Mapping Biological Risks Related to Necropsy Activities: Old Concerns and Novel Issues for the Safety of Health Professionals
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaires
2.2. Cadaveric Samples
- ▪
- a search for common germs and fungi; each sample was seeded on general (blood Agar) and specific (Mac Conkey, Sabouraud, Mannitol Salt Agar) solid media and incubated for 24–48 h at 37 °C; the quantification of microorganisms was expressed in Colony Forming Units (CFU)/mL;
- ▪
- a search for alcohol-acid fast bacilli (direct and cultural examination); each sample, after an adequate decontamination and fluidization procedure, was subjected to direct examination with Ziehl-Neelsen staining and subsequent incubation for 40 days at 37 °C on a specific medium for mycobacteria (Lowenstein-Jensen).
2.3. Environmental Samples
2.4. Research of SARS-CoV-2
3. Results
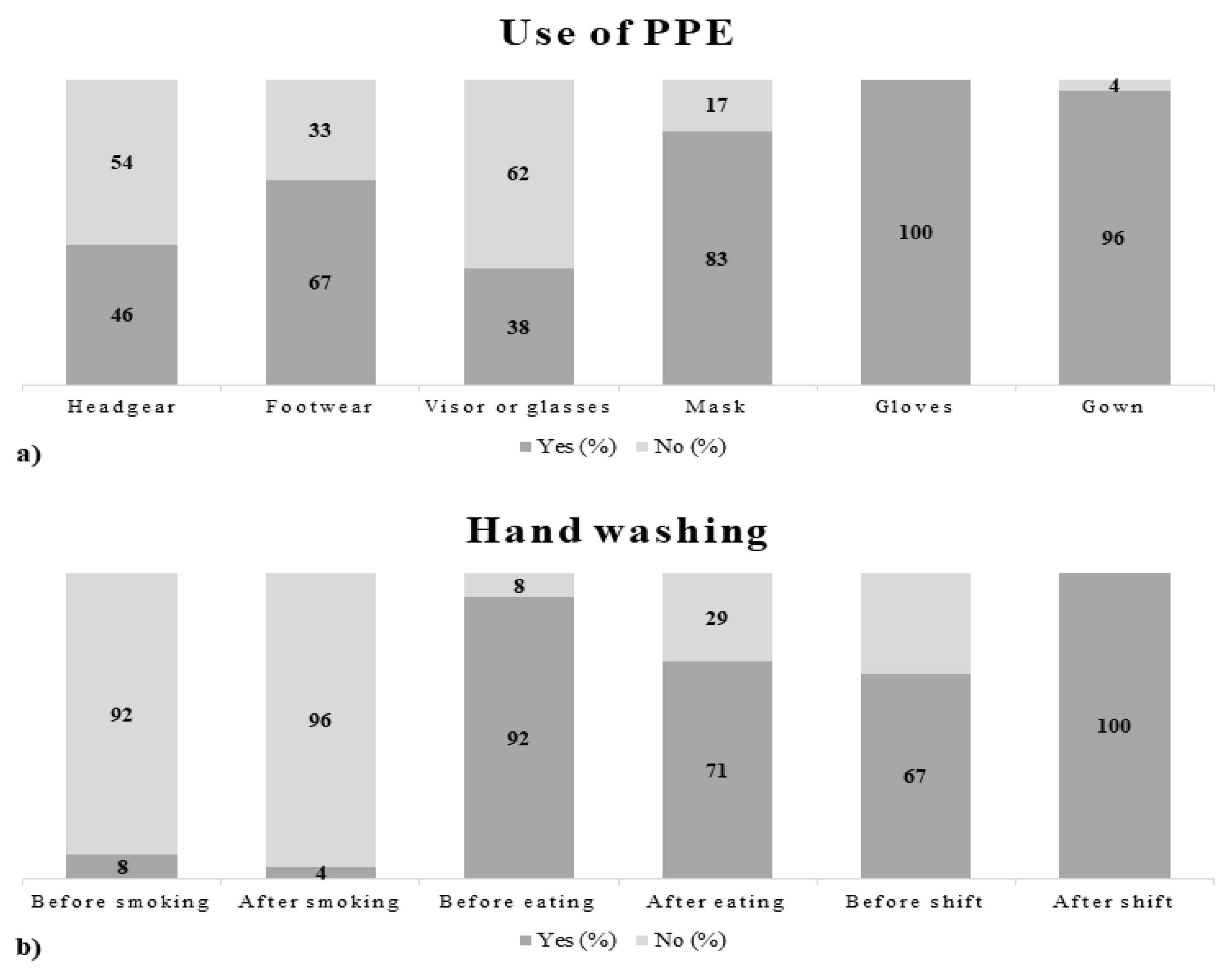
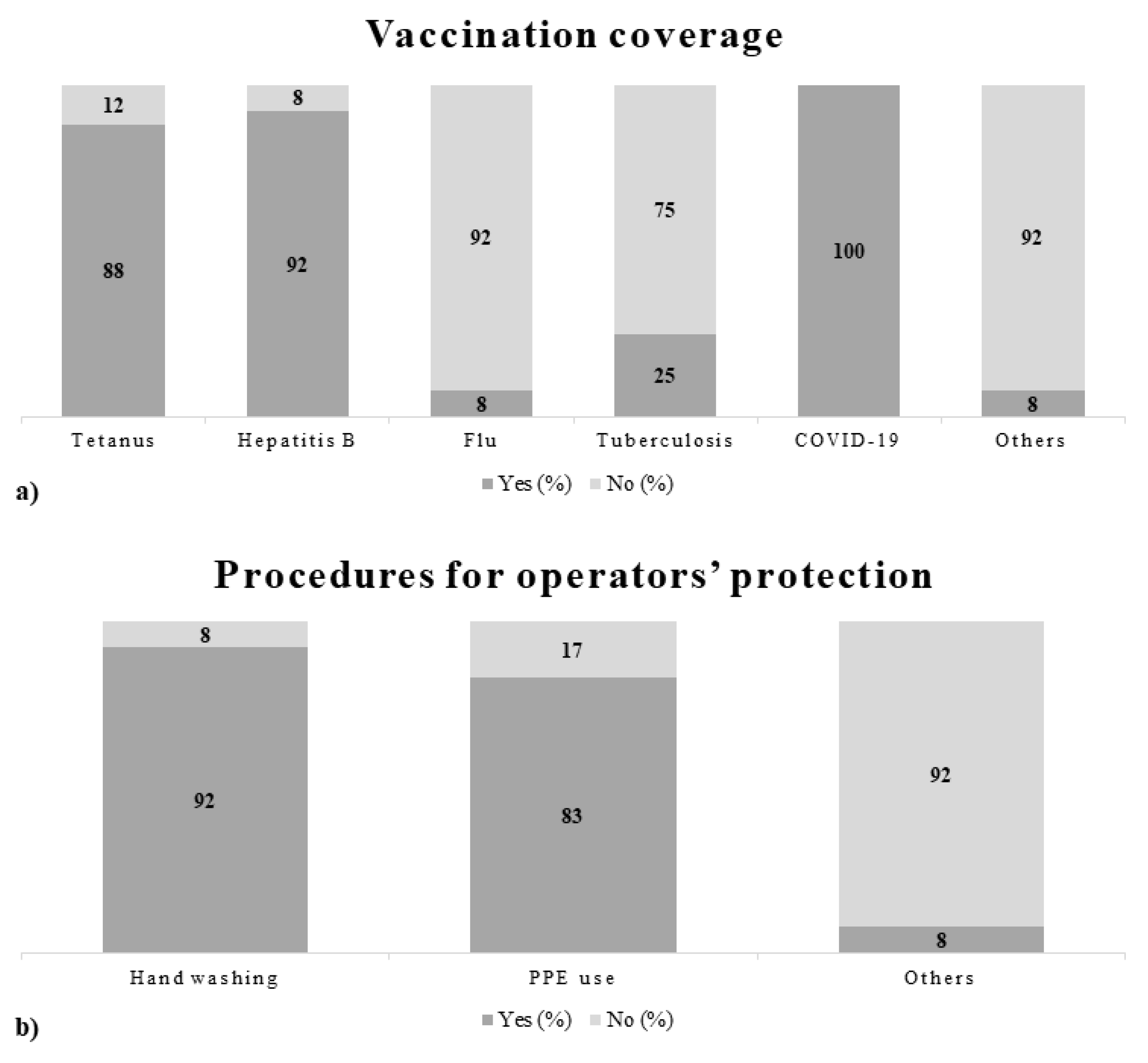
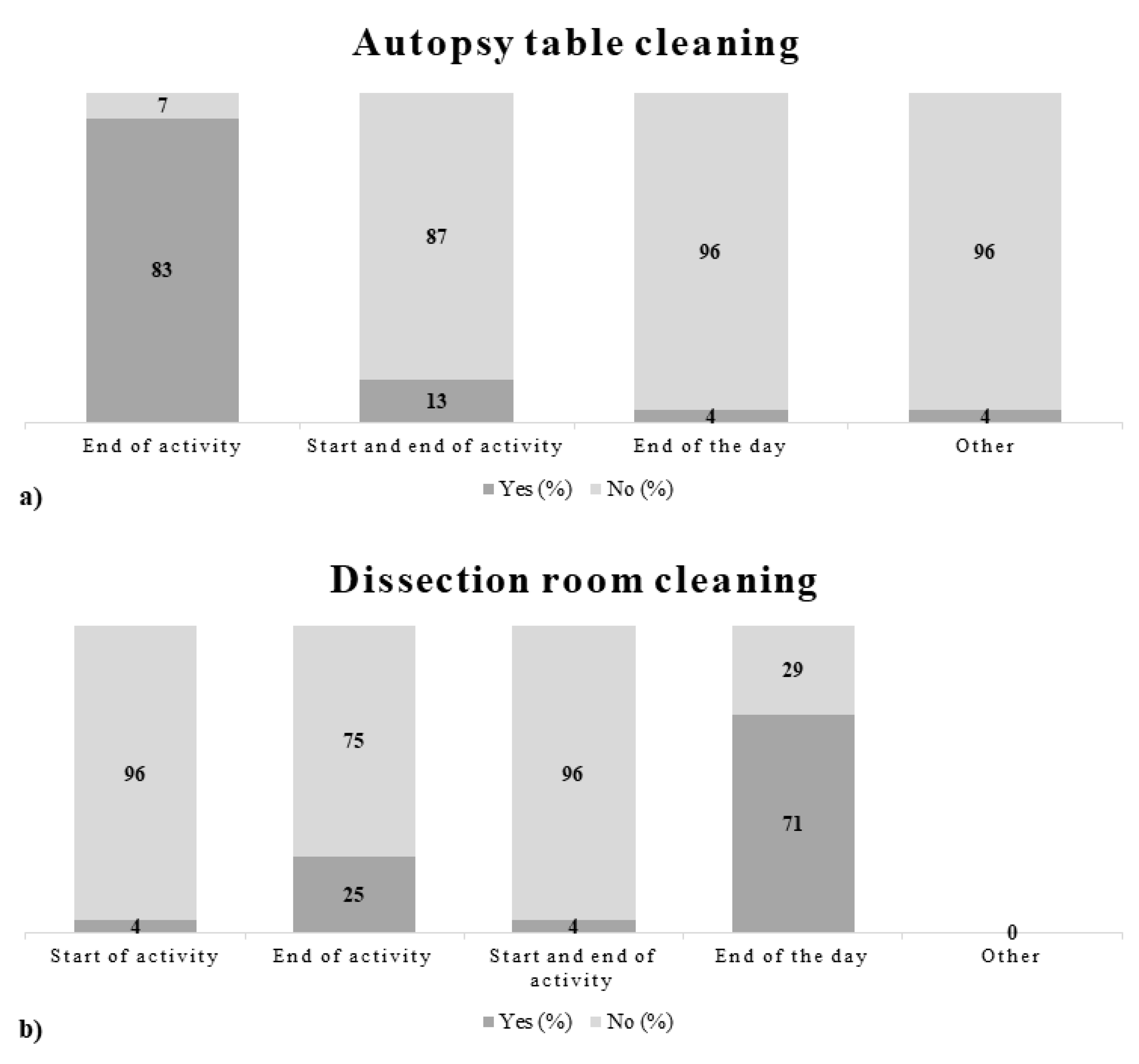
3.1. Questionnaires
3.2. Cadaveric Samples
3.3. Environmental Samples
3.4. Research of SARS-CoV-2
4. Discussion
- ▪
- the opening of the cranial cavity and the extraction of the brain are particularly risky because of the release of bone dust at risk of inhalation, the possibility of injury by bone fragments with sharp edges (fracture lesions or section surfaces);
- ▪
- the opening of the thoracic cavity can be risky owing to the possible presence of metal sutures, fracture lesions that are not easily visible;
- ▪
