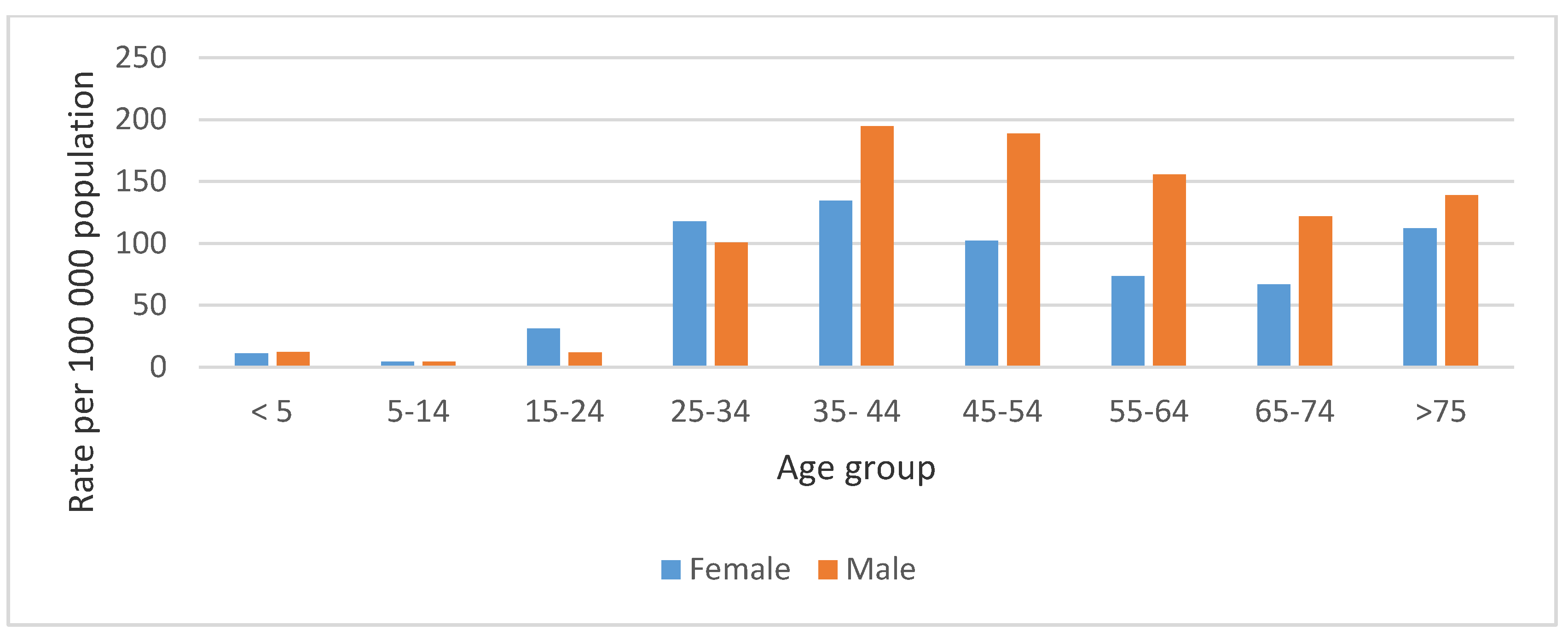1. Introduction
Tuberculosis has been a preventable disease since 1921 when the Bacillus Calmette-Guerin (BCG) vaccine was discovered, but it still poses a major global health problem. Annually, the number of TB prevalent cases is approximately 10 million people globally [
1] with more than one million deaths attributed to TB [
2]. Most of these deaths are in low-income countries. South Africa remains one of the most TB burdened countries with an estimated annual TB incidence of 301,000 cases in 2018. In 2019, the World Health Organisation estimated the mortality rate of all forms of TB for South Africa, excluding deaths among those coinfected with TB/HIV, to be 37 and 73 per 100,000 population, respectively [
1]. It is estimated that there is a new infection with the TB bacterium every second globally [
3]. For almost a decade, TB has caused more deaths than HIV/Acquired Immunodeficiency Syndrome (HIV/AIDS), making it the single leading cause of death from an infectious disease [
1].
South Africa is one of the most unequal countries in the world with respect to income variation with a large proportion of South Africans who are affected by extreme poverty. Almost half (45%) of the South African population is still living on less than USD 2 per day. Poverty has affected the health of most South Africans predominantly due to an inability to access the basic requirements of life; for instance, sufficient nutrition, adequate sanitation, and appropriate housing conditions [
4,
5,
6,
7]. Household crowding, usually because of poverty, has been related to diseases of the respiratory system such as TB [
8]. South Africa has one of the world’s worst TB epidemics, which is fueled by HIV [
9]. It has been shown that for HIV-infected individuals with clinically symptomatic TB, the risk of death is about three to seven-fold higher than in those individuals who are infected with TB but are HIV negative [
10,
11], whereas in those individuals with AIDS, developing TB will increase the overall mortality by one-third [
12].
