removed (Table 2). Intraoperative blood loss ranged between 200 cc and 1400 cc, but blood transfusion was only necessary in one case (Table 2) for severe anemia. The mean duration of the surgical intervention was 105 min (ranging from 90 to 135 min). Postoperative hospitalization ranged from 4 to 7 days. The histologic diagnosis demonstrated the benign nature of all 27 excised masses. Regarding the obstetric outcome, in all but one case the pregnancies were carried to full term without maternal or fetal complications. As for the only adverse outcome, 8 h after surgical treatment with excision of multiple myomas, of which two were larger than 10 cm and four were larger than 5 cm, spontaneous abortion occurred with spontaneous fetal expulsion. The blood loss was substantial (1400 cc) and required perioperative transfusions. No hysterectomy was needed.
In the other eight successful cases, women were closely followed up with ultrasound and physical examination every four weeks until delivery, and a cesarean section was performed after the 37th gestational week. More specifically, in seven cases the delivery was through an elective cesarean section, while one was a scheduled cesarean section due to premature rupture of membranes (PROM). No complications related to the cesarean sections and the feto-neonatal outcomes were reported.
4. Discussion
The relationship between fibroids and pregnancy outcome is not supported by high-quality data. However, the available information, mainly from observational case series, reports that the presence of myoma(s) during pregnancy is associated with an increased risk of maternal, fetal and neonatal complications [5], and the risk of pregnancy loss or preterm delivery may be higher when there are multiple fibroids [5,11].
The appropriate therapeutic approach of myomas/masses during pregnancy depends on several factors, considering the severity of symptoms, size, number and site of masses. Moreover, if a large (>10 cm) uterine mass is diagnosed for the first time in pregnancy, there are no ultrasonographic or MRI features to decisively differentiate a benign lesion (uterine leiomyoma) from a malignant variant (uterine sarcoma) [12].
To the best of our knowledge, two main approaches for the management of myomas during pregnancy have been described: (i) conservative treatment with observation and, when required, analgesic and tocolytic treatments [13]; and (ii) surgical treatment, mainly after the failure of conservative approaches and/or when a rapid abnormal growth and increase in size with displacement of the surrounding organs is observed.
The data in the literature describe both laparoscopic and laparotomic surgical procedures. However, in cases with large masses, the laparotomic approach gives the advantage of achieving the best control of the surgical field and extracting the myoma intact, especially when the malignant nature cannot be ruled out. Indeed, in 2014, the Food and Drug Administration released a statement advising against the use of laparoscopic morcellation in cases with large myomas of unknown origin [12,14,15,16].
Nevertheless, in our research we decided to surgically treat the asymptomatic patient as well, which was first performed during pregnancy, due to the large size at diagnosis, the uncertain nature of the detected mass and the potential pregnancy complications. In the cases considered here, we removed small myomas only when they were subserous or pedunculated, and strictly close to the large myoma. Although a surgical approach for uterine myomas during pregnancy is not the preferred choice, our experience suggests that it can be performed, in selected cases, by an experienced team of surgeons after an accurate preoperative multidisciplinary planning.
In our study, we selected cases where the women presented large pelvic mass(es) that were detected for the first time during the first trimester of pregnancy and were unknown in the preconception period. Preoperative planning included an accurate ultrasound study to assess the number, site, dimensions and characteristics of large mass(es); the myometrial thickness between the mass and the uterine cavity; the evaluation of risks and benefits; a multidisciplinary board with experienced surgeons and patient consent.
The timing of interventions reported in previous studies ranged between the first and second trimester of pregnancy. In our cases, we decided to perform myomectomy during the second trimester, when the risks of spontaneous abortion and preterm contractions are lower. This approach was made possible by the fact that none of our cases needed an urgent procedure, making it the optimal time for non-urgent surgical treatment. Moreover, existing data on anesthetic agents have not been associated with teratogenic effects in humans when standard concentrations are used at any gestational age [17].
Regarding the surgical outcomes, in line with the data in the literature, no major postoperative complications occurred and no hysterectomy was needed. Regarding the obstetric outcomes, spontaneous abortion occurred with spontaneous fetal expulsion in only one case, followed by curettage, on the same day of the myomectomy.
The abovementioned case, characterized by fetal demise, differed from the others because of 1. hemorrhagic episodes during the first trimester; 2. the presence of more than 10 myomas and 3. conspicuous intraoperative blood loss leading to anemia, requiring transfusions.
Although this is a single case, such an event suggests that preoperative counseling probably needs to be focused on the risk of abortion and/or hysterectomy if the number of myomas exceeds 10 and/or if the pregnancy is at risk of an abortion due to a hemorrhage in the first trimester.
Indeed, according to a recent review [18], myomectomy in pregnancy can have a success rate as high as 90%, while the risk of miscarriage or fetal demise after myomectomy is reportedly about 6–7% (13 cases/198 cases). The procedure was successful in eight out of nine of our cases (88.8%), which is consistent with the reported data.
Intraoperative ultrasounds are useful for assistance during myomectomy in pregnant women [19].