- the opening of the abdominal cavity with extraction of the organs can involve risks from the possible presence of foreign bodies of a medical nature or of other origin (metal fragments, splinters, retained projectiles, etc.) as well as the possible presence of bone stumps from any fracture, especially in the pelvis.
- ▪
- the anatomopathological activity consists of cases mostly studied clinically;
- ▪
- the forensic activity involves exposure to different and additional risks (contaminated corpses, foreign bodies, etc.).
4.1. Questionnaires
4.2. Cadaveric Samples
4.3. Environmental Samples
4.4. Research of SARS-CoV-2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Guidance on Regulations for the Transport of Infectious Substances 2021–2022; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Gańczak, M.; Boroń-Kaczmarska, A.; Dziuba, I. Pathologist and HIV—Are safe autopsies possible? Pol. J. Pathol. 2003, 54, 143–146. [Google Scholar]
- Bagheri Amiri, F.; Mostafavi, E.; Mirzazadeh, A. HIV, HBV and HCV Coinfection Prevalence in Iran--A Systematic Review and Meta-Analysis. PloS one. 2016, 11(3), e0151946. [Google Scholar] [CrossRef] [PubMed]
- Flavin, R.J.; Gibbons, N.; O’Briain, D.S. Mycobacterium tuberculosis at autopsy—Exposure and protection: An old adversary revisited. J. Clin. Pathol. 2007, 60, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Nolte, K.B.; Taylor, D.G.; Richmond, J.Y. Biosafety considerations for autopsy. Am. J. Forensic Med. Pathol. 2002, 23, 107–122. [Google Scholar] [CrossRef]
- Teppo, L.; Ojajärvi, J.; Brander, E. The tuberculosis morbidity among pathologists in Finland. Scand. J. Respir. Dis. 1974, 55, 257–261. [Google Scholar] [PubMed]