Therefore, this research aims to contribute to TB control programs in South Africa by showing the value of understanding the spatial distribution of TB deaths in South Africa. There is a lack of previous research papers on TB spatial analysis and regression. Furthermore, the study aims to confirm an association between TB, Social Economic Status (SES) (using the South African Multidimensional Poverty Index), and HIV.
2. Materials and Methods
2.1. Data Sources
This research used secondary data from four data sources, namely, mortality and causes of death in South Africa from Statistics South Africa, the South African Multidimensional Poverty Index (SAMPI) from Statistics South Africa, HIV data from the South African national Department of Health, and census and mid-year population estimates from Statistics South Africa. In South Africa, national mortality statistics are captured in the civil registration system. In recent years, the country has adopted the Africa Programme on Accelerated Improvement of Civil Registration and Vital Statistics (APAI-CRVS) [
13]. The mortality data are based on death notification forms (Form BI-1663) that are submitted to the Department of Home Affairs offices for death registration as a requirement by the country’s Births and Deaths Registration Act No 51 of 1992 [
14]. We extracted data on TB deaths from the civil registration system in South Africa. We used ICD-10 codes A16-A19 for TB deaths.
SAMPI was constructed by Statistics South Africa to profile poverty at a household level using variables from censuses from the years 2001 and 2011. The SAMPI score is derived from the product of the headcount, and the intensity of the poverty experienced by households [
15]. The antenatal HIV prevalence data for 2010 were obtained from the national Department of Health of South Africa.
2.2. Methods of Data Analysis
We first conducted a descriptive epidemiological analysis of TB deaths for the period 2005–2015 with respect to gender, age group, provinces, and district municipalities in South Africa. This was followed by a spatial autocorrelation analysis and a spatial lag regression (also known as the spatial autoregressive (SAR)) model of TB deaths for 2010. Spatial autocorrelation was conducted to establish the degree of similarity between TB death rates in a district municipality to TB death rates in neighbouring district municipalities. We conducted a spatial regression analysis (SAR) to investigate a potential association in the spatial variations of TB deaths (dependent variable), in instances where there were hot spot clusters of district municipalities. The independent variables for this study were the SAMPI and HIV.
To enable comparison of TB mortality for population groups that may have a different age structure, as may be the case for South Africa’s district municipalities, age-sex-standardised rates were computed. The direct standardisation technique was used to derive the age-sex-standardised TB death rates using the age distribution of the 2011 population census as the standard age structure.
The presence of spatial autocorrelation was determined using both Global and Local Moran’s Indices methods (Moran’s I) [
16]. Suppose that
are observations of the TB mortality rates for the
districts in South Africa for the year 2010. We also have
as an adjacency matrix, which quantifies the connection between the districts. Global Moran’s I is defined as follows:
where
and
. One approach is to define a neighboring district would be when it shares a boundary (i.e., contiguous districts) or it is within a certain distance or it100 km) or it is among the nearest district (e.g., the 3 closest districts). For this study, we adopt the first definition where we take
if the districts share a boundary, otherwise it is 0. By its construction, the Global Moran’s
varies from −1 to +1, where positive values would mean that the districts close together had similar TB mortality rates, while negative values would means that districts close together had more dissimilar rates than those districts further away. For local spatial dependence, the Local Moran’s
for district
defined as
A positive Local Moran’s would indicate that ditrsict has neighboring districts with similarly high or low TB mortality rates.
To model the incident of TB related mortality using predictors SAMPI and HIV, the spatial lag regression (also known as the spatial autoregressive (SAR)) model was used. The SAR model accounts for dependence between TB deaths data in a municipality and neighboring municipalities’ TB mortality data. Under the normality assumption on Y, the spatial lag model is defined as [
16].
where
,
is the spatial lag parameter which measures the strength of spatial dependence in the TB death rates, vector
is covariate vector consisting of poverty, and HIV burden, and they are associated with a
vector
of regression parameters. Most GIS packages default to using row-standardized weights, with
, in which the spatially lagged predictor would be the average of the TB mortality rates of neighboring districts. Thus, the SAR model adds a spatially averaged vector as a covariate, reflecting the TB mortality data from the neighbouring districts to aid in explaining the variation in the TB death rates between the districts. Spatial analysis was performed using the Geographic Data Analysis (GeoDa) version 1.14.
