Short-Term Acute Exposure to Wildfire Smoke and Lung Function among Royal Canadian Mounted Police (RCMP) Officers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Demographic, Job, and Health-Related Information
2.3. Exposure Assessment
2.4. Lung Function
2.5. Statistical Analyses
3. Results
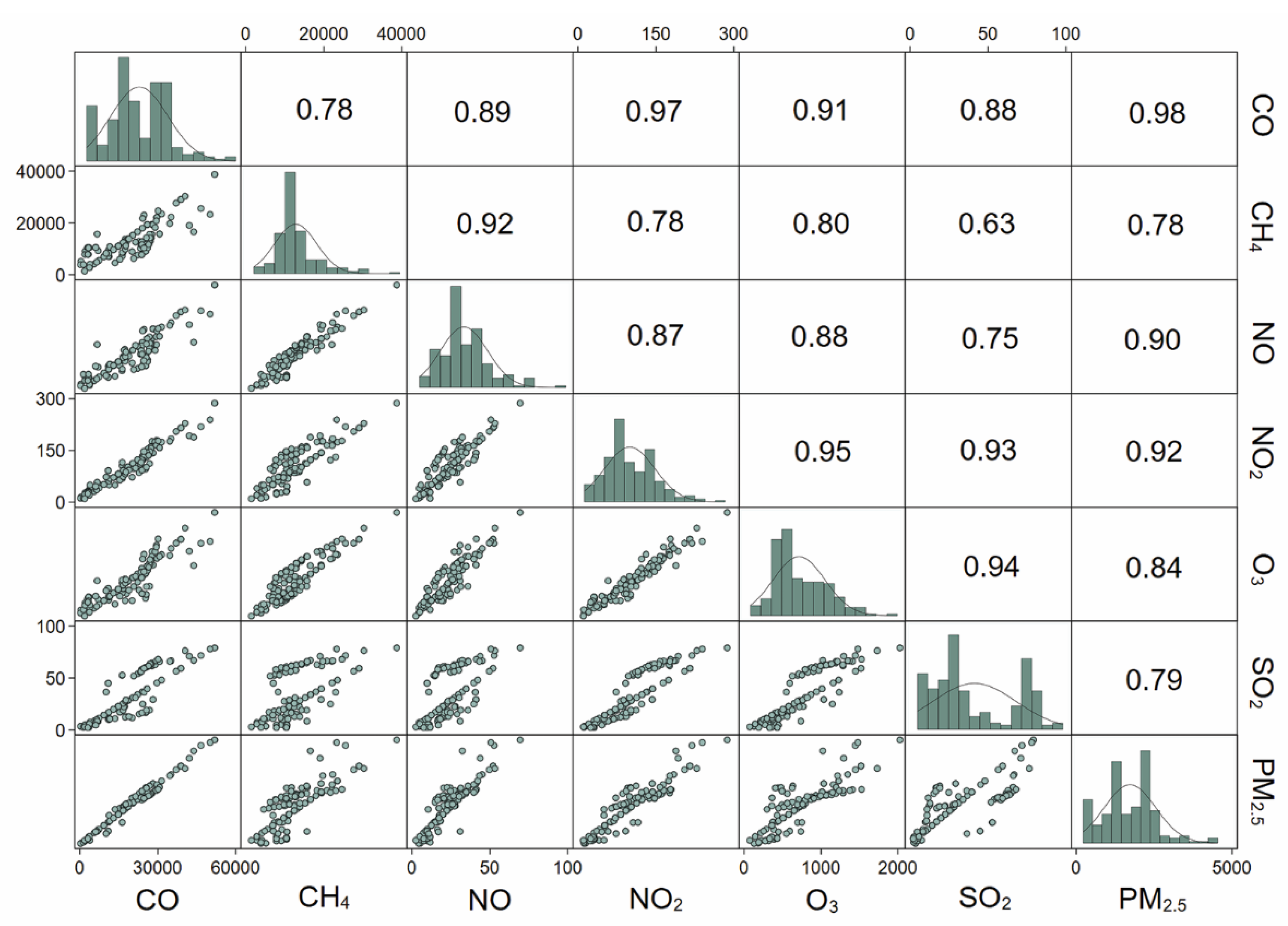
3.1. Study Population Characteristics and Air Pollution Exposure
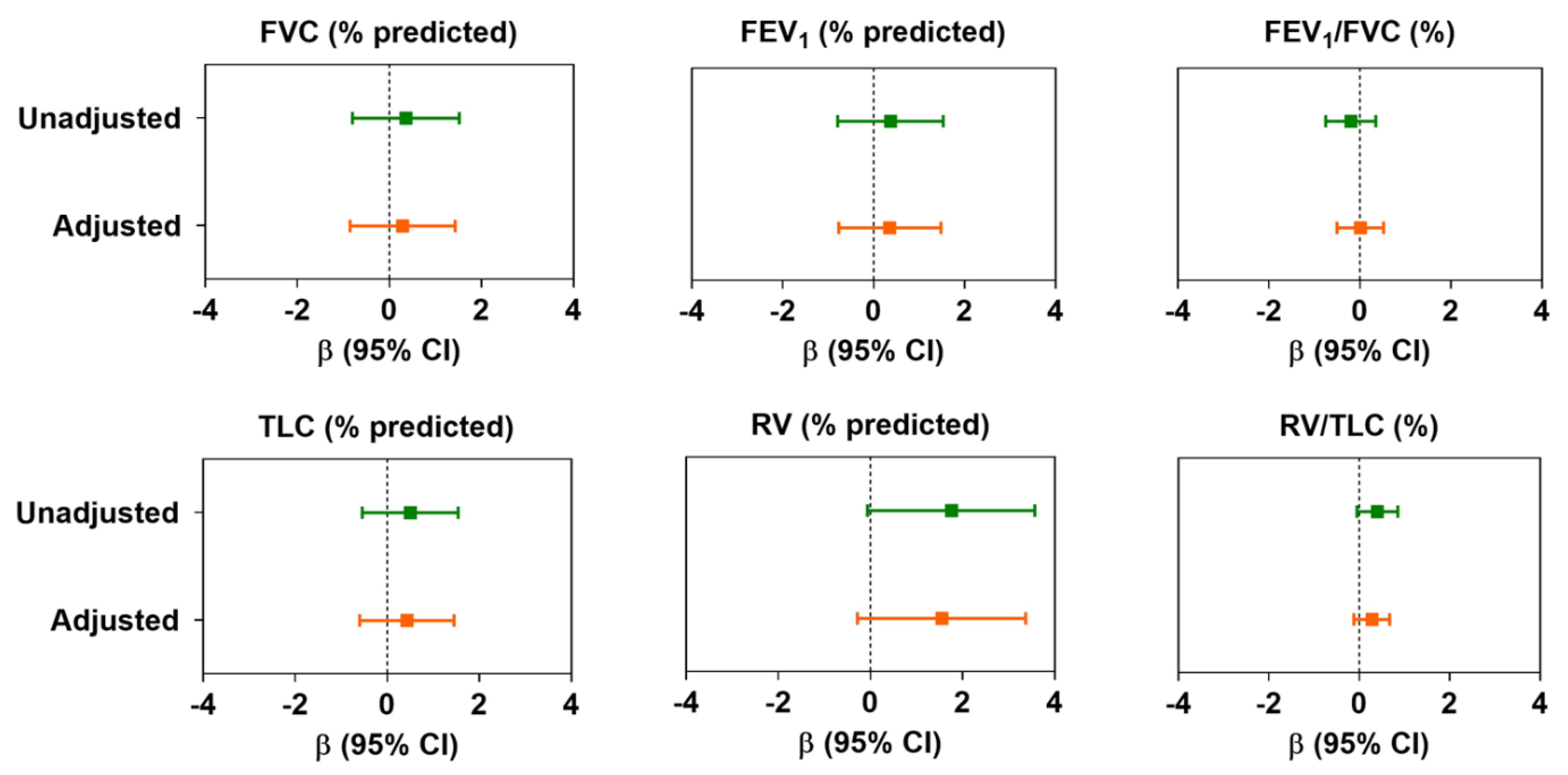
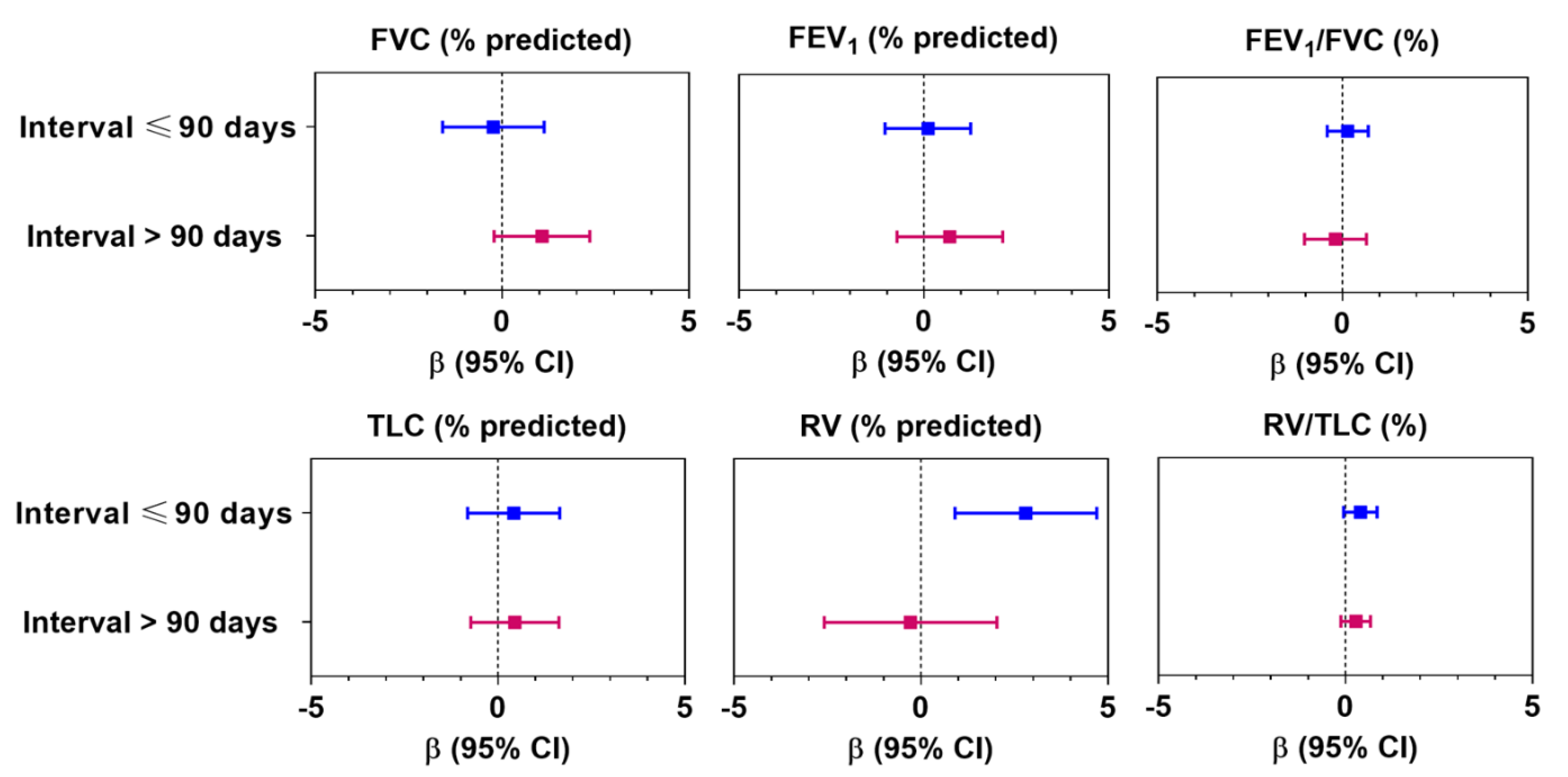
3.2. Association between Air Pollution and Lung Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Government of Canada. National Wildland Fire Situation Report. Available online: https://cwfis.cfs.nrcan.gc.ca/report (accessed on 27 November 2020).