- Safar, J.G.; Geschwind, M.D.; Deering, C.; Didorenko, S.; Sattavat, M.; Sanchez, H.; Serban, A.; Vey, M.; Baron, H.; Giles, K.; et al. Diagnosis of human prion disease. Proc. Natl. Acad. Sci. USA 2005, 102, 3501–3506. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M. Preventing accidental transmission of human transmissible spongiform encephalopathies. Br. Med. Bull. 2003, 66, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, E.M.; Williams, I.T.; Shapiro, C.N.; Chamberland, M.E. Risk and management of blood-borne infections in health care workers. Clin. Microbiol. Rev. 2000, 13, 385–407. [Google Scholar] [CrossRef]
- Morgan, E.; Skaathun, B.; Schneider, J.A. Sexual, Social, and Genetic Network Overlap: A Socio-Molecular Approach Toward Public Health Intervention of HIV. Am. J. Public Health 2018, 108, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Redondo, R.; Ozer, E.A.; Achenbach, C.J.; D’Aquila, R.T.; Hultquist, J.F. Molecular epidemiology in the HIV and SARS-CoV-2 pandemics. Curr. Opin. HIV AIDS 2021, 16, 11–24. [Google Scholar] [CrossRef]
- Hemelaar, J.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Fleminger, I.; Kirtley, S.; Williams, B.; Gouws-Williams, E.; Ghys, P.D.; WHO–UNAIDS Network for HIV Isolation Characterisation. Global and regional molecular epidemiology of HIV-1, 1990–2015: A systematic review, global survey, and trend analysis. Lancet Infect. Dis. 2019, 19, 143–155. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A. Occupational Health Update: Focus on Preventing the Acquisition of Infections with Pre-exposure Prophylaxis and Postexposure Prophylaxis. Infect. Dis. Clin. N. Am. 2016, 30, 729–757. [Google Scholar] [CrossRef]
