Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters
Abstract
:1. Introduction
2. Particulate Matter, US Air Pollution Standards, Ultrafine Particles, Engineered Nanoparticles, and Internal Combustion Engine Emissions
3. Engineered Nanoparticles
4. Portals of Entry to the Brain and Key Neural Damage Mechanisms
5. Stroke and Air Pollution
6. Neurodegenerative Diseases and Nanoparticles
7. Psychiatric Symptoms in Neurodegenerative Diseases
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Ambient Air Quality Standards (NAAQS) for Particulate Matter. Available online: https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm (accessed on 9 July 2021).
- Rajagopalan, S.; Brauer, M.; Bhatnagar, A.; Deepak, L.B.; Brook, J.R.; Huang, W.; Münzel, T.; Newby, D.; Siegel, J.; Brook, R.D.; et al. Personal-Level Protective Actions against Particulate Matter Air Pollution Exposure: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e411–e431. [Google Scholar] [CrossRef]
- WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Independent Particulate Matter Review Panel; Frey, H.C.; Adams, P.J.; Adgate, J.L.; Allen, G.A.; Balmes, J.; Boyle, K.; Chow, J.C.; Dockery, D.W.; Felton, H.D. The Need for a Tighter Particulate-Matter Air-Quality Standard. N. Engl. J. Med. 2020, 383, 680–683. [Google Scholar]
- Rhew, S.H.; Kravchenko, J.; Lyerly, H.K. Exposure to low-dose ambient fine particulate matter PM2.5 and Alzheimer’s disease, non-Alzheimer’s dementia, and Parkinson’s disease in North Carolina. 2021, 16, e0253253. PLoS ONE 2021, 16, e0253253. [Google Scholar] [CrossRef]
- Johnson, K.C.; Durbin, T.D.; Jung, H.; Chaudhary, A.; Cocker, D.R.; Herner, J.D.; Robertson, W.H.; Huai, T.; Ayala, A.; Kittelson, D. Evaluation of the European PMP Methodologies during On-road and Chassis Dynamometer Testing for DPF Equipped Heavy Duty Diesel Vehicles. Aerosol Sci. Technol. 2009, 43, 962–969. [Google Scholar] [CrossRef]