3. Results
The mean annual TB death rate for the study period was 142.2 per 100,000 population, 95% CI [114.2, 170.2]. Overall, there was a statistically significant decrease in TB deaths of less than 1 TB death (0.01) per 100 000 population annually at 5% significance (F (1,10) = 93.58,
p = 0.001) for the period 2005–2015. The mean annual TB death rates for males and females were 161.5 per 100,000 population, 95% CI [132.9, 190.0] and 123.2 per 100,000 population, 95% CI [95.6, 150.8], respectively. The annual TB death rate for both male and females decreased over the study period (
Table 1).
The highest mean annual TB death rate for both males and females was recorded in the 35–44 age group, 194.7 per 100,000 population (129,724 deaths), and 134.3 per 100,000 population (89,480 deaths), respectively (
Figure 1). The mean annual TB death rate was also highest in the Black African population group at 136.3 per 100,000 population, followed by the Coloured population group at 63.8 per 100,000 population for the period of study.
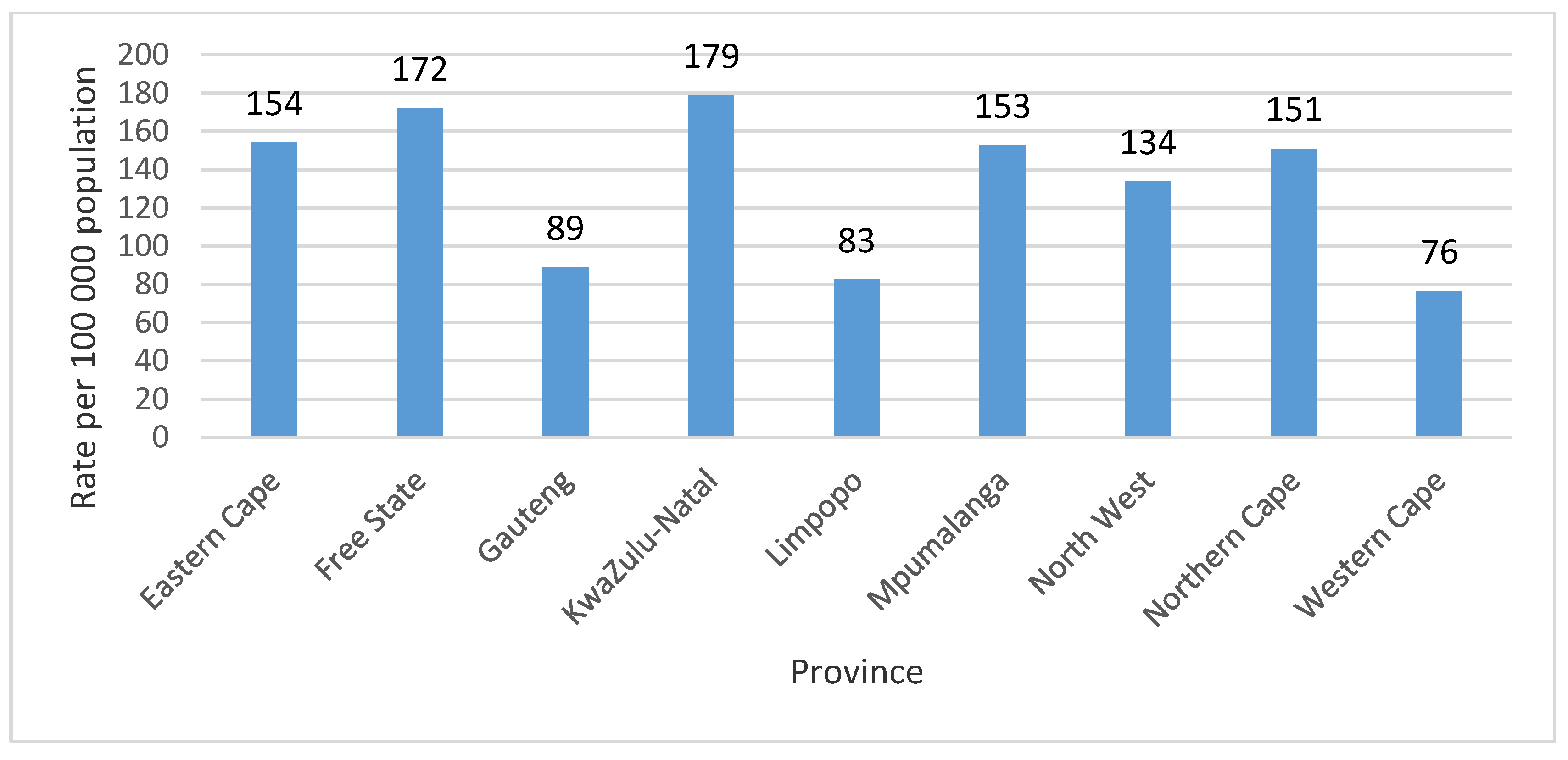
For the study period, the mean annual TB death rate for the provinces was 132.3 per 100,000 population at 95% CI [101.9, 162.4]. The highest mean annual TB death rate for the period 2005–2015 was recorded in the KwaZulu-Natal province at 178.9 per 100,000 population, closely followed by Free State province at 172.1 per 100,000 population (53,940 TB deaths). The Western Cape province recorded the lowest mean annual TB death rate 76.5 per 100,000 population (45,513 TB deaths) for the period of study (
Figure 2).
