Inputs of Total and Labile Dissolved Metals from Six Facilities Continuously Discharging Treated Wastewaters to the Marine Environment of Gran Canaria Island (Canary Islands, Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Facilities
- TP-1: WWTP, which mainly collects household wastewater from the city of Las Palmas de Gran Canaria. This plant was considered as a reference for a typical large Canarian WWTP with domestic inputs.
- TP-2 and TP-3: medium size WWTP, which collects mixed household and industrial sewage inlets.
- TP-4: WWTP, which collects only industrial wastewater from two industrial areas of the island.
- TP-5: a coastal thermal power plant, which produces one of the largest discharges of cooling wastewater in the Canary Islands.
- TP-6: an indoor seawater aquaculture (fish) farm.
2.2. Sample Collection
- DGT-Deployment
- Spot-sampling
- In situ parameters measurements
2.3. Sample Analyses
- Trace elements in DGTs by ICP-MS
- Trace elements in spot water samples by ICP-MS
2.4. Data Processing
- Treatment of DGT-labile-fraction metal concentration data
- Calculation of the mass of metal (M), in g units, accumulated in the resin-gel layer, according to Equation (1):where:M = Ce ∗ (VHNO3 + Vgel)/fe
- Ce is the concentration of metals, in g·L−1 units, in the 1 M HNO3 elution solution
- VHNO3 is the volume of HNO3 added to the resin gel (1.25 mL in this study)
- Vgel is the volume of the resin gel (typically 0.15 mL)
- fe is the elution factor for each metal (typically 0.8)
- Calculation of the concentration of metal in water, in g·L−1 units, measured by the DGT device (CDGT), according to Equation (2):where:CDGT = (M ∗ Δg)/(D ∗ t ∗ A)
- Δg is the thickness, in cm units, of the diffusive gel (approx. 0.08 cm) plus the thickness of the filter membrane (0.014 cm)
- D is the diffusion coefficient of metal in the gel, available at [30]:
- t is deployment time (in s units)
- A is the exposure area (3.14 cm2)
- Treatment of Spot-sampling dissolved metal concentration data
- Verify that the mean concentrations of each metal in the exposed DGTs at each sampling site were higher than those in the DGTs blanks at the laboratory.
- Verify that, in the TP-6 results, the labile-fraction metal concentration (based on DGTs results, in triplicate) was higher than the total dissolved metal concentration (based on spot sampling results, in triplicate).
3. Results and Discussion
3.1. DGT Blanks
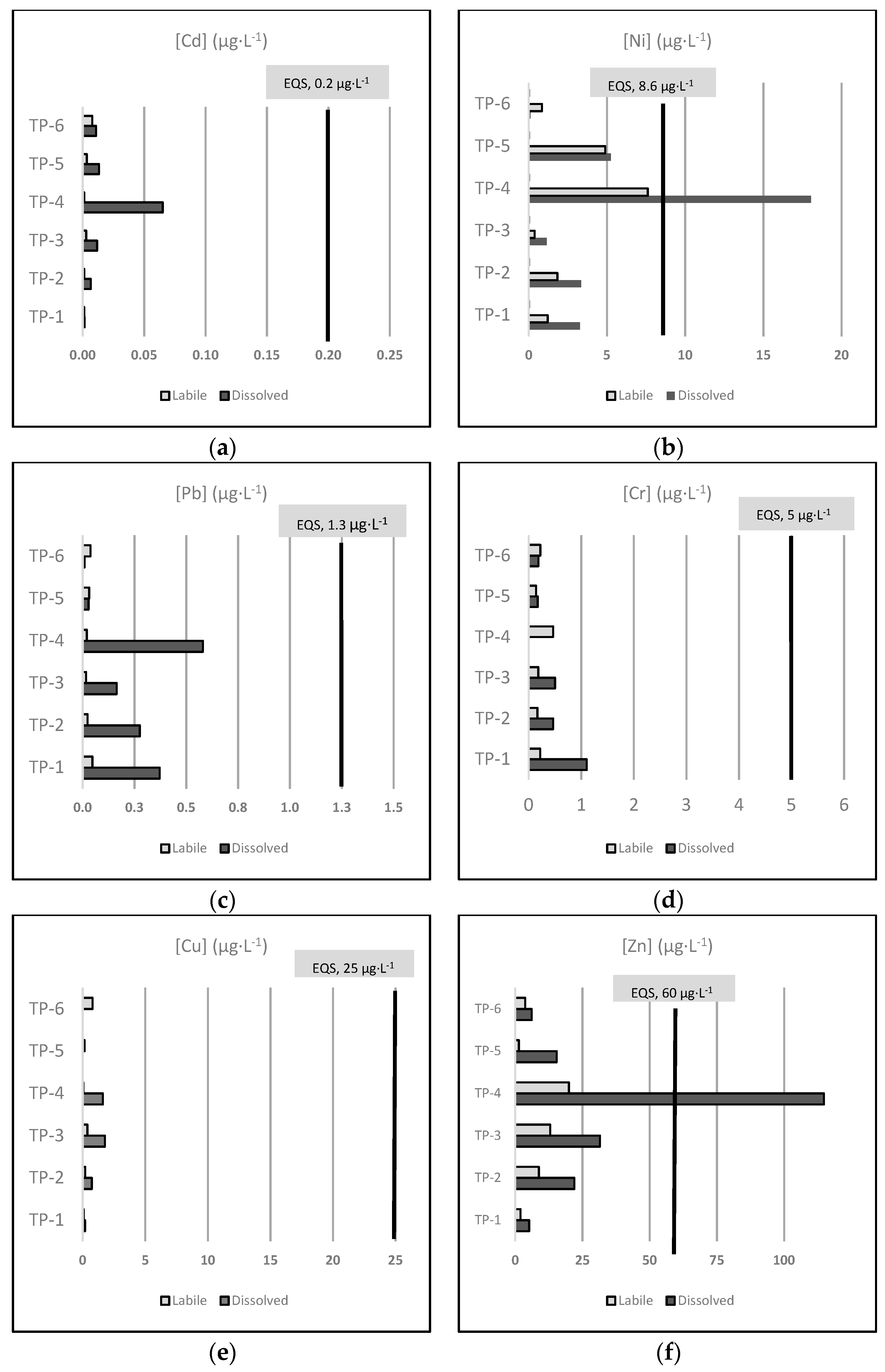
3.2. Concentrations of Total Dissolved and Dissolved Labile Metals
3.2.1. Total Dissolved Metals
3.2.2. Dissolved Labile Metals
3.2.3. Comparison between the Concentrations of the Total-Dissolved and the Labile Metal Fractions