- The Economist. Why the North American West Is on Fire. Available online: https://www.economist.com/the-economist-explains/2017/10/13/why-the-north-american-west-is-on-fire (accessed on 27 November 2020).
- Spracklen, D.V.; Mickley, L.J.; Logan, J.A.; Hudman, R.C.; Yevich, R.; Flannigan, M.D.; Westerling, A.L. Impacts of climate change from 2000 to 2050 on wildfire activity and carbonaceous aerosol concentrations in the western United States. J. Geophys. Res. 2009, 114, D20301. [Google Scholar] [CrossRef]
- Radeloff, V.C.; Helmers, D.P.; Kramer, H.A.; Mockrin, M.H.; Alexandre, P.M.; Bar-Massada, A.; Butsic, V.; Hawbaker, T.J.; Martinuzzi, S.; Syphard, A.D.; et al. Rapid growth of the US wildland-urban interface raises wildfire risk. Proc. Natl. Acad. Sci. USA 2018, 115, 3314–3319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, D.M.J.S.; Williamson, G.J.; Abatzoglou, J.T.; Kolden, C.A.; Cochrane, M.A.; Smith, A.M.S. Human exposure and sensitivity to globally extreme wildfire events. Nat. Ecol. Evol. 2017, 1, 0058. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Fort McMurray 2016 Wildfire—Economic Impact. Available online: https://www150.statcan.gc.ca/n1/en/pub/11-627-m/11-627-m2017007-eng.pdf (accessed on 27 November 2020).
- Jaffe, D.; Hafner, W.; Chand, D.; Westerling, A.; Spracklen, D. Interannual Variations in PM2.5 due to Wildfires in the Western United States. Environ. Sci. Technol. 2008, 42, 2812–2818. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, D.; Chand, D.; Hafner, W.; Westerling, A.; Spracklen, D. Influence of Fires on O3Concentrations in the Western U.S. Environ. Sci. Technol. 2008, 42, 5885–5891. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.E.; Considine, E.M.; Watson, G.L.; Telesca, D.; Pfister, G.G.; Jerrett, M. Associations between respiratory health and ozone and fine particulate matter during a wildfire event. Environ. Int. 2019, 129, 291–298. [Google Scholar] [CrossRef]
- Ontawong, A.; Saokaew, S.; Jamroendararasame, B.; Duangjai, A. Impact of long-term exposure wildfire smog on respiratory health outcomes. Expert Rev. Respir. Med. 2020, 14, 527–531. [Google Scholar] [CrossRef]
- Vasileva, A.; Moiseenko, K. Methane emissions from 2000 to 2011 wildfires in Northeast Eurasia estimated with MODIS burned area data. Atmos. Environ. 2013, 71, 115–121. [Google Scholar] [CrossRef]
- Fiore, A.M.; Jacob, D.J.; Field, B.D.; Streets, D.G.; Fernandes, S.D.; Jang, C. Linking ozone pollution and climate change: The case for controlling methane. Geophys. Res. Lett. 2002, 29, 25-21–25-24. [Google Scholar] [CrossRef]
- Kasischke, E.S.; Bruhwiler, L.P. Emissions of carbon dioxide, carbon monoxide, and methane from boreal forest fires in 1998. J. Geophys. Res. 2002, 107, FFR 2-1–FFR 2-14. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Warren, S.H.; Krantz, Q.T.; King, C.; Jaskot, R.; Preston, W.T.; George, B.J.; Hays, M.D.; Landis, M.S.; Higuchi, M.; et al. Mutagenicity and Lung Toxicity of Smoldering vs. Flaming Emissions from Various Biomass Fuels: Implications for Health Effects from Wildland Fires. Environ. Health Perspect. 2018, 126, 017011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, M.; De Roos, A.; White, L.; Heilman, W.; Mockrin, M.; Gross-Davis, C.; Burstyn, I. Meta-Analysis of Heterogeneity in the Effects of Wildfire Smoke Exposure on Respiratory Health in North America. Int. J. Environ. Res. Public Health 2019, 16, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, C.; Tesfaigzi, Y.; Bassein, J.A.; Miller, L.A. Wildfire smoke exposure and human health: Significant gaps in research for a growing public health issue. Environ. Toxicol. Pharmacol. 2017, 55, 186–195. [Google Scholar] [CrossRef]
- Adetona, O.; Reinhardt, T.E.; Domitrovich, J.; Broyles, G.; Adetona, A.M.; Kleinman, M.T.; Ottmar, R.D.; Naeher, L.P. Review of the health effects of wildland fire smoke on wildland firefighters and the public. Inhal. Toxicol. 2016, 28, 95–139. [Google Scholar] [CrossRef]