The mean annual TB death rate for the district municipalities for the study period was 155.5 per 100,000 population at 95% CI [139.6, 171.4]. Ugu district municipality had the highest mean annual TB death rate of 312.0 per 100,000 population (22,722 TB deaths); followed by Amathole district municipality 233.1 per 100,000 population (21,791 TB deaths). Vhembe and Overberg district municipalities had the lowest mean annual TB death rate 66.1 per 100,000 population (9348 TB deaths) and 66.3 per 100,000 population (1800 TB deaths), respectively, for the period of study.
3.1. Spatial Autocorrelation
The results for both the Moran’s I and Z values were positive and greater than zero for the overall, male, and female age-sex-standardised TB death rates. In addition, the results were significant at the 99% confidence interval. These results are indicative of a presence of autocorrelation for the overall, male, and female age-sex-standardised TB death rates. Therefore, there was significant similarity between neighbouring district municipalities for the overall, male, and female age-sex-standardised TB death rates (
Table 2).
3.2. Local Spatial Clustering for TB Death Rates
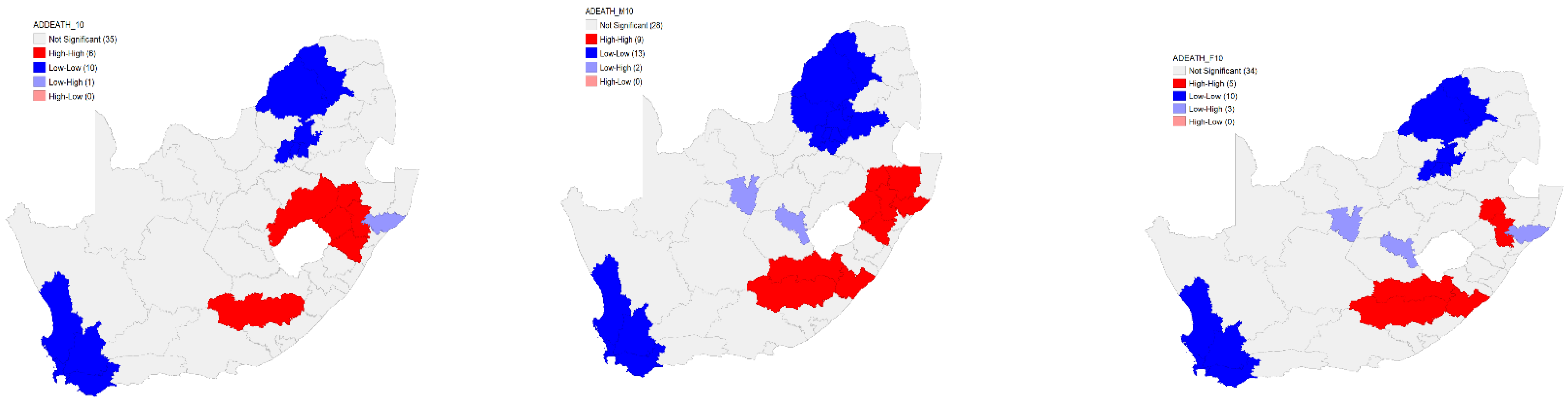
The Local Moran’s I analysis for clustering for the overall age-sex-standardised TB death rates revealed six high–high clusters. Ten low–low clusters for the overall age-sex-standardised TB death rates were identified. Furthermore, the Local Moran’s I analysis identified one low–high cluster. However, there were no high–low clusters that were identified for the overall age-sex-standardised TB death rates in 2010 (
Figure 3).