5. Conclusions
Our experience confirms that large uterine mass removal is a feasible option during pregnancy when the benefits exceed the risks. Surgical treatment should not be viewed as standard practice, but rather taken into consideration in case of large mass(es), especially those detected for the first time during pregnancy and/or when there is a need to obtain a pathological characterization to exclude the possibility of malignancy. Data from our experience indicated, after surgical treatment, the benign nature of the mass(es), despite their large size being undetected in the preconception period. Unless urgent, surgery should be performed during the second trimester of pregnancy by an experienced team of surgeons. Prenatal counseling should be provided during the first trimester and prior to performing any surgical treatment; the risks and benefits of a surgical procedure during pregnancy should be carefully evaluated and discussed with patients.
Author Contributions
Conceptualization, A.F.C. and G.V.; methodology, A.F.C. and G.V.; formal analysis, A.F.C., G.V. and A.V.; investigation, A.F.C.; data curation, A.V., M.C.L.M. and S.P.; writing—original draft preparation, A.F.C., A.V. and S.G.A.; writing—review and editing, A.F.C., G.V. and S.R.; supervision, G.S., A.F. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because the surgical procedure described is part of normal clinical practice and is one of the possible therapeutic choices for the patient.
Informed Consent Statement
General informed consent for surgical procedure was obtained from all subjects involved in the study, as required by normal clinical practice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef]
- Borah, B.J.; Nicholson, W.K.; Bradley, L.; Stewart, E.A. The impact of uterine leiomyomas: A national survey of affected women. Am. J. Obstet. Gynecol. 2013, 209, 319.e1–319.e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.; Zhang, W.; Chames, M.C.; Guo, J. Myomectomy during cesarean delivery. Int. J. Gynaecol. Obstet. 2013, 121, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Padula, F.; Gulino, F.A. Management of uterine fibroids in pregnancy: Recent trends. Curr. Opin. Obstet. Gynecol. 2015, 27, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, D.; Norwitz, E.R. Are Women With Uterine Fibroids at Increased Risk for Adverse Pregnancy Outcome? Clin. Obstet. Gynecol. 2016, 59, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Tarazona, M.; Dasí Carrasco, J.; Estaca, G.; Cristóbal, I.; Monleón, J. Updated approaches for management of uterine fibroids. Int. J. Womens Health 2017, 9, 607–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milazzo, G.N.; Catalano, A.; Badia, V.; Mallozzi, M.; Caserta, D. Myoma and myomectomy: Poor evidence concern in pregnancy. J. Obstet/Gynaecol. Res. 2017, 43, 1789–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Cruz, M.S.; Buchanan, E.M. Uterine Fibroids: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 100–107. [Google Scholar] [PubMed]
- Pritts, E.A.; Vanness, D.J.; Berek, J.S.; Parker, W.; Feinberg, R.; Feinberg, J.; Olive, D.L. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: A meta-analysis. Gynecol. Surg. 2015, 12, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Luigi, G.; D’Alfonso, A.; Patacchiola, F.; Di Stefano, L.; Palermo, P.; Carta, G. Leiomyosarcoma: A rare malignant transformation of a uterine leiomyoma. Eur. J. Gynaecol. Oncol. 2015, 36, 84–87. [Google Scholar] [PubMed]
- Klatsky, P.C.; Tran, N.D.; Caughey, A.B.; Fujimoto, V.Y. Fibroids and reproductive outcomes: A systematic literature review from conception to delivery. Am. J. Obstet. Gynecol. 2008, 198, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kaganov, H.; Ades, A.; Fraser, D.S. Preoperative Magnetic Resonance Imaging Diagnostic Features of Uterine Leiomyosarcomas: A Systematic Review. Int. J. Technol. Assess. Health Care 2018, 34, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Tropea, A.; Rossetti, D.; Carnelli, M.; Cianci, A. Management of uterine leiomyomas in pregnancy: Review of literature. Updates Surg. 2013, 65, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wallis, L. FDA warns against power morcellation for hysterectomy and fibroids. Am. J. Nurs. 2014, 114, 16. [Google Scholar] [CrossRef] [PubMed]
- Mynbaev, O.A.; Sparic, R.; Stark, M.; Malvasi, A.; Marinelli, E.; Zaami, S.; Tinelli, A. The Medical Device Applied to Uterine Fibroids Morcellation: Analysis of Critical Biological Issues and Drawbacks from A Medical-Legal Prospective. Curr. Pharm. Des. 2020, 26, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S.; Zupi, E.; Lazzeri, L.; Stark, M.; Malvasi, A.; Signore, F.; Marinelli, E. Medicolegal Issues in Power Morcellation: Cautionary Rules for Gynecologists to Avoid Unfavorable Outcomes. J. Minim. Invasive Gynecol. 2020, 27, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Committee Opinion No. 696: Nonobstetric Surgery During Pregnancy. Obstet. Gynecol. 2017, 129, 777–778. [CrossRef] [PubMed]
- Basso, A.; Catalano, M.R.; Loverro, G.; Nocera, S.; Di Naro, E.; Loverro, M.; Natrella, M.; Mastrolia, S.A. Uterine Fibroid Torsion during Pregnancy: A Case of Laparotomic Myomectomy at 18 Weeks’ Gestation with Systematic Review of the Literature. Case Rep. Obstet. Gynecol. 2017, 2017, 4970802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moruzzi, M.C.; Moro, F.; Bolomini, G.; Macchi, C.; Cavaliere, A.F.; Fagotti, A.; Scambia, G.; Testa, A.C. Intraoperative ultrasound assistance during myomectomy in pregnant woman. Ultrasound Obstet. Gynecol. 2020, 55, 840–841. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).