- Cascio, W.E. Wildland fire smoke and human health. Sci. Total Environ. 2018, 624, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Groot, E.; Caturay, A.; Khan, Y.; Copes, R. A systematic review of the health impacts of occupational exposure to wildland fires. Int. J. Occup. Med. Environ. Health 2019, 32, 121–140. [Google Scholar] [CrossRef]
- Kim, Y.H.; Tong, H.; Daniels, M.; Boykin, E.; Krantz, Q.T.; McGee, J.; Hays, M.; Kovalcik, K.; Dye, J.A.; Gilmour, M.I. Cardiopulmonary toxicity of peat wildfire particulate matter and the predictive utility of precision cut lung slices. Part. Fibre Toxicol. 2014, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Reid, C.E.; Brauer, M.; Johnston, F.H.; Jerrett, M.; Balmes, J.R.; Elliott, C.T. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environ. Health Perspect. 2016, 124, 1334–1343. [Google Scholar] [CrossRef] [Green Version]
- Tse, K.; Chen, L.; Tse, M.; Zuraw, B.; Christiansen, S. Effect of catastrophic wildfires on asthmatic outcomes in obese children: Breathing fire. Ann. Allergy Asthma Immunol. 2015, 114, 308–311.e304. [Google Scholar] [CrossRef] [Green Version]
- Vora, C.; Renvall, M.J.; Chao, P.; Ferguson, P.; Ramsdell, J.W. 2007 San Diego wildfires and asthmatics. J. Asthma 2011, 48, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Künzli, N.; Avol, E.; Wu, J.; Gauderman, W.J.; Rappaport, E.; Millstein, J.; Bennion, J.; McConnell, R.; Gilliland, F.D.; Berhane, K.; et al. Health effects of the 2003 Southern California wildfires on children. Am. J. Respir. Crit. Care Med. 2006, 174, 1221–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipner, E.M.; O’Dell, K.; Brey, S.J.; Ford, B.; Pierce, J.R.; Fischer, E.V.; Crooks, J.L. The Associations between Clinical Respiratory Outcomes and Ambient Wildfire Smoke Exposure among Pediatric Asthma Patients at National Jewish Health, 2012–2015. GeoHealth 2019, 3, 146–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stowell, J.D.; Geng, G.; Saikawa, E.; Chang, H.H.; Fu, J.; Yang, C.-E.; Zhu, Q.; Liu, Y.; Strickland, M.J. Associations of wildfire smoke PM2.5 exposure with cardiorespiratory events in Colorado 2011–2014. Environ. Int. 2019, 133, 105151. [Google Scholar] [CrossRef] [PubMed]
- Youssouf, H.; Liousse, C.; Roblou, L.; Assamoi, E.-M.; Salonen, R.; Maesano, C.; Banerjee, S.; Annesi-Maesano, I. Non-Accidental Health Impacts of Wildfire Smoke. Int. J. Environ. Res. Public Health 2014, 11, 11772–11804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.C.; Wilson, A.; Mickley, L.J.; Dominici, F.; Ebisu, K.; Wang, Y.; Sulprizio, M.P.; Peng, R.D.; Yue, X.; Son, J.-Y.; et al. Wildfire-specific Fine Particulate Matter and Risk of Hospital Admissions in Urban and Rural Counties. Epidemiology 2017, 28, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.W.; Ford, B.; Lassman, W.; Pfister, G.; Vaidyanathan, A.; Fischer, E.; Volckens, J.; Pierce, J.R.; Magzamen, S. Comparison of wildfire smoke estimation methods and associations with cardiopulmonary-related hospital admissions. GeoHealth 2017, 1, 122–136. [Google Scholar] [CrossRef]
- Moitra, S.; Farshchi Tabrizi, A.; Idrissi Machichi, K.; Kamravaei, S.; Miandashti, N.; Henderson, L.; Mukherjee, M.; Khadour, F.; Naseem, M.T.; Lacy, P.; et al. Non-Malignant Respiratory Illnesses in Association with Occupational Exposure to Asbestos and Other Insulating Materials: Findings from the Alberta Insulator Cohort. Int. J. Environ. Res. Public Health 2020, 17, 7085. [Google Scholar] [CrossRef]
- GINA. Global Strategy for Asthma Management and Prevention; National Heart, Lung and Blood Institute: Bethesda, MD, USA, 2016. [Google Scholar]
- WBEA. Ambient Air Monitoring Station Site Documentation—Athabasca Valley. Available online: https://wbea.org/wp-content/uploads/2020/03/Athabasca-Valley-Site-Documentation.pdf (accessed on 2 August 2020).