For the male, nine high–high clusters were identified on the eastern side of the country. In addition to the high–high clusters, 13 low–low clusters for the male age-sex-standardised TB death rates were identified in 2010. Furthermore, the Local Moran’s I clustering analysis identified two low–high with no high–low clusters identified for the male age-sex-standardised TB death rates (
Figure 3).
In 2010, the results of the Local Moran’s I analysis for clustering for the female age-sex-standardised TB death rates in 2010 identified five high–high clusters located on the eastern part of the country. Furthermore, the Local Moran’s I revealed a presence of 10 low–low clusters. The results further revealed a presence of three low–high clusters. There were no high–low clusters identified for the female age-sex-standardised TB death rates (
Figure 3).
3.3. Intensity of TB Death Rates (Hot Spots)
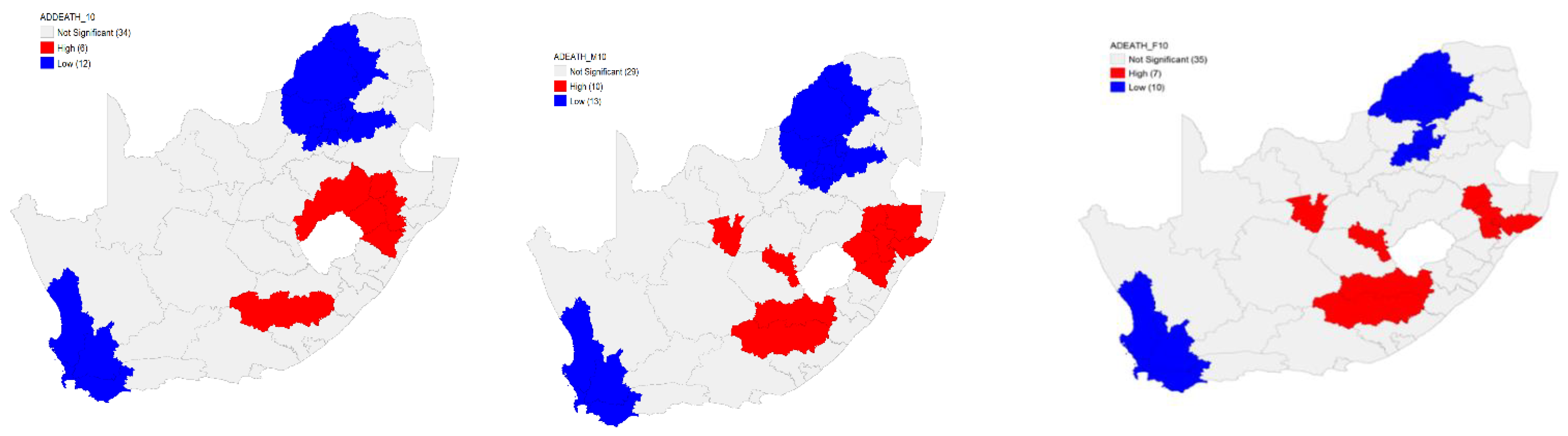
Six hot spots were identified in South Africa by the Local G (hot spots) analysis for the overall age-sex-standardised TB death rates in 2010. These hot spots were on the eastern side of the country, located in three provinces. Twelve cold spots were identified. In other words, the results revealed a high risk of TB deaths in 2010 in the South African population on the eastern part of the country with a lower risk in the northern and southern parts (
Figure 4).
The Local G hot spots analysis identified 10 hot spots for the male age-sex-standardised TB death rates. These hot spots were in the central part with the majority on the eastern side of the country. Analysis further revealed a presence of 13 cold spots in 13 district municipalities in the country. These results indicate that, in 2010, there was a high risk of TB deaths in the male in the South African population on the eastern and central parts of the country and a lower risk in the northern and southern parts (
Figure 4).
There were seven hot spots for the female age-sex-standardised TB death rates identified by the Local G hot spots analysis. Many hot spots were located on the eastern side of the country. Analysis further revealed a presence of 10 cold spots in the country (
Figure 4). The Local G hot spots analysis was able to identify more hot spots than the Local Moran’s for both the male and female age-sex-standardised TB death rates.
3.4. Spatial Regression Analysis
Spatial lag regression analysis for the overall age-sex-standardised TB death rates identified a positive association between the overall age-sex-standardised TB death rates for both HIV and SAMPI in the neighbouring district municipalities. The association between the overall age-sex-standardised TB death rates and HIV was not statistically significant at
p value 0.49995. However, the association with SAMPI was statistically significant at
p value 0.05887 (
Table 3).