- Kritselis, M.; Remick, D.G. Universal Precautions Provide Appropriate Protection during Autopsies of Patients with Infectious Diseases. Am. J. Pathol. 2020, 190, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Ciesielka, M.; Buszewicz, G.; Maciejewski, R.; Budzyńska, B.; Listos, P.; Teresiński, G. COVID-19 in the autopsy room-requirements, safety, recommendations and pathological findings. Forensic Sci. Med. Pathol. 2021, 17, 101–113. [Google Scholar] [CrossRef]
- Nolte, K.B.; Muller, T.B.; Denmark, A.M.; Burstein, R.; Villalobos, Y.A. Design and Construction of a Biosafety Level 3 Autopsy Laboratory. Arch. Pathol. Lab. Med. 2021, 145, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Dolman, G.E.; Koffas, A.; Phipps, E.; Kennedy, P.T.F. Clinical and occupational health management of healthcare workers living with chronic hepatitis B: UK policy and international comparisons. J. Viral Hepat. 2021, 28, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Falzone, L.; Rapisarda, V.; Marconi, A.; Cinà, D.; Fenga, C.; Spandidos, D.A.; Libra, M. The risk of HCV infection among health-care workers and its association with extrahepatic manifestations. Mol. Med. Rep. 2017, 15, 3336–3339. [Google Scholar] [CrossRef][Green Version]
- Fritzsche, F.R.; Ramach, C.; Soldini, D.; Caduff, R.; Tinguely, M.; Cassoly, E.; Moch, H.; Stewart, A. Occupational health risks of pathologists—Results from a nationwide online questionnaire in Switzerland. BMC Public Health 2012, 6, 1054. [Google Scholar] [CrossRef]