- Differences in the fraction measured in spot sampling (total dissolved) and by DGTs (dissolved labile). As mentioned before, different chemical forms are measured depending on the fraction considered. The concentrations found of total dissolved metals tended to be generally higher than the DGT-labile concentrations, so the percentage of the labile fraction being part of the total dissolved fraction is normally less than 100% (Table 2). Although, some exceptions were observed, mainly in the TP-6 facility, where the percentage of the labile fraction per the total dissolved concentration exceeded 100% in most of the studied metals except for Cd, Ni, and Zn (Supplementary Information S3).
- Differences in the timescale of the spot-sampling measurements and the DGTs measurements. Results do not represent the same sampling timescale (Table 2). Total dissolved metal concentrations are the average of the metal concentrations measured at three specific times (day 0, 2, and 4), whereas the DGT provides 4 days-weighted average metal concentrations. Thus, the spot sampling can miss some peaks and/or decreases in metal concentrations and may not properly monitor the wide variation in the total dissolved metal content. The advantage of using DGT devices is their ability to measure time-weighted average concentrations over the deployment period providing more representative results for highly variable systems. This seems to be especially relevant in the case of the marine fish farm facility (TP- 6), where differences in the temporal distribution of the farming processes (feeding, use of chemicals, water recirculation, waste load, etc.) may affect the temporal content and speciation of metals significantly [40].
- Differences in the physicochemical characteristics of the analyzed effluents. The physicochemical conditions may impact the forms’ distribution (speciation) for a given metal. In this study, we determined the physicochemical parameters in the different wastewater effluents at each sampling day (Supplementary Information S4). The overall temperature in the 6 outlets ranged between 21.9 and 29.0 °C. The highest values in temperature were measured at TP-3 (with domestic and industrial influents), whereas the lowest temperatures were registered at the marine fish farm (TP-6). The pH values ranged between 4.73, measured in the TP-4 effluent (treating industrial influents by flocculation), and 7.73, in TP-3 (with mixed domestic and industrial influents). Furthermore, the lowest dissolved oxygen values were registered at the TP-4 facility (0.36 mg·L−1), while, at the other facilities, the dissolved oxygen ranged between 6.19 and 7.31 mg·L−1. In addition, as some of the sampled effluents’ water source was seawater (TP-5 cooling water and TP-6 marine fish farm), the overall recorded conductivity (25 °C) ranged widely, between 1.66 and 55.67 mS·cm−1.