The spatial lag regression analysis for the male and female age-sex-standardised TB death rates revealed a positive association between the male and female TB death rates for both HIV and SAMPI in the neighbouring district municipalities. The association between the male age-sex-standardised TB death rates with HIV was not statistically significant at
p value 0.73237. However, the association with SAMPI was statistically significant at
p value 0.01727. The association between the female age-sex-standardised TB death rates and HIV and SAMPI were both not statistically significant at
p value 0.58862 and 0.06040 for HIV and SAMPI, respectively (
Table 3).
4. Discussion
This study set out to investigate the spatial epidemiology of TB mortality in South Africa using death notification data for the period 2005–2015. Both spatial clustering measures and spatial lag regression were used, the latter to examine the association of TB mortality rates, area-level poverty, and HIV burden. The study found a decline in the rate of TB deaths over the study period. We also found that TB death rates varied between gender and age-groups, provinces and district municipalities. Black South Africans and south-eastern parts of the country had higher TB death rates. This study revealed spatial dynamics of TB which has shown a distinct district municipality trend of hot spots occurring in areas that mostly have high levels of SAMPI and HIV. TB death rates were higher in the district that had higher levels of SAMPI.
The study showed a statistically significant decline in the rate of TB deaths over the study period. These findings were like the global trend [
17] and for studies in countries such as Japan [
18], the USA [
19], and the UK [
20]. This downward trend is an important finding, especially for the Sustainable Development Goals and the End TB strategy—both of which call for a reduction in TB deaths. The study further revealed that for the period 2005–2015, there was a higher mean annual TB death rate in males than in females. This finding is similar with other studies in other countries [
18,
21,
22]. Although this study could not independently establish why there were more male than female TB deaths, it may be argued that it is due to risk factors associated with TB that are more prevalent in men, for example, smoking [
22] and co-morbidities such as HIV [
18]. Another important finding is that for the study period, for both males and females, the mean annual TB death rate was highest in the 35–44 age group. While a similar study in South Africa had the same finding [
23], this finding is in contrast to the findings of a study in Japan where TB deaths were higher in the older age groups, believed to be due to increased exposure of the elderly to other chronic health conditions [
18]. Tuberculosis deaths in the 35–44 age group in South Africa has implications in that this is the age group that is economically active; therefore, it is important that health workers in the TB control programme pay particular attention to this age group in order to reduce the number of TB deaths.
A previous study showed similar findings of high TB deaths in KwaZulu-Natal province [
23]. Many parts of KwaZulu-Natal and Eastern Cape provinces province are rural with most inhabitants being Black Africans. According to Mayosi and colleagues, a large proportion of South Africans live in poverty with limited access to, for instance, reasonable housing conditions [
4]. Moreover, it is known that there is an association between TB burden and socio economic status (SES) [
24,
25,
26], and that the poor maybe unable to access health care services [
27]. These findings mean that public health policies on TB prevention and management should directed in south-eastern parts of the country. We have also found significant evidence of spatial closuring in the TB mortality rates. This is consistent with some of the previous studies that found strong spatial autocorrelation [
28,
29,
30]. The TB clusters could be an indication that populations in sorounding districts in share common TB risk factors [
31,
32]. The identification of these clusters and hot spots underscores the importance of understanding TB transmission dynamics between districts. This requires recognition of the local context in the efforts to prevent and control infectious diseases such as TB with inclusion of a neighbourhood approach, for instance, the district municipalities that are located next to each other. Our findings, are important in providing empirical evidence to the Department of Health to focus the identified high TB risk districts prioritised for TB interventions.
5. Study Limitations
This study used secondary TB data from the Civil registration system. This TB data source may have missing TB deaths due to the incompleteness of the Civil registration system. Despite this limitation, the study added new knowledge by investigating spatial TB deaths at a district level—a level at which primary health care services such as TB services are offered in South Africa. In addition, this study was able to identify hotspots and relate them to SES and HIV.
6. Conclusions
The findings revealed a significant decrease in TB deaths and a disproportionate distribution of TB deaths among certain groups of people in South Africa (Black African, males, and those in the 35–44 age group) and certain provinces and district municipalities. The findings further revealed strong spatial autocorrelation for the overall, male, and female age-sex-standardised TB death rates, thus revealing a significant similarity between neighbouring districts regarding TB death rates. In addition, the findings revealed a significant positive association between district level TB deaths and poverty profiles, as well as the effect of TB burden in nearby districts. The existence of these identified inequalities in the burden of TB deaths underscores the importance of developing targeted public health interventions and policies to be directed towards the most vulnerable populations in district municipalities with hot spots. Furthermore, enough resources should be directed into TB prevention activities at a district municipal level.