- Ayala, A. Ultrafine Particles and Air Pollution Policy. In Ambient Combustion Particles and Health; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; Chapter 13; ISBN 9781536188318. [Google Scholar]
- Habre, R.; Girguis, M.; Urman, R.; Fruin, S.A.; Lurmann, F.; Shafer, M.; Gorski, P.R.; Franklin, M.; McConnell, R.; Avol, E.; et al. Contribution of tailpipe and non-tailpipe traffic sources to quasi-ultrafine, fine and coarse particulate matter in southern California. J. Air Waste Manag. Assoc. 2021, 71, 209–230. [Google Scholar] [CrossRef]
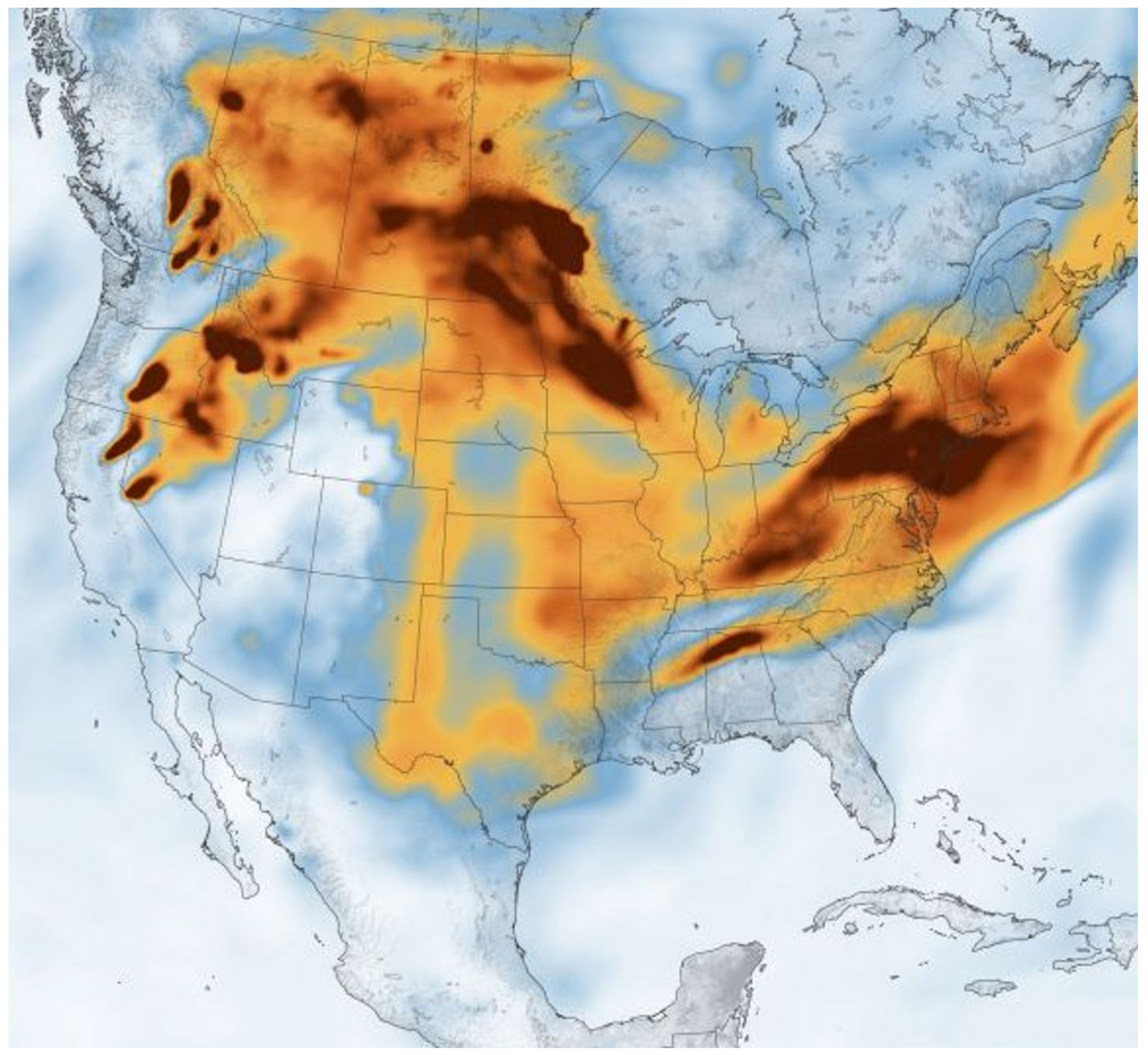
- Burke, M.; Driscoll, A.; Heft-Neal, S.; Xue, J.; Burney, J.; Wara, M. The changing risk and burden of wildfire in the United States. Proc. Natl. Acad. Sci. USA 2021, 118, e2011048118. [Google Scholar] [CrossRef]
- Cleland, S.E.; West, J.J.; Jia, Y.; Reid, S.; Raffuse, S.; O’Neill, S.; Serre, M.L. Estimating Wildfire Smoke Concentrations during the October 2017 California Fires through BME Space/Time Data Fusion of Observed, Modeled, and Satellite-Derived PM2.5. Environ. Sci. Technol. 2020, 54, 13439–13447. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Nowack, B. Size-Specific, Dynamic, Probabilistic Material Flow Analysis of Titanium Dioxide Releases into the Environment. Environ. Sci. Technol. 2021, 55, 2392–2402. [Google Scholar] [CrossRef]
- Egamaram, O.P.; Pillai, S.K.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2020, 96, 779–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
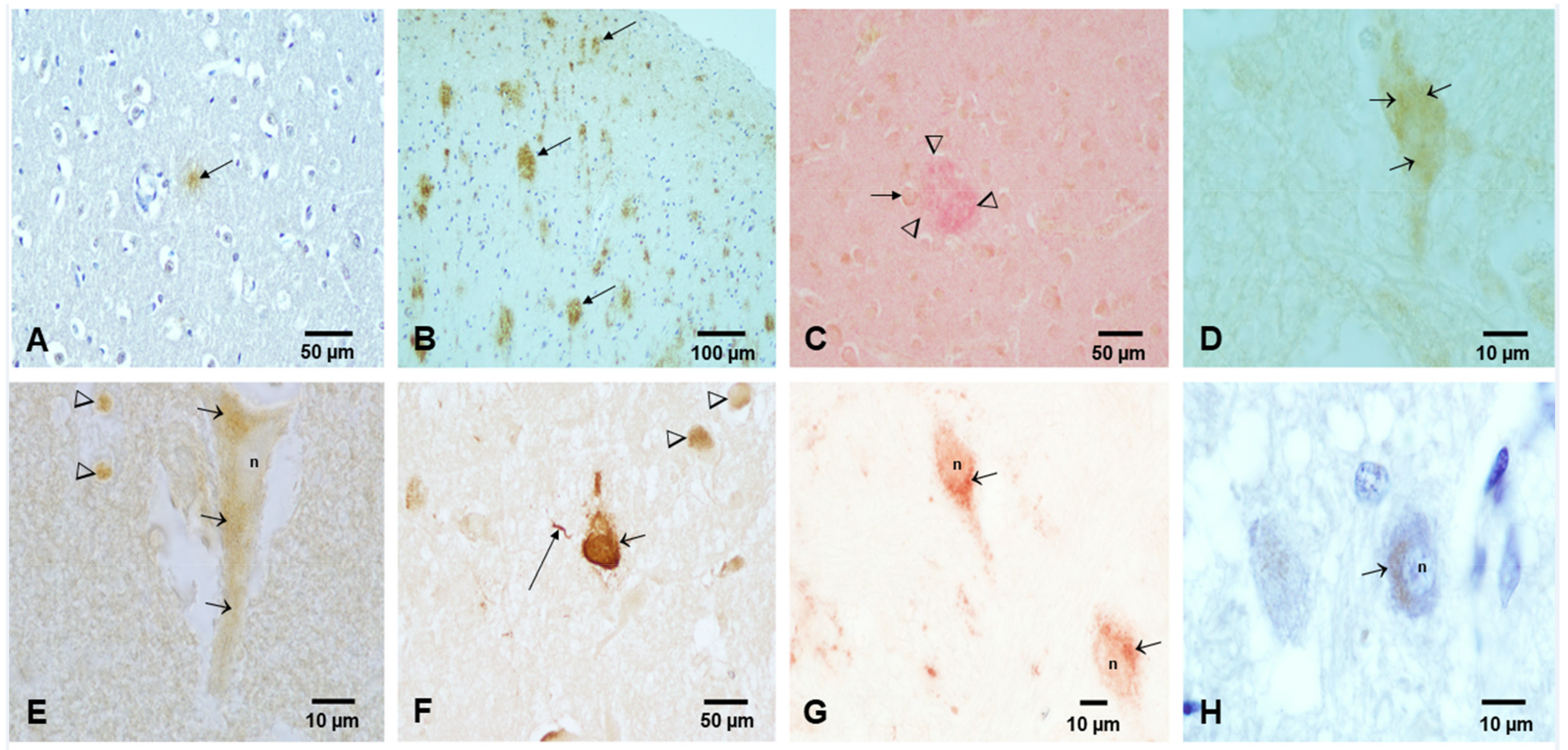
- Calderón-Garcidueñas, L.; Reynoso-Robles, R.; González-Maciel, A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: The culprit of Alzheimer and Parkinson’s diseases. Environ. Res. 2019, 176, 108574. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Delgado-Chávez, R.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Ávila-Ramírez, J.; Villarreal-Ríos, R. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤40 years of age. Environ. Res. 2018, 164, 475–487. [Google Scholar] [CrossRef]