3.3. Effluent Discharge Impact on Coastal Water Bodies
- ci is the mean concentration (total dissolved or labile) of each metal in the ith sample measured in this study for each facility
- qi is the daily average flow from each facility, and ti is the time interval (1 day).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Carletti, G.; Fatone, F.; Bolzonella, D.; Cecchi, F. Occurrence and fate of heavy metals in large wastewater treatment plants treating municipal and industrial wastewaters. Water Sci. Technol. 2008, 57, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Cantinho, P.; Matos, M.; Trancoso, M.A.; Dos Santos, M.M.C. Behaviour and fate of metals in urban wastewater treatment plants: A review. Int. J. Environ. Sci. Technol. 2016, 13, 359–386. [Google Scholar] [CrossRef]
- Ida, S.; Eva, T. Removal of Heavy Metals during Primary Treatment of Municipal Wastewater and Possibilities of Enhanced Removal: A Review. Water 2021, 13, 1121. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future, 1st ed.; Sobti, R., Arora, N., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Chaudhary, M.; Walker, T.R.; Willis, R.; Oakes, K. Baseline characterization of sediments and marine biota near industrial effluent discharge in Northumberland Strait, Nova Scotia, Canada. Mar. Pollut. Bull. 2020, 157, 111372. [Google Scholar] [CrossRef]
- Industrial Waste Water Treatment—Pressures on Europe’s Environment. EEA Report No 23/2018. Available online: https://www.eea.europa.eu/publications/industrial-waste-water-treatment-pressures (accessed on 14 July 2021).
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Union 2000, 327, 1–73. [Google Scholar]
- Martin Ruel, S.; Choubert, J.M.; Budzinski, H.; Miege, C.; Esperanza, M.; Coquery, M. Occurrence and fate of relevant substances in wastewater treatment plants regarding Water Framework Directive and future legislations. Water Sci. Technol. 2012, 65, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, 226, 1–17. [Google Scholar]
- Ministerio de Agricultura, Alimentación y Medio Ambiente. Real Decreto 817/2015, de 11 de septiembre, por el que se establecen los criterios de seguimiento y evaluación del estado de las aguas superficiales y las normas de calidad ambiental. B.O.E. 2015, 219, 80582–80677. [Google Scholar]
- Tercier Waeber, M.L.; Stoll, S.; Slaveykova, V. Trace metal behaviour in surface waters: Emphasis on dynamic speciation, sorption processes and bioavailability. Arch. Sci. 2012, 65, 119–142. Available online: https://archive-ouverte.unige.ch/unige:27739 (accessed on 6 October 2021).
- Buzier, R.; Tusseau-Vuillemin, M.H.; Keirsbulck, M.; Mouchel, J.M. Inputs of total and labile trace metals from wastewater treatment plants effluents to the Seine River. Phys. Chem. Earth. 2011, 36, 500–505. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Oropeza-Guzman, M.T.; Jurado-Baizaval, J.L.; Ochoa-Terán, A. Coagulation–flocculation mechanisms in wastewater treatment plants through zeta potential measurements. J. Hazard. Mater. 2014, 279, 1–10. [Google Scholar] [CrossRef]
- Bubb, J.M.; Lester, J.N. The significance of sediment metal concentrations in two eroding Essex salt marshes. Mar. Pollut. Bull. 1995, 30, 190–199. [Google Scholar] [CrossRef]
- Gagnon, C.; Saulnier, I. Distribution and fate of metals in the dispersion plume of a major municipal effluent. Environ. Pollut. 2003, 124, 47–55. [Google Scholar] [CrossRef]
- Buzier, R.; Tusseau-Vuillemin, M.H.; dit Meriadec, C.M.; Rousselot, O.; Mouchel, J.M. Trace metal speciation and fluxes within a major French wastewater treatment plant: Impact of the successive treatments stages. Chemosphere 2006, 65, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Gourlay-Francé, C.; Bressy, A.; Uher, E.; Lorgeoux, C. Labile, dissolved and particulate PAHs and trace metals in wastewater: Passive sampling, occurrence, partitioning in treatment plants. Water Sci. Technol. 2011, 63, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Merrington, G.; Leverett, D.; Ellor, B.; Lofts, S.; Gravell, A. The effect of advanced treatment of sewage effluents on metal speciation and (bio) availability. Bull. Environ. Contam. Toxicol. 2014, 92, 248–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yapici, T.; Fasfous, I.I.; Murimboh, J.; Chakrabarti, C.L. Investigation of DGT as a metal speciation technique for municipal wastes and aqueous mine effluents. Anal. Chim. Acta. 2008, 622, 70–76. [Google Scholar] [CrossRef]
- Thomas, P. Metals pollution tracing in the sewerage network using the diffusive gradients in thin films technique. Water Sci. Technol. 2009, 60, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Buzier, R.; Tusseau-Vuillemin, M.H.; Mouchel, J.M. Evaluation of DGT as a metal speciation tool in wastewater. Sci. Total Environ. 2006, 358, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Davison, W.; Zhang, H. In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Zhao, C.M.; Campbell, P.C.G.; Wilkinson, K.J. When are metal complexes bioavailable? Environ. Chem. 2016, 13, 425–433. [Google Scholar] [CrossRef]