- Ogden, T.L. The ISO and ACGIH Standardized Size Fractions and their Relation to Human Deposition Data. Ann. Occup. Hyg. 1988, 32, 413–421. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Sanna, T.; Dallolio, L.; Raggi, A.; Mazzetti, M.; Lorusso, G.; Zanni, A.; Farruggia, P.; Leoni, E. ATP bioluminescence assay for evaluating cleaning practices in operating theatres: Applicability and limitations. BMC Infect. Dis. 2018, 18, 583. [Google Scholar] [CrossRef]
- Santurro, A.; Scopetti, M.; D’Errico, S.; Fineschi, V. A technical report from the Italian SARS-CoV-2 outbreak. Postmortem sampling and autopsy investigation in cases of suspected or probable COVID-19. Forensic Sci. Med. Pathol. 2020, 16, 471–476. [Google Scholar] [CrossRef]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef]
- Legislative Decree no. 81 of 9 April 2008—Consolidated Law on Safety in the Workplace. Available online: http://www.cip.srl/documenti/Testo%20Unico%20Salute%20e%20Sicurezza%20sul%20lavoro%20-%20D.lgs.%2081-2008.pdf (accessed on 18 August 2021).
- Health Council of the Netherlands. Endotoxins. Health-Based Recommended Occupational Exposure Limit. Publication no. 2010/04OSH, 2010. Available online: https://www.healthcouncil.nl/binaries/healthcouncil/documents/advisory-reports/2010/07/15/endotoxins-health-based-recommended-occupational-exposure-limit/advisory-report-endotoxins-health-based-recommended-occupational-exposure-limit.pdf (accessed on 15 July 2021).
