Association between Smoking during Pregnancy and Short Root Anomaly in Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Settings
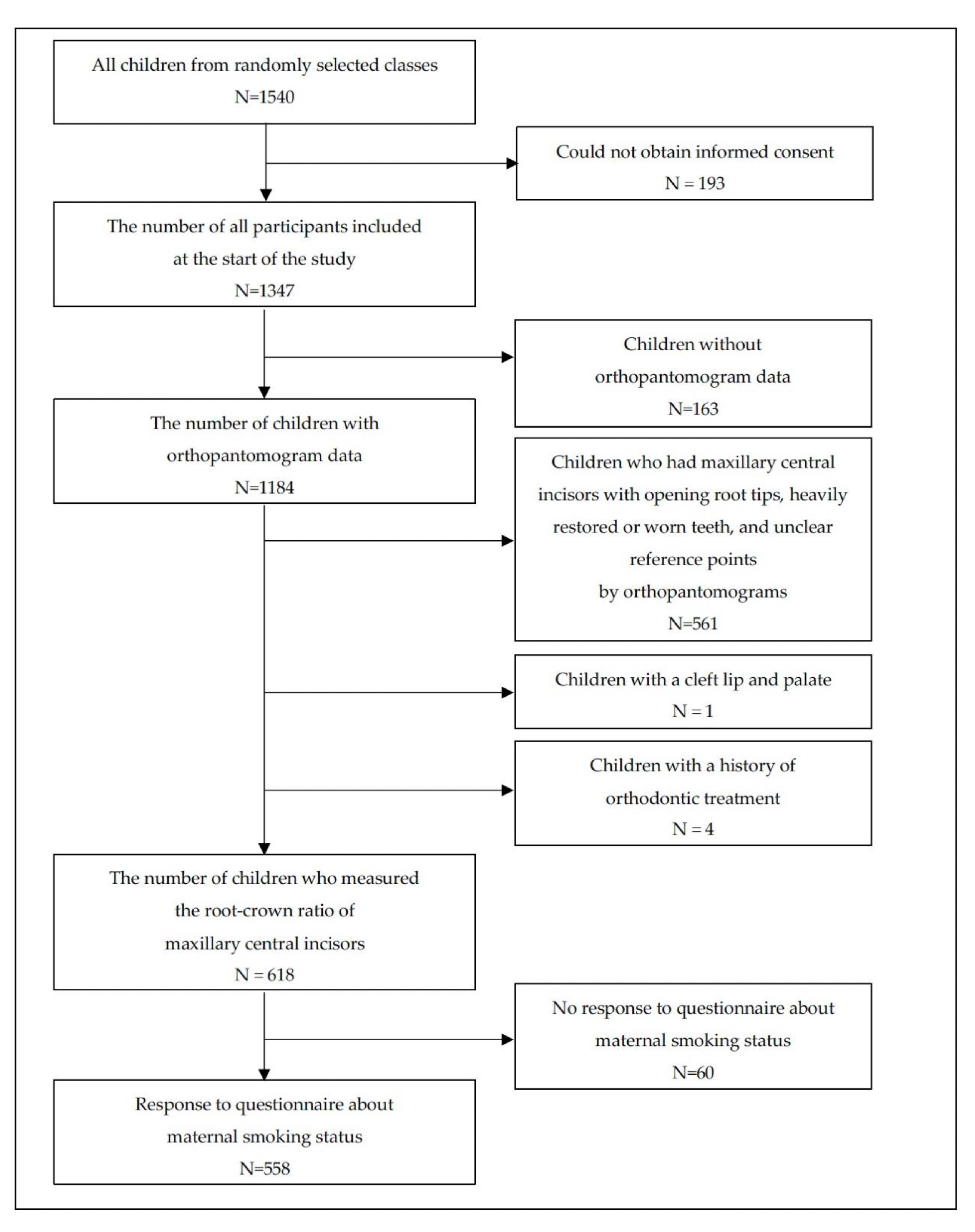
2.2. Sampling and Recruitment
2.3. Measurement of Root-Crown Ratio
2.4. Maternal Smoking Status
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Parada, C.; Chai, Y. Cellular and Molecular Mechanisms of Tooth Root Development. Development 2017, 144, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Lind, V. Short Root Anomaly. Scand. J. Dent. Res. 1972, 80, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Apajalahti, S.; Hölttä, P.; Turtola, L.; Pirinen, S. Prevalence of Short-Root Anomaly in Healthy Young Adults. Acta Odontol. Scand. 2002, 60, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, R.; Lind, V. Variation in Root Length of The Permanent Maxillary Central Incisor. Scand. J. Dent. Res. 1973, 81, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Aizawa, K.; Oshima, S.; Nakamura, Y.; Sato, A.; Suzuki, Y.; Arita, I.; Kaida, Y.; Nagayama, R. Studies on The Consecutive Survey of Succedaneous and Permanent Dentition in The Japanese Children. Part 11. On The Difference Between Variations in The Eruptive Time of Mandibular First Premolars and Their Root Formation. J. Nihon Univ. Sch. Dent. 1976, 18, 25–28. [Google Scholar] [CrossRef][Green Version]
- Turner, C.G. Major Features of Sundadonty and Sinodonty, Including Suggestions About East Asian Microevolution, Population History, and Late Pleistocene Relationships With Australian Aboriginals. Am. J. Phys. Anthropol. 1990, 82, 295–317. [Google Scholar] [CrossRef]
- Park, J.H.; Yamaguchi, T.; Watanabe, C.; Kawaguchi, A.; Haneji, K.; Takeda, M.; Kim, Y.I.; Tomoyasu, Y.; Watanabe, M.; Oota, H.; et al. Effects of An Asian-Specific Nonsynonymous EDAR Variant on Multiple Dental Traits. J. Hum. Genet. 2012, 57, 508–514. [Google Scholar] [CrossRef]
- Yun, H.J.; Jeong, J.S.; Pang, N.S.; Kwon, I.K.; Jung, B.Y. Radiographic Assessment of Clinical Root-Crown Ratios of Permanent Teeth in a Healthy Korean Population. J. Adv. Prosthodont. 2014, 6, 171–176. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, J.S.; Kim, C.S.; Yu, H.S.; Hwang, C.J. Cone-Beam Computed Tomography for The Assessment of Root-Crown Ratios of The Maxillary and Mandibular Incisors in A Korean Population. Korean J. Orthod. 2017, 47, 39–49. [Google Scholar] [CrossRef]