- Menegário, A.A.; Yabuki, L.N.M.; Luko, K.S.; Williams, P.N.; Blackburn, D.M. Use of diffusive gradient in thin films for in situ measurements: A review on the progress in chemical fractionation, speciation and bioavailability of metals in waters. Anal. Chim. Acta 2017, 983, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Strivens, J.; Hayman, N.; Rosen, G.; Myers-Pigg, A. Toward validation of toxicological interpretation of diffusive gradients in thin films in marine waters impacted by copper. Environ. Toxicol. Chem. 2020, 39, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gao, B.; Xu, D.; Liu, L. DGT: A promising technology for in-situ measurement of metal speciation in the environment. Sci. Total Environ. 2020, 715, 136810. [Google Scholar] [CrossRef]
- Bersuder, P.; Amouroux, I.; Belzunce-Segarra, M.J.; Bolam, T.; Caetano, M.; Carvalho, I.; dos Santose, M.C.; Fones, G.R.; Gonzalez, J.-L.; Guesdon, S.; et al. Concurrent sampling of transitional and coastal waters by Diffusive Gradient in Thin-films (DGT) and spot sampling for trace metals analysis. MethodsX 2021, 8, 101462. [Google Scholar] [CrossRef]
- Henríquez-Hernández, L.A.; Romero, D.; González-Antuña, A.; Gonzalez-Alzaga, B.; Zumbado, M.; Boada, L.D.; Hernández, A.F.; López-Flores, I.; Luzardo, O.P.; Lacasaña, M. Biomonitoring of 45 inorganic elements measured in plasma from Spanish subjects: A cross-sectional study in Andalusian population. Sci. Total Environ. 2020, 706, 135750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davison, W. Performance characteristics of the technique of diffusion gradients in thin-films (DGT) for the measurement of trace metals in aqueous solution. Anal. Chem. 1995, 67, 3391. [Google Scholar] [CrossRef]
- DGT® Research. Available online: https://www.dgtresearch.com/diffusion-coefficients/ (accessed on 10 July 2020).
- Panther, J.G.; Bennett, W.W.; Teasdale, P.R.; Welsh, D.T.; Zhao, H. DGT measurement of dissolved aluminum species in waters: Comparing Chelex-100 and titanium dioxide-based adsorbents. Environ. Sci. Technol. 2012, 46, 2267–2275. [Google Scholar] [CrossRef]
- Shiva, A.H.; Teasdale, P.R.; Welsh, D.T.; Bennett, W.W. Evaluation of the DGT technique for selective measurement of aluminium and trace metal concentrations in an acid drainage-impacted coastal waterway. Environ. Sci. Process. Impacts 2017, 19, 742–751. [Google Scholar] [CrossRef]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O. Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in Eastern Cape province, South Africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Iloms, E.; Ololade, O.O.; Ogola, H.J.; Selvarajan, R. Investigating industrial effluent impact on municipal wastewater treatment plant in Vaal, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1096. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, D. Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological; CRC Press: Boca Raton, FL, USA, 2016; pp. 191–235. [Google Scholar]
- Howe, P.; Malcolm, H.; Dobson, S. Manganese and Its Compounds: Environmental Aspects. World Health Organization, 2004. Available online: https://apps.who.int/iris/handle/10665/42992 (accessed on 6 October 2021).
- Rule, K.L.; Comber, S.D.W.M.; Ross, D.; Thornton, A.; Makropoulos, C.K.; Rautiu, R. Diffuse sources of heavy metals entering an urban wastewater catchment. Chemosphere 2006, 63, 64–72. [Google Scholar] [CrossRef]
- Gimpel, J.; Zhang, H.; Hutchinson, W.; Davison, W. Effect of solution composition, flow and deployment time on the measurement of trace metals by the diffusive gradient in thin films technique. Anal. Chim. Acta 2001, 448, 93–103. [Google Scholar] [CrossRef]
- Dragun, Z.; Raspor, B.; Roje, V. The labile metal concentrations in Sava River water assessed by diffusive gradients in thin films. Chem. Speciat. Bioavailab. 2008, 20, 33–46. [Google Scholar] [CrossRef]
- Wang, X.; Cuthbertson, A.; Gualtieri, C.; Shao, D. A review on mariculture effluent: Characterization and management tools. Water 2020, 12, 2991. [Google Scholar] [CrossRef]
- Ziolko, D.; Martin, O.V.; Scrimshaw, M.D.; Lester, J.N. An evaluation of metal removal during wastewater treatment: The potential to achieve more stringent final effluent standards. Crit. Rev. Environ. Sci. Technol. 2011, 41, 733–769. [Google Scholar] [CrossRef]
- European Commission. Guidance Document No. 27: Technical Guidance for Deriving Environmental Quality Standards. Available online: https://op.europa.eu/en/publication-detail/-/publication/d5b2b9b9-32fb-11e8-b5fe-01aa75ed71a1 (accessed on 6 October 2021).