- Nolte, K.B.; Yoon, S.S. Theoretical risk for occupational blood-borne infections in forensic pathologists. Infect. Control Hosp. Epidemiol. 2003, 24, 772–773. [Google Scholar] [CrossRef]
- Pluim, J.M.E.; Jimenez-Bou, L.; Gerretsen, R.R.R.; Loeve, A.J. Aerosol production during autopsies: The risk of sawing in bone. Forensic Sci. Int. 2018, 289, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Gerston, K.F.; Blumberg, L.; Tshabalala, V.A.; Murray, J. Viability of mycobacteria in formalin-fixed lungs. Hum. Pathol. 2004, 35, 571–575. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services, Centers for Disease Control, and National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories; US Department of Health and Human Services: Washington, DC, USA, 1999; pp. 1–250. Available online: http://www.cdc.gov/biosafety/publications/bmbl5/index.htm (accessed on 18 July 2021).
- Brooks, E.G.; Utley-Bobak, S.R. Autopsy Biosafety: Recommendations for Prevention of Meningococcal Disease. Acad. Forensic Pathol. 2018, 8, 328–339. [Google Scholar] [CrossRef]
- Ortega, R.; Gonzalez, M.; Nozari, A.; Canelli, R. Personal Protective Equipment and Covid-19. N. Engl. J. Med. 2020, 382, e105. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Rutherford, G.W. Facial Masking for Covid-19—Potential for “Variolation” as We Await a Vaccine. N. Engl. J. Med. 2020, 383, e101. [Google Scholar] [CrossRef]
- Burton, J.L. Health and safety at necropsy. J. Clin. Pathol. 2003, 56, 254–260. [Google Scholar] [CrossRef]
- Gharehdaghi, J.; Abedi Khorasgani, M.H.; Ghadiani, M.H.; Kazemifar, A.M.; Solhi, H.; Solhi, S. Prevalence of HCV, HBV, and HIV Seropositivity among Cadavers Referred to Autopsy Hall of Legal Medicine Bureau of Tehran, Iran. Adv. Prev. Med. 2017, 2017, 2043840. [Google Scholar] [CrossRef]
- Giraldi, G.; Montesano, M.; Napoli, C.; Frati, P.; La Russa, R.; Santurro, A.; Scopetti, M.; Orsi, G.B. Healthcare-Associated Infections Due to Multidrug-Resistant Organisms: A Surveillance Study on Extra Hospital Stay and Direct Costs. Curr. Pharm. Biotechnol. 2019, 20, 643–652. [Google Scholar] [CrossRef]
- Gatto, V.; Scopetti, M.; La Russa, R.; Santurro, A.; Cipolloni, L.; Viola, R.V.; Di Sanzo, M.; Frati, P.; Fineschi, V. Advanced Loss Eventuality Assessment and Technical Estimates: An Integrated Approach for Management of Healthcare-Associated Infections. Curr. Pharm. Biotechnol. 2019, 20, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Ünal, Ö. During COVID-19, which is more effective in work accident prevention behavior of healthcare professionals: Safety awareness or fatalism perception? Work 2020, 67, 783–790. [Google Scholar] [CrossRef]
- Blokker, B.M.; Wagensveld, I.M.; Weustink, A.C.; Oosterhuis, J.W.; Hunink, M.G. Non-invasive or minimally invasive autopsy compared to conventional autopsy of suspected natural deaths in adults: A systematic review. Eur. Radiol. 2016, 26, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Dirnhofer, R.; Jackowski, C.; Vock, P.; Potter, K.; Thali, M.J. VIRTOPSY: Minimally invasive, imaging-guided virtual autopsy. Radiographics 2006, 26, 1305–1333. [Google Scholar] [CrossRef]
- Jackowski, C.; Sonnenschein, M.; Thali, M.J.; Aghayev, E.; von Allmen, G.; Yen, K.; Dirnhofer, R.; Vock, P. Virtopsy: Postmortem minimally invasive angiography using cross section techniques--implementation and preliminary results. J. Forensic Sci. 2005, 50, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Pomara, C.; Fineschi, V.; Scalzo, G.; Guglielmi, G. Virtopsy versus digital autopsy: Virtuous autopsy. Radiol. Med. 2009, 114, 1367–1382. [Google Scholar] [CrossRef]