- Rappaport, S.M. Selection of the Measures of Exposure for Epidemiology Studies. Appl. Occup. Environ. Hyg. 1991, 6, 448–457. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef]
- Tan, W.C.; Bourbeau, J.; Hernandez, P.; Chapman, K.; Cowie, R.; FitzGerald, M.J.; Aaron, S.; Marciniuk, D.D.; Maltais, F.; O’Donnell, D.E.; et al. Canadian prediction equations of spirometric lung function for Caucasian adults 20 to 90 years of age: Results from the Canadian Obstructive Lung Disease (COLD) study and the Lung Health Canadian Environment (LHCE) study. Can. Respir. J. 2011, 18, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, C.; Ghezzo, R.H.; Abboud, R.T.; Cosio, M.G.; Dill, J.R.; Martin, R.R.; McCarthy, D.S.; Morse, J.L.; Zamel, N. Reference values of pulmonary function tests for Canadian Caucasians. Can. Respir. J. 2004, 11, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Vestbo, J.; Hurd, S.S.; Agusti, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Cook, R.D.; Weisberg, S. Diagnostics for heteroscedasticity in regression. Biometrika 1983, 70, 1–10. [Google Scholar] [CrossRef]
- Salkind, N.J. (Ed.) Akaike Information Criterion; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2011. [Google Scholar] [CrossRef]
- Lei, D.K.; Grammer, L.C. Occupational immunologic lung disease. Allergy Asthma Proc. 2019, 40, 418–420. [Google Scholar] [CrossRef]
- Chow, G.C. Tests of Equality Between Sets of Coefficients in Two Linear Regressions. Econometrica 1960, 28, 591. [Google Scholar] [CrossRef]
- Dashdendev, B.; Fukushima, L.K.; Woo, M.S.; Ganbaatar, E.; Warburton, D. Carbon monoxide pollution and lung function in urban compared with rural Mongolian children. Respirology 2011, 16, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Canova, C.; Torresan, S.; Simonato, L.; Scapellato, M.L.; Tessari, R.; Visentin, A.; Lotti, M.; Maestrelli, P. Carbon monoxide pollution is associated with decreased lung function in asthmatic adults. Eur. Respir. J. 2009, 35, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kesavachandran, C.N.; Kamal, R.; Bihari, V.; Ansari, A.; Azeez, P.A.; Saxena, P.N.; Ks, A.K.; Khan, A.H. Indoor air pollution and its association with poor lung function, microalbuminuria and variations in blood pressure among kitchen workers in India: A cross-sectional study. Environ. Health 2017, 16, 33. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.Y.; Kwon, Y.S.; Lee, J.W.; Park, J.S.; Rho, B.H.; Choi, W.-I. Acute Respiratory Distress Due to Methane Inhalation. Tuberc. Respir. Dis. 2013, 74, 120. [Google Scholar] [CrossRef] [Green Version]
- Ngajilo, D. Respiratory health effects in poultry workers. Curr. Allergy Clin. Immunol. 2014, 27, 116–124. [Google Scholar]
- Yu, Y.; Yu, Z.; Sun, P.; Lin, B.; Li, L.; Wang, Z.; Ma, R.; Xiang, M.; Li, H.; Guo, S. Effects of ambient air pollution from municipal solid waste landfill on children’s non-specific immunity and respiratory health. Environ. Pollut. 2018, 236, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, H.; Chen, Q.; Gu, J.; Ding, Z.; Xu, Y. Effects of individual ozone exposure on lung function in the elderly: A cross-sectional study in China. Environ Sci. Pollut. Res. 2019, 26, 11690–11695. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, G.G.; Liu, M.C.; Proud, D.; Weidenbach-Gerbase, M.; Hubbard, W.; Frank, R. Ozone exposure in humans: Inflammatory, small and peripheral airway responses. Am. J. Respir. Crit. Care Med. 1995, 152, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Tager, I.B.; Balmes, J.; Lurmann, F.; Ngo, L.; Alcorn, S.; Künzli, N. Chronic Exposure to Ambient Ozone and Lung Function in Young Adults. Epidemiology 2005, 16, 751–759. [Google Scholar] [CrossRef]
- Frank, R.; Liu, M.; Spannhake, E.; Mlynarek, S.; Macri, K.; Weinmann, G. Repetitive ozone exposure of young adults: Evidence of persistent small airway dysfunction. Am. J. Respir. Crit. Care Med. 2001, 164, 1253–1260. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chan, C.-C.; Chen, B.-Y.; Cheng, T.-J.; Leon Guo, Y. Effects of particulate air pollution and ozone on lung function in non-asthmatic children. Environ. Res. 2015, 137, 40–48. [Google Scholar] [CrossRef]
- Bhalla, D.K. Ozone-Induced Lung Inflammation and Mucosal Barrier Disruption: Toxicology, Mechanisms, and Implications. J. Toxicol. Environ. Health B Crit. Rev. 1999, 2, 31–86. [Google Scholar] [CrossRef]
- Fleming, G.M.; Chester, E.H.; Montenegro, H.D. Dysfunction of Small Airways following Pulmonary Injury due to Nitrogen Dioxide. Chest 1979, 75, 720–721. [Google Scholar] [CrossRef]
- Gong, H., Jr.; Linn, W.S.; Clark, K.W.; Anderson, K.R.; Geller, M.D.; Sioutas, C. Respiratory Responses to Exposures With Fine Particulates and Nitrogen Dioxide in the Elderly With and Without COPD. Inhal. Toxicol. 2008, 17, 123–132. [Google Scholar] [CrossRef]
- Havet, A.; Hulo, S.; Cuny, D.; Riant, M.; Occelli, F.; Cherot-Kornobis, N.; Giovannelli, J.; Matran, R.; Amouyel, P.; Edmé, J.-L.; et al. Residential exposure to outdoor air pollution and adult lung function, with focus on small airway obstruction. Environ. Res. 2020, 183, 109161. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Brauer, M. Ambient atmospheric particles in the airways of human lungs. Ultrastruct. Pathol. 2000, 24, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Wen, B.; Han, F.; Wang, C.; Zhang, S.P.; Wang, J.; Xu, D.Q.; Wang, Q. Acute Effects of Individual Exposure to Fine Particulate Matter on Pulmonary Function in Schoolchildren. Biomed. Environ. Sci. 2020, 33, 647–659. [Google Scholar] [CrossRef]
- Cui, X.; Li, Z.; Teng, Y.; Barkjohn, K.K.; Norris, C.L.; Fang, L.; Daniel, G.N.; He, L.; Lin, L.; Wang, Q.; et al. Association Between Bedroom Particulate Matter Filtration and Changes in Airway Pathophysiology in Children with Asthma. JAMA Pediatr. 2020, 174, 533. [Google Scholar] [CrossRef]
- Buick, J.B.; Lowry, R.C.; Magee, T.R.A. Is a reduction in residual volume a sub-clinical manifestation of hydrogen sulfide intoxication? Am. J. Ind. Med. 2000, 37, 296–299. [Google Scholar] [CrossRef]
- Zheng, R.; Tao, L.; Jian, H.; Chang, Y.; Cheng, Y.; Feng, Y.; Zhang, H. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol. Environ. Saf. 2018, 163, 612–619. [Google Scholar] [CrossRef]
- Statistics Canada. Table 13-10-0096-08 Asthma, by Age Group. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009608 (accessed on 27 November 2020).