- MONITOOL Project, New Tools for Monitoring the Chemical Status in Transitional and Coastal Waters under the WFD, Was Co-Financed by the European Regional Development Fund through the Interreg Atlantic Area Programme (n° Contract: EAPA_565/2016). Available online: www.monitoolproject.eu (accessed on 6 October 2021).
- Gardner, M.; Comber, S.; Scrimshaw, M.D.; Cartmell, E.; Lester, J.; Ellor, B. The significance of hazardous chemicals in wastewater treatment works effluents. Sci. Total Environ. 2012, 437, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Nezlin, N.P.; Beegan, C.; Feit, A.; Gully, J.R.; Latker, A.; McLaughlin, K.; Mengel, M.J.; Robertson, G.L.; Steele, A.; Weisberg, S.B. Colored Dissolved Organic Matter (CDOM) as a tracer of effluent plumes in the coastal ocean. Reg. Stud. Mar. Sci. 2020, 35, 101163. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Gilbert, A. Developing adaptive and integrated strategies for managing the electricity-water nexus. Univ. Richmond Law Rev. 2013, 48, 997. Available online: https://scholarship.richmond.edu/lawreview/vol48/iss3/6 (accessed on 6 October 2021).
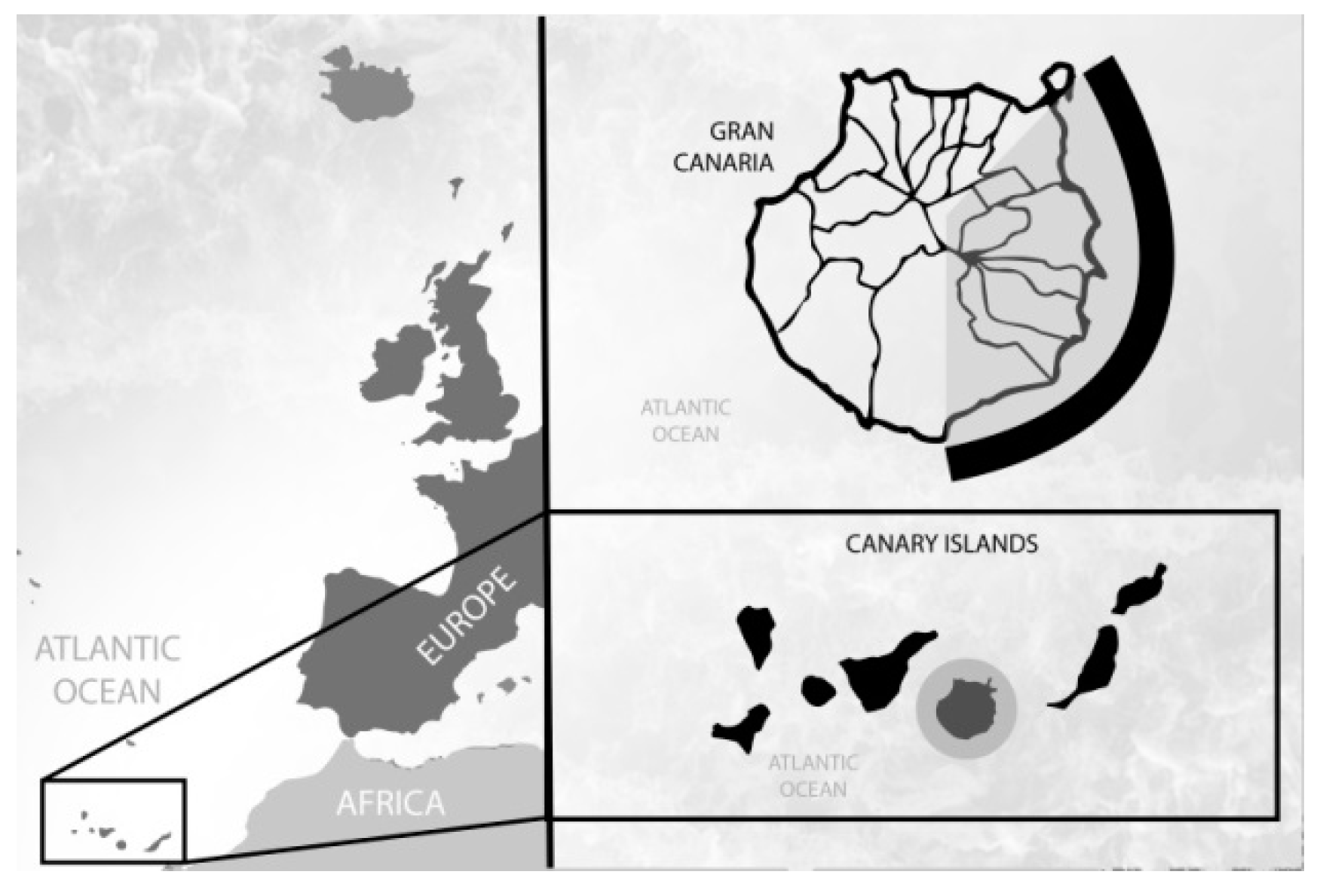
| Label | Type of Facility 1 | Wastewater Source | Size (p.e.) 2 | Main Treatment | Discharge Flow (m3/h) |
|---|---|---|---|---|---|
| TP-1 | WWTP | Household | 600,000 | Pre-treatment/Settling Activated sludge Chlorination | 1100 |
| TP-2 | WWTP | Household + industry (10%) | 171,600 | Pre-treatment/Settling Activated sludge Chlorination | 345 |
| TP-3 | WWTP | Household + industry (25%) | 50,000 | Pre-treatment Membrane bioreactor (MBR) Chlorination | 136 |
| TP-4 | WWTP | Industry | 4600 | Pre-treatment Coagulation–flocculation settling | 30 |
| TP-5 | TPP | Industry (cooling water of a thermal power plant) | No data | Aeration tanks/Settling | 27,500 |
| TP-6 | MFF | Aquaculture (indoor marine fish-farm) | No data | Settling | 75 |
| Metal | Fraction | Facility | |||||
|---|---|---|---|---|---|---|---|
| TP-1 | TP-2 | TP-3 | TP-4 | TP-5 | TP-6 | ||
| Cd | Dissolved, day 0 | 0.002 | 0.005 | 0.009 | 0.090 | 0.010 | 0.010 |
| Dissolved, day 2 | 0.002 | 0.010 | 0.013 | 0.088 | 0.017 | 0.012 | |
| Dissolved, day 4 | 0.001 | 0.004 | 0.013 | 0.017 | 0.013 | 0.010 | |
| Dissolved (mean ± SD) | 0.002 ± 0 | 0.007 ± 0.003 | 0.012 ± 0.002 | 0.065 ± 0.041 | 0.013 ± 0.004 | 0.011 ± 0.001 | |
| Labile | 0.001 | 0.001 | 0.003 | 0.001 | 0.003 | 0.008 | |