- Diao, J.; Xia, Y.; Jiang, X.; Qiu, J.; Cheng, S.; Su, J.; Duan, X.; Gao, M.; Qin, X.; Zhang, J.; et al. Silicon dioxide nanoparticles induced neurobehavioral impairments by disrupting microbiota-gut-brain axis. J. Nanobiotechnol. 2021, 19, 174. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Reynoso-Robles, R.; Pérez-Guillé, B.; Mukherjee, P.S.; Gónzalez-Maciel, A. Combustion-derived nanoparticles, the neuroenteric system, cervical vagus, hyperphosphorylated alpha synuclein and tau in young Mexico City residents. Environ. Res. 2017, 159, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, J.S.; Pan, D.C.; Myerson, J.W.; Marcos-Contreras, O.A.; Villa, C.H.; Patel, P.; Hekierski, H.; Chatterjee, S.; Tao, J.Q.; Parhiz, H.; et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018, 9, 2684. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Pretini, V.; Koenen, M.H.; Kaestner, L.; Fens, M.H.A.M.; Schiffelers, R.M.; Bartels, M.; Van Wijk, R. Red Blood Cells: Chasing Interactions. Front. Physiol. 2019, 10, 945. [Google Scholar] [CrossRef] [Green Version]
- Morton, H.; Kshirsagar, S.; Orlov, E.; Bunquin, L.E.; Sawant, N.; Boleng, L.; George, M.; Basu, T.; Ramasubramanian, B.; Pradeepkiran, J.A.; et al. Defective mitophagy and synaptic degeneration in Alzheimer’s disease: Focus on aging, mitochondria and synapse. Free Radic. Biol. Med. 2021, 172, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, L.; de la Cueva, L.; Moros, M.; Mazarío, E.; de Bernardo, S.; de la Fuente, J.M.; Morales, M.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parveen, R.; Shamsi, T.N.; Fatima, S. Nanoparticles-protein interaction: Role in protein aggregation and clinical implications. Int. J. Biol. Macromol. 2017, 94 Pt A, 386–395. [Google Scholar] [CrossRef]
- Hartl, F.U. Protein Misfolding Diseases. Annu. Rev. Biochem. 2017, 86, 21–26. [Google Scholar] [CrossRef] [Green Version]
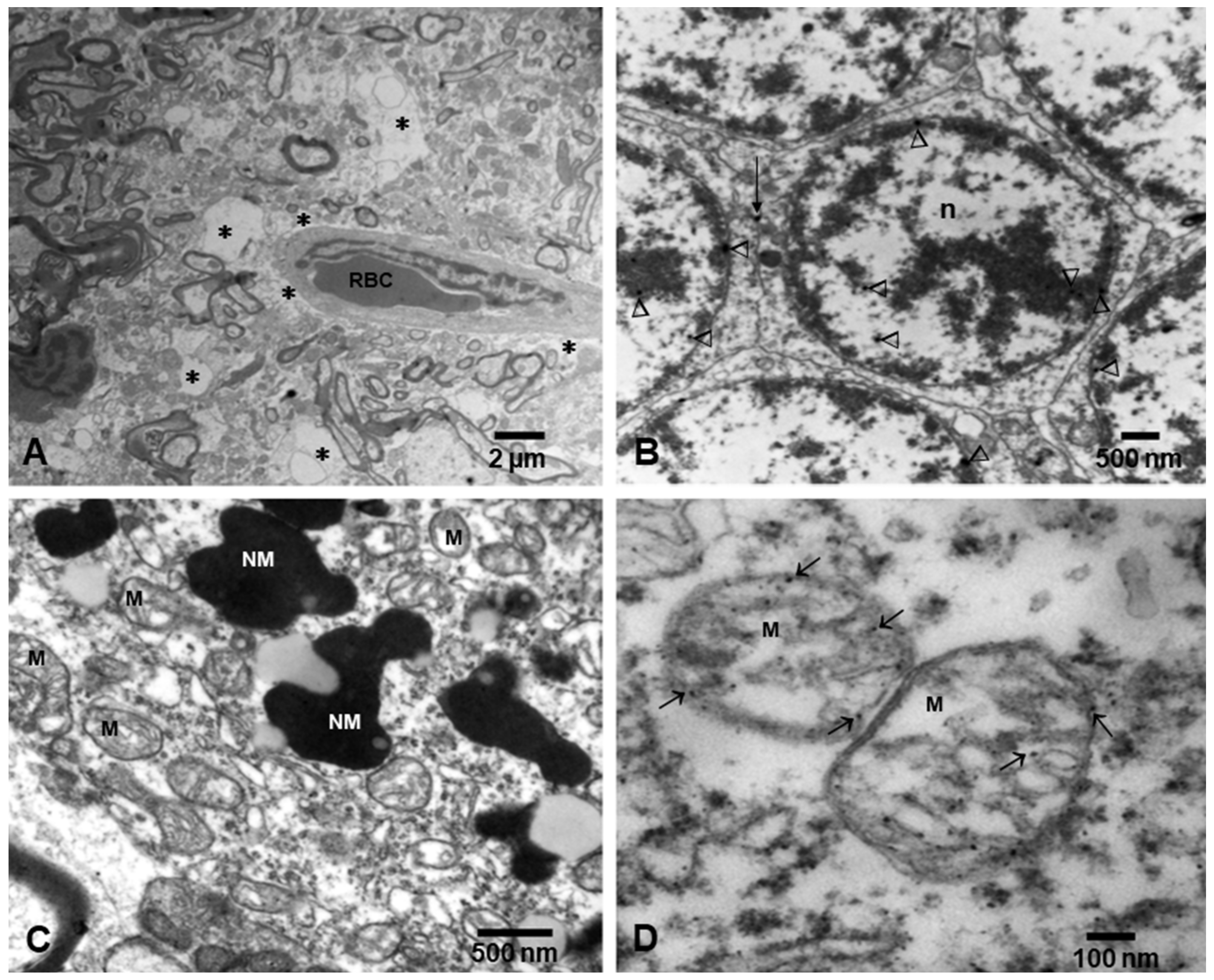