- Apajalahti, S.; Arte, S.; Pirinen, S. Short Root Anomaly in Families and Its Association with Other Dental Anomalies. Eur. J. Oral Sci. 1999, 107, 97–101. [Google Scholar] [CrossRef]
- Puranik, C.P.; Hill, A.; Henderson Jeffries, K.; Harrell, S.N.; Taylor, R.W.; Frazier-Bowers, S.A. Characterization of Short Root Anomaly in A Mexican Cohort—Hereditary Idiopathic Root Malformation. Orthod. Craniofacial Res. 2015, 18 (Suppl. 1), 62–70. [Google Scholar] [CrossRef]
- Näsman, M.; Björk, O.; Söderhäll, S.; Ringdén, O.; Dahllöf, G. Disturbances in The Oral Cavity in Pediatric Long-Term Survivors After Different Forms of Antineoplastic Therapy. Pediatric Dent. 1994, 16, 217–223. [Google Scholar]
- Näsman, M.; Forsberg, C.M.; Dahllöf, G. Long-Term Dental Development in Children After Treatment for Malignant Disease. Eur. J. Orthod. 1997, 19, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.T.; Onetto, J.E. How does orofacial trauma in children affect the developing dentition? Long-term treatment and associated complications. Dent. Traumatol. 2019, 35, 312–323. [Google Scholar] [CrossRef]
- GBD 2015 Tobacco Collaborators. Smoking Prevalence and Attributable Disease Burden In 195 Countries and Territories, 1990–2015: A Systematic Analysis from The Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- Pagano, S.; Negri, P.; Coniglio, M.; Bruscoli, S.; Di Michele, A.; Marchetti, M.C.; Valenti, C.; Gambelunghe, A.; Fanasca, L.; Billi, M.; et al. Heat-Not-Burn Tobacco (IQOS), Oral Fibroblasts and Keratinocytes: Cytotoxicity, Morphological Analysis, Apoptosis and Cellular Cycle. An In Vitro Study. J. Periodontal Res. 2021, 56, 917–928. [Google Scholar] [CrossRef]
- Zaitsu, M.; Hosokawa, Y.; Okawa, S.; Hori, A.; Kobashi, G.; Tabuchi, T. Heated Tobacco Product Use and Hypertensive Disorders of Pregnancy and Low Birth Weight: Analysis of A Cross-Sectional, Web-Based Survey in Japan. BMJ Open 2021, 11, e052976. [Google Scholar] [CrossRef]
- Xuan, Z.; Zhongpeng, Y.; Yanjun, G.; Jiaqi, D.; Yuchi, Z.; Bing, S.; Chenghao, L. Maternal Active Smoking and Risk of Oral Clefts: A Meta-Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 680–690. [Google Scholar] [CrossRef]
- Al-Ani, A.H.; Antoun, J.S.; Thomson, W.M.; Merriman, T.R.; Farella, M. Maternal Smoking During Pregnancy Is Associated with Offspring Hypodontia. J. Dent. Res. 2017, 96, 1014–1019. [Google Scholar] [CrossRef]