| % Labile | 80 | 18 | 25 | 2 | 25 | 71 | |
| Ni | Dissolved, day 0 | 2.242 | 2.820 | 1.104 | 18.319 | 3.700 | 0.146 |
| Dissolved, day 2 | 3.665 | 4.304 | 1.157 | 21.829 | 2.707 | 0.117 | |
| Dissolved, day 4 | 3.939 | 2.904 | 1.169 | 13.984 | 9.347 | 0.069 | |
| Dissolved (mean ± SD) | 3.282 ± 0.911 | 3.343 ± 0.833 | 1.143 ± 0.035 | 18.044 ± 3.930 | 5.251 ± 3.581 | 0.011 ± 0.001 | |
| Labile | 1.214 | 1.831 | 0.376 | 7.61 | 4.886 | 0.008 | |
| % Labile | 37 | 55 | 33 | 42 | 93 | 71 | |
| Pb | Dissolved, day 0 | 0.350 | 0.219 | 0.205 | 0.901 | 0.009 | 0.009 |
| Dissolved, day 2 | 0.377 | 0.353 | 0.130 | 0.764 | 0.067 | 0.009 | |
| Dissolved, day 4 | 0.387 | 0.254 | 0.157 | 0.076 | 0.009 | 0.009 | |
| Dissolved (mean ± SD) | 0.371 ± 0.019 | 0.276 ± 0.069 | 0.164 ± 0.038 | 0.580 ± 0.442 | 0.028 ± 0.033 | 0.009 ± 0 | |
| Labile | 0.047 | 0.024 | 0.017 | 0.02 | 0.031 | 0.037 | |
| % Labile | 13 | 9 | 10 | 3 | >100 | >100 | |
| Cr | Dissolved, day 0 | 0.786 | 0.447 | 0.500 | 4.296 | 0.133 | 0.196 |
| Dissolved, day 2 | 1.207 | 0.582 | 0.503 | 4.730 | 0.170 | 0.190 | |
| Dissolved, day 4 | 1.312 | 0.360 | 0.498 | 1.612 | 0.212 | 0.167 | |
| Dissolved (mean ± SD) | 1.102 ± 0.278 | 0.463 ± 0.112 | 0.5 ± 0.003 | 3.546 ± 1.689 | 0.172 ± 0.040 | 0.185 ± 0.015 | |
| Labile | 0.218 | 0.165 | 0.181 | 0.465 | 0.139 | 0.222 | |
| % Labile | 20 | 36 | 36 | 13 | 81 | >100 | |
| Cu | Dissolved, day 0 | 0.242 | 0.438 | 2.300 | 2.385 | 0.015 | 0.015 |
| Dissolved, day 2 | 0.135 | 1.330 | 1.666 | 1.998 | 0.015 | 0.015 | |
| Dissolved, day 4 | 0.182 | 0.403 | 1.356 | 0.440 | 0.015 | 0.015 | |
| Dissolved (mean ± SD) | 0.186 ± 0.054 | 0.724 ± 0.526 | 1.774 ± 0.481 | 1.608 ± 1.03 | 0.015 ± 0 | 0.015 ± 0 | |
| Labile | 0.068 | 0.196 | 0.38 | nd | 0.146 | 0.791 | |
| % Labile | 36 | 27 | 21 | - | >100 | >100 | |
| Zn | Dissolved, day 0 | 5.328 | 19.201 | 30.643 | 125.712 | 20.115 | 6.518 |
| Dissolved, day 2 | 5.249 | 26.584 | 31.016 | 136.248 | 21.053 | 6.413 | |
| Dissolved, day 4 | 4.903 | 20.147 | 32.818 | 82.488 | 5.059 | 5.294 | |
| Dissolved (mean ± SD) | 5.160 ± 0.226 | 21.977 ± 4.017 | 31.492 ± 1.163 | 114.816 ± 28.488 | 15.409 ± 8.976 | 6.075 ± 0.679 | |
| Labile | 1.92 | 8.799 | 12.997 | 19.943 | 1.361 | 3.675 | |
| % Labile | 37 | 40 | 41 | 17 | 9 | 60 | |
| Al | Dissolved, day 0 | 29.823 | 72.254 | 17.724 | 911.029 | 0.861 | 0.861 |
| Dissolved, day 2 | 32.419 | 44.602 | 20.245 | 1056.066 | 0.861 | 0.748 | |