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Hammond, J.; Kulesza, R.; Lachmann, I.; Torres-Jardón, R.; Mukherjee, P.S.; Maher, B.A. Quadruple abnormal protein aggregates in brainstem pathology and exogenous metal-rich magnetic nanoparticles (and engineered Ti-rich nanorods). The substantia nigrae is a very early target in young urbanites and the gastrointestinal tract a key brainstem portal. Environ. Res. 2020, 191, 110139. [Google Scholar] [CrossRef]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M.; et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Boot, E.; Ekker, M.S.; Putaala, J.; Kittner, S.; De Leeuw, F.E.; Tuladhar, A.M. Ischaemic stroke in young adults: A global perspective. J. Neurol. Neurosurg. Psychiatry 2020, 91, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, E.B.; Sloan, M.A. Recent racial/ethnic disparities in stroke hospitalizations and outcomes for young adults in Florida, 2001–2006. Neuroepidemiology 2009, 32, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Shi, Y.; Chen, N.; Wang, H.; Chen, T. Ambient fine particulate matter and hospital admissions for ischemic and hemorrhagic strokes and transient ischemic attack in 248 Chinese cities. Sci. Total Environ. 2020, 715, 136896. [Google Scholar] [CrossRef]
- Byrne, C.P.; Bennett, K.E.; Hickey, P.; Broderick, B.; O’Mahony, M.; Williams, D.J. Short-Term Air Pollution as a Risk for Stroke Admission: A Time-Series Analysis. Cerebrovasc. Dis. 2020, 49, 404–411. [Google Scholar] [CrossRef]
- Ljungman, P.L.S.; Andersson, N.; Stockfelt, L.; Andersson, E.M.; Sommar, J.N.; Eneroth, K.; Gidhagen, L.; Johansson, C.; Lager, A.; Leander, K.; et al. Long-Term Exposure to Particulate Air Pollution, Black Carbon, and Their Source Components in Relation to Ischemic Heart Disease and Stroke. Environ. Health Perspect. 2019, 127, 107012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.R.; Lin, Y.T.; Hwang, B.F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. J. Alzheimer’s. Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Russ, T.C.; Cherrie, M.P.C.; Dibben, C.; Tomlinson, S.; Reis, S.; Dragosits, U.; Vieno, M.; Beck, R.; Carnell, E.; Niamh K Shortt, N.K.; et al. Life course air pollution exposure and cognitive decline: Modelled historical air pollution data and the Lothian birth cohort 1936. J. Alzheimer’s Dis. 2021, 79, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Ljungman, P.L.S.; Eneroth, K.; Bellander, T.; Rizzuto, D. Association between Cardiovascular Disease and Long-term Exposure to Air Pollution with the Risk of Dementia. JAMA Neurol. 2020, 77, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, A.; Burtscher, H.; Loretz, S.; Kasper, M.; Czerwinski, J.C. High air pollution in vehicle cabins due to traffic nanoparticle emission exposure and a solution for in-use vehicles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 421, 032018. [Google Scholar] [CrossRef]
- Lee, P.C.; Liu, L.L.; Sun, Y.; Chen, Y.A.; Liu, C.C.; Li, C.Y.; Yu, H.L.; Ritz, B. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environ. Int. 2016, 96, 75–81. [Google Scholar] [CrossRef]
- Fleury, V.; Himsl, R.; Joost, S.; Nicastro, N.; Bereau, M.; Guessous, I.; Burkhard, P.R. Geospatial analysis of individual-based Parkinson’s disease data supports a link with air pollution: A case-control study. Parkinsonism Relat. Disord. 2021, 83, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wu, X.; Yazdi, M.D.; Braun, D.; Awad, Y.A.; Wei, Y.; Liu, P.; Di, Q.; Wang, Y.; Schwartz, J.; et al. Long-term effects of PM2.5 on neurological disorders in the American Medicare population: A longitudinal cohort study. Lancet Planet. Health 2020, 4, e557–e565. [Google Scholar] [CrossRef]
- Baker, M.G.; Criswell, S.R.; Racette, B.A.; Simpson, C.D.; Sheppard, L.; Checkoway, H.; Seixas, N.S. Neurological outcomes associated with low-level manganese exposure in an inception cohort of asymptomatic welding trainees. Scand. J. Work Environ. Health 2015, 41, 94–101. [Google Scholar] [CrossRef]
- Racette, B.A.; Nelson, G.; Dlamini, W.W.; Prathibha, P.; Turner, J.R.; Ushe, M.; Checkoway, H.; Sheppard, L.; Nielsen, S.S. Severity of parkinsonism associated with environmental manganese exposure. Environ. Health 2021, 20, 27. [Google Scholar] [CrossRef]