- Nakagawa, K.J.; Unnai, Y.Y.; Ogawa, T.; Sato, M.; Yamagata, Z.; Fujiwara, T.; Moriyama, K. Association Between Maternal Smoking During Pregnancy and Missing Teeth in Adolescents. Int. J. Environ. Res. Public Health 2019, 16, 4536. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, F.; Sheldon, E.; Sharma, J.; Canturk, K.M.; Otu, H.H.; Nawshad, A. Nicotine Exposure During Pregnancy Results in Persistent Midline Epithelial Seam with Improper Palatal Fusion. Nicotine Tob. Res. 2016, 18, 604–612. [Google Scholar] [CrossRef]
- Braun, M.; Klingelhöfer, D.; Oremek, G.M.; Quarcoo, D.; Groneberg, D.A. Influence of Second-Hand Smoke and Prenatal Tobacco Smoke Exposure on Biomarkers, Genetics and Physiological Processes in Children—An Overview in Research Insights of The Last Few Years. Int. J. Environ. Res. Public Health 2020, 17, 3212. [Google Scholar] [CrossRef] [PubMed]
- Kjær, I. Morphological Characteristics of Dentitions Developing Excessive Root Resorption During Orthodontic Treatment. Eur. J. Orthod. 1995, 17, 25–34. [Google Scholar] [CrossRef]
- Tada, S.; Allen, P.F.; Ikebe, K.; Zheng, H.; Shintani, A.; Maeda, Y. The Impact of The Crown-Root Ratio on Survival of Abutment Teeth for Dentures. J. Dent. Res. 2015, 94, 220S–225S. [Google Scholar] [CrossRef] [PubMed]
- Tumurkhuu, T.; Fujiwara, T.; Komazaki, Y.; Kawaguchi, Y.; Tanaka, T.; Inazawa, J.; Ganburged, G.; Bazar, A.; Ogawa, T.; Moriyama, K. Association Between Maternal Education and Malocclusion in Mongolian Adolescents: A Cross-Sectional Study. BMJ Open 2016, 6, 902. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Yasuda, Y.; Ogawa, T.; Tumurkhuu, T.; Ganburged, G.; Bazar, A.; Fujiwara, T.; Moriyama, K. Associations Between Malocclusion and Oral Health-Related Quality of Life Among Mongolian Adolescents. Int. J. Environ. Res. Public Health 2017, 14, 902. [Google Scholar] [CrossRef]
- Nakahara, N.; Matsuyama, Y.; Kino, S.; Badrakhkhuu, N.; Ogawa, T.; Moriyama, K.; Fujiwara, T.; Kawachi, I. The Consumption of Sweets and Academic Performance Among Mongolian Children. Int. J. Environ. Res. Public Health 2020, 17, 8912. [Google Scholar] [CrossRef] [PubMed]