- De Marco, E.; Vacchiano, G.; Frati, P.; La Russa, R.; Santurro, A.; Scopetti, M.; Guglielmi, G.; Fineschi, V. Evolution of post-mortem coronary imaging: From selective coronary arteriography to post-mortem CT-angiography and beyond. Radiol. Med. 2018, 123, 351–358. [Google Scholar] [CrossRef]
- La Russa, R.; Catalano, C.; Di Sanzo, M.; Scopetti, M.; Gatto, V.; Santurro, A.; Viola, R.V.; Panebianco, V.; Frati, P.; Fineschi, V. Postmortem computed tomography angiography (PMCTA) and traditional autopsy in cases of sudden cardiac death due to coronary artery disease: A systematic review and meta-analysis. Radiol. Med. 2019, 124, 109–117. [Google Scholar] [CrossRef]
- Boariu, D.I.; Armean, P. Role of Risk Assessment in Prevention of Work-Related Accidents and Diseases in Hospital Staff. J. Med. Life 2020, 13, 410–417. [Google Scholar] [PubMed]
- Loibner, M.; Langner, C.; Regitnig, P.; Gorkiewicz, G.; Zatloukal, K. Biosafety Requirements for Autopsies of Patients with COVID-19: Example of a BSL-3 Autopsy Facility Designed for Highly Pathogenic Agents. Pathobiology 2021, 88, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, V.; Aprile, A.; Aquila, I.; Arcangeli, M.; Asmundo, A.; Bacci, M.; Cingolani, M.; Cipolloni, L.; D’Errico, S.; De Casamassimi, I.; et al. Management of the corpse with suspect, probable or confirmed COVID-19 respiratory infection - Italian interim recommendations for personnel potentially exposed to material from corpses, including body fluids, in morgue structures and during autopsy practice. Pathologica 2021, 112(2), 64–77. [Google Scholar]
- Frisoni, P.; Neri, M.; D’Errico, S.; Alfieri, L.; Bonuccelli, D.; Cingolani, M.; Di Paolo, M.; Gaudio, R.M.; Lestani, M.; Marti, M.; et al. Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: From viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci. Med. Pathol. 2021, 1–15. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Fossarello, M.; d’Aloja, E. Autopsies and asymptomatic patients during the COVID-19 pandemic: Balancing risk and reward. Front. Public Health 2020, 8, 595405. [Google Scholar] [CrossRef]
- Hanley, B.; Lucas, S.B.; Youd, E.; Swift, B.; Osborn, M. Autopsy in suspected COVID-19 cases. J. Clin. Pathol. 2020, 73, 239–242. [Google Scholar] [CrossRef]
- Del Fante, Z.; Di Fazio, N.; Papale, A.; Tomao, P.; Del Duca, F.; Frati, P.; Fineschi, V. Evaluation of Physical Risk during Necropsy and Morgue Activities as Risk Management Strategy. Int. J. Environ. Res. Public Health 2021, 18, 8266. [Google Scholar] [CrossRef] [PubMed]

| Areas and Equipment Sampled |
|---|
| 90 morgue refrigeration units (+5 °C) |
| UV lamps for room disinfection |
| Full-air ventilation system with emission filters capable of ensuring 12.5 changes/h |
| No. 3 autopsy tables complete with accessories |
| 1 refrigerator (+4 °C) and 1 freezer (−20 °C) |
| Autoclave |
| Hood for histology complete with accessories |
| Vacuum tissue processing machine |
| Semi-automatic microtome and automatic colorator |
| Microscope |
| B-βCoV (Target E Gene) | SARS-CoV-2 (Target S Gene) | Internal Control | Result Interpretation |
|---|---|---|---|
| + | + | + | Positive: B-βCoV (target E gene) and SARS-CoV-2 (target S gene) specific RNA detected |