- Mehta, P.; Kaye, W.; Raymond, J.; Punjani, R.; Larson, T.; Cohen, J.; Muravov, O.; Horton, K. Prevalence of Amyotrophic Lateral Sclerosis-United States, 2015 MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Malagoli, C.; Violi, F.; Mandrioli, J.; Consonni, D.; Rothman, K.J.; Wise, L.A. Amyotrophic lateral sclerosis incidence following exposure to inorganic selenium in drinking water: A long-term follow-up. Environ Res. 2019, 179 Pt A, 108742. [Google Scholar] [CrossRef]
- Tesauro, M.; Bruschi, M.; Filippini, T.; D’Alfonso, S.; Mazzini, L.; Corrado, L.; Consonni, M.; Vinceti, M.; Fusi, P.; Urani, C. Metal(loid)s role in the pathogenesis of amyotrophic lateral sclerosis: Environmental, epidemiological, and genetic data. Environ. Res. 2021, 192, 110292. [Google Scholar] [CrossRef]
- Andrew, A.S.; Bradley, W.G.; Peipert, D.; Butt, T.; Amoako, K.; Pioro, E.P.; Tandan, R.; Novak, J.; Quick, A.; Pugar, K.D.; et al. Risk factors for amyotrophic lateral sclerosis: A regional United States case-control study. Muscle Nerve 2021, 63, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.M.; Malek, A.M.; Foulds, A.; Rager, J.; Deperrior, S.E.; Vena, J.E.; Larson, T.C.; Mehta, P.; Horton, D.K.; Talbott, E.O. Recruitment of population-based controls for ALS cases from the National ALS Registry. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 395–400. [Google Scholar]
- Haley, R.W. Excess incidence of ALS in young Gulf War veterans. Neurology 2003, 61, 750–756. [Google Scholar] [CrossRef] [PubMed]
- McKay, K.A.; Smith, K.A.; Smertinaite, L.; Fang, F.; Ingre, C.; Taube, F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol. Scand. 2021, 143, 39–50. [Google Scholar] [CrossRef]
- Numan, M.S.; Brown, J.P.; Michou, L. Impact of air pollutants on oxidative stress in common autophagy-mediated aging diseases. Int. J. Environ. Res. Public Health 2015, 12, 2289–2305. [Google Scholar] [CrossRef] [Green Version]