| Dissolved, day 4 | 32.103 | 95.000 | 21.680 | 124.072 | 6.898 | 0.748 | |
| Dissolved (mean ± SD) | 31.448 ± 1.417 | 70.619 ± 25.238 | 19.883 ± 2.003 | 697.056 ± 501.489 | 2.874 ± 3.486 | 0.786 ± 0.065 | |
| Labile | ND | ND | ND | ND | ND | ND | |
| % Labile | - | - | - | - | - | - | |
| Fe | Dissolved, day 0 | 101.001 | 58.643 | 59.049 | 13,048.876 | 0.207 | 0.482 |
| Dissolved, day 2 | 114.755 | 108.238 | 52.918 | 11,627.790 | 0.207 | 0.207 | |
| Dissolved, day 4 | 118.142 | 73.133 | 56.386 | 4890.290 | 1.118 | 0.207 | |
| Dissolved (mean ± SD) | 111.299 ± 9.078 | 80.005 ± 25.501 | 56.118 ± 3.074 | 9855.652 ± 4358.438 | 0.511 ± 0.526 | 0.299 ± 0.159 | |
| Labile | 18.37 | 15.171 | 5.972 | 6200.034 | 3.07 | 4.575 | |
| % Labile | 17 | 19 | 11 | 63 | >100 | >100 | |
| Mn | Dissolved, day 0 | 30.919 | 14.048 | 1.328 | 829.592 | 1.335 | 1.234 |
| Dissolved, day 2 | 32.832 | 62.795 | 0.223 | 718.726 | 1.432 | 1.283 | |
| Dissolved, day 4 | 35.490 | 43.483 | 3.285 | 381.830 | 1.971 | 1.116 | |
| Dissolved (mean ± SD) | 33.080 ± 2.295 | 40.109 ± 24.548 | 1.612 ± 1.550 | 643.383 ± 233.195 | 1.579 ± 0.342 | 1.211 ± 0.086 | |
| Labile | 43.479 | 66.146 | 6.694 | 17.129 | 1.306 | 3.043 | |
| % Labile | >100 | >100 | >100 | 3 | 83 | >100 | |
| Co | Dissolved, day 0 | 0.251 | 0.613 | 0.283 | 7.344 | 0.360 | 0.007 |
| Dissolved, day 2 | 0.649 | 0.866 | 0.299 | 8.488 | 0.247 | 0.006 | |
| Dissolved, day 4 | 0.646 | 0.544 | 0.321 | 4.146 | 1.049 | 0.008 | |
| Dissolved (mean ± SD) | 0.515 ± 0.229 | 0.674 ± 0.17 | 0.301 ± 0.019 | 6.659 ± 2.251 | 0.552 ± 0.434 | 0.007 ± 0.001 | |
| Labile | 0.033 | 0.116 | 0.024 | 2.372 | 0.498 | 0.057 | |
| % Labile | 6 | 17 | 8 | 36 | 90 | >100 | |
| Metal | Fraction | Facility | |||||
|---|---|---|---|---|---|---|---|
| TP-1 | TP-2 | TP-3 | TP-4 | TP-5 | TP-6 | ||
| Cd | Total dissolved | 0.040 | 0.055 | 0.038 | 0.047 | 8.735 | 0.020 |
| Dissolved labile | 0.032 | 0.010 | 0.009 | 0.001 | 2.188 | 0.014 | |
| Ni | Total dissolved | 86.639 | 27.680 | 3.732 | 12.992 | 3465.770 | 0.199 |
| Dissolved labile | 32.041 | 15.164 | 1.226 | 5.479 | 3224.948 | 1.518 | |
| Pb | Total dissolved | 9.801 | 2.282 | 0.535 | 0.418 | 18.563 | 0.016 |
| Dissolved labile | 1.241 | 0.196 | 0.054 | 0.014 | 20.527 | 0.067 | |
| Cr | Total dissolved | 29.086 | 3.835 | 1.633 | 2.553 | 113.199 | 0.331 |
| Dissolved labile | 5.753 | 1.364 | 0.592 | 0.335 | 91.451 | 0.400 | |