- Badrakhkhuu, N.; Matsuyama, Y.; Araki, M.Y.; Yasuda, Y.U.; Ogawa, T.; Tumurkhuu, T.; Ganburged, G.; Bazar, A.; Fujiwara, T.; Moriyama, K. Association Between Malocclusion and Academic Performance Among Mongolian Adolescents. Front. Dent. Med. 2021, 1, 27. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Miyake, K.; Kawaguchi, A.; Miura, R.; Kobayashi, S.; Tran, N.Q.V.; Kobayashi, S.; Miyashita, C.; Araki, A.; Kubota, T.; Yamagata, Z.; et al. Association Between DNA Methylation in Cord Blood and Maternal Smoking: The Hokkaido Study on Environment and Children’s Health. Sci. Rep. 2018, 8, 5654. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Hussain, M.; Xu, C.; Lu, M.; Wu, X.; Tang, L.; Wu, X. Wnt/β-catenin Signaling Links Embryonic Lung Development and Asthmatic Airway Remodeling. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 3226–3242. [Google Scholar] [CrossRef] [PubMed]
- Rehan, V.K.; Asotra, K.; Torday, J.S. The Effects of Smoking on The Developing Lung: Insights from A Biologic Model for Lung Development, Homeostasis, and Repair. Lung 2009, 187, 281–289. [Google Scholar] [CrossRef]
- Galdzicka, M.; Patnala, S.; Hirshman, M.G.; Cai, J.F.; Nitowsky, H.; Egeland, J.A.; Ginns, E. A New Gene, EVC2, Is Mutated in Ellis-Van Creveld Syndrome. Mol. Genet. Metab. 2002, 77, 291–295. [Google Scholar] [CrossRef]
- Zhang, H.; Takeda, H.; Tsuji, T.; Kamiya, N.; Kunieda, T.; Mochida, Y.; Mishina, Y. Loss of Function of Evc2 in Dental Mesenchyme Leads to Hypomorphic Enamel. J. Dent. Res. 2017, 96, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Nakatomi, M.; Hovorakova, M.; Gritli-Linde, A.; Blair, H.J.; MacArthur, K.; Peterka, M.; Lesot, H.; Peterkova, R.; Ruiz-Perez, V.L.; Goodship, J.A.; et al. Evc Regulates a Symmetrical Response to Shh Signaling in Molar Development. J. Dent. Res. 2013, 92, 222–228. [Google Scholar] [CrossRef]
- Manaseki, S. Mongolia: A Health System in Transition. Br. Med J. 1993, 307, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Hikita, N.; Haruna, M.; Matsuzaki, M.; Sasagawa, E.; Murata, M.; Oidovsuren, O.; Yura, A. Prevalence and Risk Factors of Secondhand Smoke (SHS) Exposure Among Pregnant Women in Mongolia. Sci. Rep. 2017, 7, 16426. [Google Scholar] [CrossRef]
- Demaio, A.R.; Nehme, J.; Otgontuya, D.; Meyrowitsch, D.W.; Enkhtuya, P. Tobacco Smoking in Mongolia: Findings of A National Knowledge, Attitudes and Practices Study. BMC Public Health 2014, 14, 213. [Google Scholar] [CrossRef]
| ALL | Male | Female | p Value for t-Test | ||||
|---|---|---|---|---|---|---|---|
| (N = 558, 100%) | (N = 237, 42.5%) | (N = 321, 57.5%) | |||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | ||
| Root-crown ratio | 1.21 | 0.18 | 1.23 | 0.19 | 1.20 | 0.18 | 0.09 |