| Demographics | N = 218 |
| Sex (male), n (%) | 155 (71) |
| Age (years), mean (SD) | 38 (9) |
| BMI (kg/m2), mean (SD) | 29.8 (5.2) |
| Ethnicity, n (%) | |
| Caucasian | 209 (96) |
| Others | 9 (4) |
| Never smokers, n (%) | 176 (81) |
| Passively smoke exposure at childhood, n (%) | 131 (60) |
| Parental lung disease, n (%) | 33 (15) |
| Family history of cancer, n (%) | 49 (23) |
| Personal PPE used while deployed, n (%) | 147 (68) |
| Days spent amid wildfire, median (IQR) | 8 (7, 10) |
| Interval (days), median (IQR) † | 60 (3, 627) |
| Asthma, n (%) | 65 (30) |
| COPD, n (%) | 5 (2) |
| Personal exposure details | |
| CO (μg/m3), median (IQR) | 17,386.8 (11,509.3, 25,945.0) |
| CH4 (μg/m3), median (IQR) | 11,063.6 (9680.7, 13,413.1) |
| NO (μg/m3), median (IQR) | 19.9 (16.9, 28.8) |
| NO2 (μg/m3), median (IQR) | 90.3 (68.8, 140.0) |
| O3 (μg/m3), median (IQR) | 590.8 (446.8, 992.1) |
| SO2 (μg/m3), median (IQR) | 22.6 (14.6, 57.8) |
| PM2.5 (μg/m3), median (IQR) | 1632.9 (1014.3, 2142.6) |
| Lung function | |
| FEV1 (% predicted), mean (SD) | 96.2 (12.4) |
| FVC (% predicted), mean (SD) | 100.8 (12.3) |
| FEV1/FVC (%), mean (SD) | 76.5 (5.9) |
| TLC (% predicted), mean (SD) | 95.3 (11.1) |
| RV (% predicted), mean (SD) | 80.1 (19.5) |
| RV/TLC (%), mean (SD) | 22.4 (4.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moitra, S.; Tabrizi, A.F.; Fathy, D.; Kamravaei, S.; Miandashti, N.; Henderson, L.; Khadour, F.; Naseem, M.T.; Murgia, N.; Melenka, L.; et al. Short-Term Acute Exposure to Wildfire Smoke and Lung Function among Royal Canadian Mounted Police (RCMP) Officers. Int. J. Environ. Res. Public Health 2021, 18, 11787. https://doi.org/10.3390/ijerph182211787
Moitra S, Tabrizi AF, Fathy D, Kamravaei S, Miandashti N, Henderson L, Khadour F, Naseem MT, Murgia N, Melenka L, et al. Short-Term Acute Exposure to Wildfire Smoke and Lung Function among Royal Canadian Mounted Police (RCMP) Officers. International Journal of Environmental Research and Public Health. 2021; 18(22):11787. https://doi.org/10.3390/ijerph182211787
Chicago/Turabian StyleMoitra, Subhabrata, Ali Farshchi Tabrizi, Dina Fathy, Samineh Kamravaei, Noushin Miandashti, Linda Henderson, Fadi Khadour, Muhammad T. Naseem, Nicola Murgia, Lyle Melenka, and et al. 2021. "Short-Term Acute Exposure to Wildfire Smoke and Lung Function among Royal Canadian Mounted Police (RCMP) Officers" International Journal of Environmental Research and Public Health 18, no. 22: 11787. https://doi.org/10.3390/ijerph182211787
APA StyleMoitra, S., Tabrizi, A. F., Fathy, D., Kamravaei, S., Miandashti, N., Henderson, L., Khadour, F., Naseem, M. T., Murgia, N., Melenka, L., & Lacy, P. (2021). Short-Term Acute Exposure to Wildfire Smoke and Lung Function among Royal Canadian Mounted Police (RCMP) Officers. International Journal of Environmental Research and Public Health, 18(22), 11787. https://doi.org/10.3390/ijerph182211787