Author Contributions
D.K., P.N. and S.M. jointly conceived the study. D.K. and S.M. carried out the analysis. D.K. drafted the manuscript. C.M., P.N. and S.M. contributed to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by The South African Medical Research Council-National Health Scholars Programme and The Auckland University of Technology, Faculty of Health and Environment Sciences, Doctoral fees scholarship. funding, New Zealand.
Institutional Review Board Statement
The study was approved by the Auckland University of Technology Ethics Committee (AUTEC) in New Zealand, reference number 17/369 and by the human research ethics committee of the South African Medical Research Council, reference number EC006-5/2018.
Informed Consent Statement
The study used secondary TB data of deceased persons, no informed consent was collected from the subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Kyu, H.H.; Maddison, E.R.; Henry, N.J.; Mumford, J.E.; Barber, R.; Shields, C.; Brown, J.C.; Nguyen, G.; Carter, A.; Wolock, T.M.; et al. The global burden of tuberculosis: Results from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2018, 18, 261–284. [Google Scholar] [CrossRef]
- Butler, R.; Carr, J. Tuberculosis: Department of Molecular Virology and Microbiology; Baylor College of Medicine: Houston, TX, USA, 2013. [Google Scholar]
- Mayosi, B.M.; Benatar, S.R. Health and health care in South Africa—20 years after Mandela. N. Engl. J. Med. 2014, 371, 1344–1353. [Google Scholar] [CrossRef]
- Taylor, V. Transforming the Present—Protecting the Future. In Report of the Committee of Inquiry into a Comprehensive System of Social Security for South Africa; Ministry of Social Development: Pretoria, South Africa, 2002. [Google Scholar]
- Leibbrandt, M.; Woolard, I.; Finn, A.; Argent, J. Trends in South African Income Distribution and Poverty since the Fall of Apartheid. In OECD Social, Employment and Migration Working Papers; OECD Publishing: Paris, France, 2010. [Google Scholar]
- Benatar, S.R. The challenges of health disparities in South Africa. S. Afr. Med. J. 2013, 103, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.C.; Kumar, S. Health Inequalities and Infectious Disease Epidemics: A Challenge for Global Health Security. Biosecur. Bioterror. Biodef. Strat. Pract. Sci. 2014, 12, 263–273. [Google Scholar] [CrossRef]
- Churchyard, G.; Mametja, L.D.; Mvusi, L.; Ndjeka, N.; Hesseling, A.C.; Reid, A.; Babatunde, S.; Pillay, Y. Tuberculosis control in South Africa: Successes, challenges and recommendations. S. Afr. Med. J. 2014, 104, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Nunn, P.; Brindle, R.; Carpenter, L.; Odhiambo, J.; Wasunna, K.; Newnham, R.; Githui, W.; Gathua, S.; Omwega, M.; McAdam, K. Cohort Study of Human Immunodeficiency Virus Infection in Patients with Tuberculosis in Nairobi, Kenya: Analysis of Early (6-Month) Mortality. Am. Rev. Respir. Dis. 1992, 146, 849–854. [Google Scholar] [CrossRef]
- Perriëns, J.H.; Colebunders, R.L.; Karahunga, C.; Willame, J.-C.; Jeugmans, J.; Kaboto, M.; Mukadi, Y.; Pauwels, P.; Ryder, R.W.; Prignot, J.; et al. Increased mortality and tuberculosis treatment failure rate among human immunodeficiency virus (HIV) Seropositive compared with HIV Seronegative patients with pulmonary tuberculosis treated with “Standard” chemotherapy in Kinshasa, Zaire. Am. Rev. Respir. Dis. 1991, 144, 750–755. [Google Scholar] [CrossRef]
- Perneger, T.V.; Sudre, P.; Lundgren, J.D.; Hirschel, B. Does the onset of tuberculosis in AIDS predict shorter survival? Results of a cohort study in 17 European countries over 13 years. BMJ 1995, 311, 1468–1471. [Google Scholar] [CrossRef]
- Kabudula, C.W.; Joubert, J.D.; Tuoane-Nkhasi, M.; Kahn, K.; Rao, C.; Gmez-Oliv, F.X.; Mee, P.; Tollman, S.; Lopez, A.D.; Vos, T.; et al. Evaluation of record linkage of mortality data between a health and demographic surveillance system and national civil registration system in South Africa. Popul. Health Metr. 2014, 12, 23. [Google Scholar] [CrossRef]
- Republic of South Africa. Births and Deaths Registration Act 51 of 1992, in Government Gazette No. 13953; Government Printing Works: Pretoria, South Africa, 2010.