| + | - | + | Positive: B-βCoV (target E gene) specific RNA detected |
| − | + | + | Positive: SARS-CoV-2 (target S gene) specific RNA detected |
| − | − | + | Negative: Neither B-βCoV (target E gene) nor SARS-CoV-2 (target S gene) specific RNA detected |
| Cases (No.) | Microorganisms | CFU |
|---|---|---|
| 1 | Candida spp. Stenotrophomonas maltophilia | >106 CFU/mL >106 CFU/mL |
| 1 | non-MDR Klesbiella pneumoniae | 104 CFU/mL |
| 2 | non-MDR Klesbiella pneumoniae Candida spp. | >106 CFU/mL 102 CFU/mL |
| 1 | Methicillin-susceptible Staphylococcus aureus | 104 CFU/mL |
| 1 | non-MDR Klesbiella pneumoniae Pseudomonas aeruginosa | 104 CFU/mL 104 CFU/mL |
| Activity | Bacteria |
|---|---|
| Skull opening | Staphylococcus aureus |
| Thoracic cavity opening | Escherichia coli, Staphylococcus aureus, Enterococcus faecalis |
| Abdominal cavity opening | Escherichia coli, Enterobacter cloacae, Klebsiella oxytoca, Staphylococcus aureus, Enterococcus faecalis |
| Event | Related Activities | Bioaerosol Exposure Risk | Fluid Exposure Risk | Impact of PPE on Risk Prevention | ||||
|---|---|---|---|---|---|---|---|---|
| Headgear | Visor or Glasses | Mask | Gown | Footwear | ||||
| Acupuncture | Mobilization of the corpse, blood collection, tissue sampling | None | High | High | Low | Low | Low | Medium |
| Cut | Cavities opening, tissue sampling | None | High | High | Low | Low | Low | Medium |
| Jetting and leaking of biological fluid and organic material | External examination, cavities opening, tissue sampling, washing of stretchers | Low | High | High | Low | High | High | High |
| Sample droplets | Cavities opening, tissue sampling, washing of stretchers | High | Medium | Medium | Low | High | High | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomao, P.; La Russa, R.; Oliva, A.; De Angelis, M.; Mansi, A.; Paba, E.; Marcelloni, A.M.; Chiominto, A.; Padovano, M.; Maiese, A.; et al. Mapping Biological Risks Related to Necropsy Activities: Old Concerns and Novel Issues for the Safety of Health Professionals. Int. J. Environ. Res. Public Health 2021, 18, 11947. https://doi.org/10.3390/ijerph182211947
Tomao P, La Russa R, Oliva A, De Angelis M, Mansi A, Paba E, Marcelloni AM, Chiominto A, Padovano M, Maiese A, et al. Mapping Biological Risks Related to Necropsy Activities: Old Concerns and Novel Issues for the Safety of Health Professionals. International Journal of Environmental Research and Public Health. 2021; 18(22):11947. https://doi.org/10.3390/ijerph182211947
Chicago/Turabian StyleTomao, Paola, Raffaele La Russa, Alessandra Oliva, Massimiliano De Angelis, Antonella Mansi, Emilia Paba, Anna Maria Marcelloni, Alessandra Chiominto, Martina Padovano, Aniello Maiese, and et al. 2021. "Mapping Biological Risks Related to Necropsy Activities: Old Concerns and Novel Issues for the Safety of Health Professionals" International Journal of Environmental Research and Public Health 18, no. 22: 11947. https://doi.org/10.3390/ijerph182211947
APA StyleTomao, P., La Russa, R., Oliva, A., De Angelis, M., Mansi, A., Paba, E., Marcelloni, A. M., Chiominto, A., Padovano, M., Maiese, A., Scopetti, M., Frati, P., & Fineschi, V. (2021). Mapping Biological Risks Related to Necropsy Activities: Old Concerns and Novel Issues for the Safety of Health Professionals. International Journal of Environmental Research and Public Health, 18(22), 11947. https://doi.org/10.3390/ijerph182211947