| Factors | All | SRA (−) | SRA (+) | p Value for the Chi-Squared Test | ||||
|---|---|---|---|---|---|---|---|---|
| N = 558 | N = 479 (85.8%) | N = 79 (14.2%) | ||||||
| N | % | N | % | N | % | |||
| Sex of participants | ||||||||
| Male | 237 | 42.5 | 207 | 43.2 | 30 | 38.0 | 0.38 | |
| Female | 321 | 57.5 | 272 | 56.8 | 49 | 62.0 | ||
| Drinking habit of mother during pregnancy | ||||||||
| (−) | 502 | 96.5 | 429 | 96.4 | 73 | 97.3 | 0.68 | |
| (+) | 18 | 3.5 | 16 | 3.6 | 2 | 2.7 | ||
| X-ray of mother during pregnancy | ||||||||
| (−) | 361 | 76.2 | 310 | 75.6 | 51 | 79.7 | 0.48 | |
| (+) | 113 | 23.8 | 100 | 24.4 | 13 | 20.3 | ||
| Mother taking vitamin supplements during pregnancy | ||||||||
| (−) | 354 | 63.4 | 300 | 62.6 | 54 | 68.4 | 0.33 | |
| (+) | 204 | 36.6 | 179 | 37.4 | 25 | 31.6 | ||
| Birth weight | ||||||||
| Low (<2500 g) | 16 | 3.3 | 13 | 3.1 | 3 | 4.3 | 0.60 | |
| Normal (≥2500 g) | 475 | 96.7 | 408 | 96.9 | 67 | 95.7 | ||
| Delivery | ||||||||
| Normal | 428 | 80.0 | 374 | 81.1 | 54 | 73.0 | 0.20 | |
| Difficult | 26 | 4.9 | 20 | 4.4 | 6 | 8.1 | ||
| Caesarean | 81 | 15.1 | 67 | 14.5 | 14 | 18.9 | ||
| Gestational age | ||||||||
| Pre-term (<37 weeks) | 28 | 5.2 | 25 | 5.5 | 3 | 4.0 | 0.86 | |
| Full term | 444 | 83.2 | 380 | 83.0 | 64 | 84.2 | ||
| Post-term (>42 weeks) | 62 | 11.6 | 53 | 11.5 | 9 | 11.8 | ||
| Family income level | ||||||||
| Low | 165 | 26.6 | 139 | 30.0 | 26 | 35.1 | 0.36 | |
| Average | 281 | 55.3 | 248 | 53.4 | 33 | 44.6 | ||
| High | 92 | 18.1 | 77 | 16.6 | 15 | 20.3 | ||
| Educational level of mother | ||||||||
| Low | 109 | 20.0 | 98 | 20.9 | 11 | 14.9 | 0.48 | |
| Intermediate | 224 | 41.2 | 191 | 40.6 | 33 | 44.6 | ||
| High | 211 | 38.8 | 181 | 38.5 | 30 | 40.5 | ||
| Educational level of father | ||||||||
| Low | 130 | 25.8 | 111 | 25.6 | 19 | 26.4 | 0.98 | |
| Intermediate | 245 | 48.5 | 210 | 48.5 | 35 | 48.6 | ||
| High | 130 | 25.7 | 112 | 25.9 | 18 | 25.0 | ||
| Factors | All | SRA (−) | SRA (+) | p Value for the Chi-Squared Test | ||||
|---|---|---|---|---|---|---|---|---|
| N = 558 | N = 479 (85.8%) | N = 79 (14.2%) | ||||||
| N | % | N | % | N | % | |||
| Finger sucking habit | ||||||||
| (−) | 504 | 92.8 | 435 | 92.9 | 69 | 92.0 | 0.77 | |
| (+) | 39 | 7.2 | 33 | 7.1 | 6 | 8.0 | ||
| Open mouth habit | ||||||||
| (−) | 502 | 90.1 | 432 | 90.4 | 70 | 88.6 | 0.63 | |
| (+) | 55 | 9.9 | 46 | 9.6 | 9 | 11.4 | ||