| Cu | Total dissolved | 4.919 | 5.994 | 5.790 | 1.157 | 9.900 | 0.027 |
| Dissolved labile | 1.790 | 1.626 | 1.239 | n.a. | 96.509 | 1.423 | |
| Zn | Total dissolved | 136.217 | 181.973 | 102.791 | 82.667 | 10,169.972 | 10.935 |
| Dissolved labile | 50.686 | 72.855 | 42.421 | 14.359 | 898.025 | 6.615 | |
| Al | Total dissolved | 830.235 | 584.723 | 64.897 | 501.880 | 1896.635 | 1.415 |
| Dissolved labile | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
| Fe | Total dissolved | 2938.306 | 662.441 | 183.168 | 7096.069 | 337.149 | 0.538 |
| Dissolved labile | 484.962 | 125.615 | 19.494 | 4464.025 | 2025.898 | 8.234 | |
| Mn | Total dissolved | 873.325 | 332.102 | 5.262 | 463.236 | 1042.385 | 2.180 |
| Dissolved labile | 1147.854 | 547.690 | 21.850 | 12.333 | 861.648 | 5.478 | |
| Co | Total dissolved | 13.603 | 5.581 | 0.982 | 4.795 | 364.386 | 0.013 |
| Dissolved labile | 0.874 | 0.964 | 0.079 | 1.708 | 328.592 | 0.102 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo Sanz, M.; Millán Gabet, V.; Gonzalez, J.-L. Inputs of Total and Labile Dissolved Metals from Six Facilities Continuously Discharging Treated Wastewaters to the Marine Environment of Gran Canaria Island (Canary Islands, Spain). Int. J. Environ. Res. Public Health 2021, 18, 11582. https://doi.org/10.3390/ijerph182111582
Rodrigo Sanz M, Millán Gabet V, Gonzalez J-L. Inputs of Total and Labile Dissolved Metals from Six Facilities Continuously Discharging Treated Wastewaters to the Marine Environment of Gran Canaria Island (Canary Islands, Spain). International Journal of Environmental Research and Public Health. 2021; 18(21):11582. https://doi.org/10.3390/ijerph182111582
Chicago/Turabian StyleRodrigo Sanz, Marta, Vanessa Millán Gabet, and Jean-Louis Gonzalez. 2021. "Inputs of Total and Labile Dissolved Metals from Six Facilities Continuously Discharging Treated Wastewaters to the Marine Environment of Gran Canaria Island (Canary Islands, Spain)" International Journal of Environmental Research and Public Health 18, no. 21: 11582. https://doi.org/10.3390/ijerph182111582
APA StyleRodrigo Sanz, M., Millán Gabet, V., & Gonzalez, J.-L. (2021). Inputs of Total and Labile Dissolved Metals from Six Facilities Continuously Discharging Treated Wastewaters to the Marine Environment of Gran Canaria Island (Canary Islands, Spain). International Journal of Environmental Research and Public Health, 18(21), 11582. https://doi.org/10.3390/ijerph182111582