- Statistics South Africa. The South African MPI Creating a Multidimensional Poverty Index Using Census Data (03-10-08); Statistics South Africa: Pretoria, South Africa, 2014. Available online: http://www.statssa.gov.za/publications/Report-03-10-08/Report-03-10-082014.pdf (accessed on 15 June 2020).
- Anselin, L. GeoDa: An Introduction to Spatial Data Analysis. 2020. Available online: https://geodacenter.github.io/workbook/5a_global_auto/lab5a.html#morans-i (accessed on 13 March 2021).
- WHO. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 13 January 2020).
- Hagiya, H.; Koyama, T.; Zamami, Y.; Minato, Y.; Tatebe, Y.; Mikami, N.; Teratani, Y.; Ohshima, A.; Shinomiya, K.; Kitamura, Y.; et al. Trends in incidence and mortality of tuberculosis in Japan: A population-based study, 1997–2016. Epidemiol. Infect. 2019, 147, 1–10. [Google Scholar]
- Barnes, R.F.; Moore, M.L.; Garfein, R.S.; Brodine, S.; Strathdee, S.A.; Rodwell, T.C. Trends in Mortality of Tuberculosis Patients in the United States: The Long-Term Perspective. Ann. Epidemiol. 2011, 21, 791–795. [Google Scholar] [CrossRef]
- Glaziou, P.; Floyd, K.; Raviglione, M. Trends in tuberculosis in the UK. Thorax 2018, 73, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Llorca, J.; Dierssen-Sotos, T.; Arbaizar, B.; Gomez-Acebo, I. Mortality from Tuberculosis in Spain, 1971 to 2007: Slow Decrease in Female and in Elderly Patients. Ann. Epidemiol. 2012, 22, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.E.; Plant, A.J. Does smoking explain sex differences in the global tuberculosis epidemic? Epidemiol. Infect. 2006, 134, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kootbodien, T.; Wilson, K.; Tlotleng, N.; Ntlebi, V.; Made, F.; Rees, D.; Naicker, N. Tuberculosis Mortality by Occupation in South Africa, 2011–2015. Int. J. Environ. Res. Public Health 2018, 15, 2756. [Google Scholar] [CrossRef] [PubMed]
- Waaler, H.T. Tuberculosis and poverty. Int. J. Tuberc. Lung Dis. 2002, 6, 745–746. [Google Scholar]
- Lönnroth, K.; Jaramillo, E.; Williams, B.; Dye, C.; Raviglione, M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc. Sci. Med. 2009, 68, 2240–2246. [Google Scholar] [CrossRef]
- Muniyandi, M.; Ramachandran, R.; Pg, G.; Chandrasekaran, V.; Subramani, R.; Sadacharam, K.; Kumaran, P.; Santha, T.; Wares, D.F.; Narayanan, P.R. The prevalence of tuberculosis in different economic strata: A community survey from south India. Int. J. Tuberc. Lung Dis. 2007, 11, 1042–1045. [Google Scholar]
- Ataguba, J.E.; Akazili, J.; McIntyre, D. Socioeconomic-related health inequality in South Africa: Evidence from General Household Surveys. Int. J. Equity Health 2011, 10, 48. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Liu, Y.; Wang, R.; LI, X.; Yuan, Z.; Wang, L.; Xue, F. Spatial epidemiology and spatial ecology study of worldwide drug-resistant tuberculosis. Int. J. Health Geogr. 2011, 10, 50. [Google Scholar] [CrossRef]
- Harling, G.; Castro, M.C. A spatial analysis of social and economic determinants of tuberculosis in Brazil. Health Place 2014, 25, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xue, F.; Chen, Y.; Ma, Y.; Liu, Y. The spatial epidemiology of tuberculosis in Linyi City, China, 2005–2010. BMC Public Health 2012, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Wubuli, A.; Xue, F.; Jiang, D.; Yao, X.; Upur, H.; Wushouer, Q. Socio-Demographic Predictors and Distribution of Pulmonary Tuberculosis (TB) in Xinjiang, China: A Spatial Analysis. PLoS ONE 2015, 10, e0144010. [Google Scholar] [CrossRef]
- Kolifarhood, G.; Khorasani-Zavareh, D.; Salarilak, S.; Shoghli, A.; Khosravi, N. Spatial and non-spatial determinants of successful tuberculosis treatment outcomes: An implication of Geographical Information Systems in health policy-making in a developing country. J. Epidemiol. Glob. Health 2015, 5, 221. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).