| Nail biting habit | ||||||||
| Never | 445 | 82.1 | 386 | 82.7 | 59 | 78.7 | 0.13 | |
| Did before | 55 | 10.2 | 49 | 10.5 | 6 | 8.0 | ||
| Still does | 42 | 7.7 | 32 | 6.8 | 10 | 13.3 | ||
| Lip sucking habit | ||||||||
| Never | 507 | 92.8 | 439 | 93.6 | 68 | 88.3 | 0.12 | |
| Did before | 25 | 4.6 | 18 | 3.8 | 7 | 9.1 | ||
| Still does | 14 | 2.6 | 12 | 2.6 | 2 | 2.6 | ||
| Object biting habit | ||||||||
| Never | 427 | 78.8 | 369 | 79.0 | 58 | 77.3 | 0.84 | |
| Did before | 80 | 14.8 | 69 | 14.8 | 11 | 14.7 | ||
| Still does | 35 | 6.4 | 29 | 6.2 | 6 | 8.0 | ||
| Bruxism | ||||||||
| Never | 456 | 84.8 | 390 | 84.2 | 66 | 88.0 | 0.63 | |
| Did before | 43 | 8.0 | 39 | 8.4 | 4 | 5.3 | ||
| Still does | 39 | 7.2 | 34 | 7.4 | 5 | 6.7 | ||
| History of facial trauma | ||||||||
| (−) | 484 | 88.6 | 413 | 87.7 | 71 | 94.7 | 0.08 | |
| (+) | 62 | 11.4 | 58 | 12.3 | 4 | 5.3 | ||
| Factors | SRA (−) | SRA (+) | Crude | Adjusted | ||
|---|---|---|---|---|---|---|
| N = 479 (85.8%) | N = 79 (14.2%) | OR (95 % CI) | p Value | OR (95 % CI) | p Value | |
| Maternal smoking status | ||||||
| Never or former smokers who quit smoking before/at pregnancy | 448 | 67 | Ref | Ref | ||
| Current smokers who did not smoke during pregnancy | 21 | 5 | 1.59 (0.58–4.36) | 0.37 | 1.48 (0.52–4.21) | 0.47 |
| Current smokers who smoked during pregnancy | 10 | 7 | 4.68 (1.72–12.72) | 0.002 | 4.95 (1.65–14.79) | 0.004 |
| Sex of participants | ||||||
| Male | 207 | 30 | Ref | Ref | ||
| Female | 272 | 49 | 1.24 (0.76–2.03) | 0.38 | 1.20 (0.72–2.02) | 0.49 |
| Paternal smoking status | ||||||
| Never or current smokers not smoking around their family | 368 | 52 | Ref | Ref | ||
| Current smokers who did not smoke next to their spouse during pregnancy but were smoking around their family after birth | 13 | 2 | 1.09 (0.24–4.96) | 0.91 | 1.39 (0.29–6.61) | 0.68 |
| Current smokers who smoked next to their spouse during pregnancy | 74 | 22 | 2.10 (1.20–3.67) | 0.009 | 1.86 (1.02–3.40) | 0.04 |
| Missing | 24 | 3 | 0.88 (0.26-3.04) | 0.85 | 0.81 (0.22-3.01) | 0.76 |
| Gestational age | ||||||
| Full term | 380 | 64 | Ref | Ref | ||
| Pre-term (<37 weeks) | 25 | 3 | 0.71 (0.21–2.43) | 0.59 | 0.71 (0.20–2.51) | 0.60 |
| Post-term (>42 weeks) | 53 | 9 | 1.01 (0.47–2.14) | 0.98 | 1.02 (0.46–2.24) | 0.97 |
| Missing | 21 | 3 | 0.85 (0.25–2.93) | 0.79 | 0.58 (0.15–2.22) | 0.42 |
| Family income level | ||||||
| Low | 139 | 26 | Ref | Ref | ||
| Average | 248 | 33 | 0.71 (0.41–1.24) | 0.23 | 0.68 (0.37–1.24) | 0.21 |
| High | 77 | 15 | 1.04 (0.52–2.08) | 0.91 | 1.00 (0.46–2.20) | 0.99 |
| Missing | 15 | 5 | 1.78 (0.60–5.33) | 0.30 | 1.47 (0.37–5.87) | 0.59 |
| Educational level of mother | ||||||
| Low | 98 | 11 | Ref | Ref | ||
| Intermediate | 191 | 33 | 1.54 (0.75–3.18) | 0.24 | 1.77 (0.83–3.78) | 0.14 |
| High | 181 | 30 | 1.48 (0.71–3.07) | 0.30 | 1.86 (0.82–4.21) | 0.14 |
| Missing | 9 | 5 | 4.95 (1.41–17.42) | 0.01 | 4.52 (0.92–22.26) | 0.06 |
| Maternal age at delivery | ||||||
| ≤19 years | 47 | 7 | Ref | Ref | ||
| 20–29 years | 325 | 56 | 1.16 (0.50–2.69) | 0.74 | 1.22 (0.50–2.99) | 0.66 |
| ≥30 years | 94 | 14 | 1.00 (0.38–2.64) | 1.00 | 0.90 (0.32–2.51) | 0.85 |
| Missing | 13 | 2 | 1.03 (0.19–5.58) | 0.97 | 0.72 (0.10–5.30) | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagawa, Y.; Ogawa, T.; Matsuyama, Y.; Nakagawa Kang, J.; Yoshizawa Araki, M.; Unnai Yasuda, Y.; Tumurkhuu, T.; Ganburged, G.; Bazar, A.; Tanaka, T.; et al. Association between Smoking during Pregnancy and Short Root Anomaly in Offspring. Int. J. Environ. Res. Public Health 2021, 18, 11662. https://doi.org/10.3390/ijerph182111662
Sagawa Y, Ogawa T, Matsuyama Y, Nakagawa Kang J, Yoshizawa Araki M, Unnai Yasuda Y, Tumurkhuu T, Ganburged G, Bazar A, Tanaka T, et al. Association between Smoking during Pregnancy and Short Root Anomaly in Offspring. International Journal of Environmental Research and Public Health. 2021; 18(21):11662. https://doi.org/10.3390/ijerph182111662
Chicago/Turabian StyleSagawa, Yuki, Takuya Ogawa, Yusuke Matsuyama, Junka Nakagawa Kang, Miyu Yoshizawa Araki, Yuko Unnai Yasuda, Tsasan Tumurkhuu, Ganjargal Ganburged, Amarsaikhan Bazar, Toshihiro Tanaka, and et al. 2021. "Association between Smoking during Pregnancy and Short Root Anomaly in Offspring" International Journal of Environmental Research and Public Health 18, no. 21: 11662. https://doi.org/10.3390/ijerph182111662
APA StyleSagawa, Y., Ogawa, T., Matsuyama, Y., Nakagawa Kang, J., Yoshizawa Araki, M., Unnai Yasuda, Y., Tumurkhuu, T., Ganburged, G., Bazar, A., Tanaka, T., Fujiwara, T., & Moriyama, K. (2021). Association between Smoking during Pregnancy and Short Root Anomaly in Offspring. International Journal of Environmental Research and Public Health, 18(21), 11662. https://doi.org/10.3390/ijerph182111662