- Annor, F.B.; Bayakly, R.A.; Morrison, R.A.; Bryan, M.J.; Gilbert, L.K.; Ivey-Stephenson, A.Z.; Holland, K.M.; Simon, T.R. Suicide Among Persons with Dementia, Georgia, 2013 to 2016. J. Geriatr. Psychiatry Neurol. 2019, 32, 31–39. [Google Scholar] [CrossRef]
- Menculini, G.; Chipi, E.; Paoletti, F.P.; Gaetani, L.; Nigro, P.; Simoni, S.; Mancini, A.; Tambasco, N.; Di Filipo, M.; Tortorella, A.; et al. Insights into the Pathophysiology of Psychiatric Symptoms in Central Nervous System Disorders: Implications for Early and Differential Diagnosis. Int. J. Mol. Sci. 2021, 22, 4440. [Google Scholar] [CrossRef]
- Lund, E.M.; Hostetter, T.A.; Forster, J.E.; Hoffmire, C.A.; Sterns-Yoder, K.A.; Brenner, L.A.; Tahmasbi Sohi, M. Suicide among veterans with amyotrophic lateral sclerosis. Muscle Nerve 2021, 63, 807–811. [Google Scholar] [CrossRef]
- Fahed, M.; Steffens, D.C. Apathy: Neurobiology, Assessment and Treatment. Clin. Psychopharmacol. Neurosci. 2021, 19, 181–189. [Google Scholar] [CrossRef]
- Giannouli, V.; Tsolaki, M. Is Depression or Apathy Playing a Key Role in Predicting Financial Capacity in Parkinson’s Disease with Dementia and Frontotemporal Dementia? Brain Sci. 2021, 11, 785. [Google Scholar] [CrossRef]
- Gladka, A.; Rymaszewska, J.; Zatoński, T. Impact of air pollution on depression and suicide. Int. J. Occup. Med. Environ. Health 2018, 31, 711–721. [Google Scholar] [PubMed]
- Liu, Q.; Wang, W.; Gu, X.; Deng, F.; Wang, X.; Lin, H.; Guo, X.; Wu, S. Association between particulate matter air pollution and risk of depression and suicide: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2021, 28, 9029–9049. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Cummings, J.; Blennow, K.; Gao, P.; Jack, C.R.; Vergallo, A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 2021, 17, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Singh, S.; Patel, K.; Goyal, H.; Shah, M.; Mansuri, Z.; Patel, S.; Mahuwala, Z.K.; Goldstein, L.B.; Qureshi, A.I. Stroke in young cannabis users (18-49 years): National trends in hospitalizations and outcomes. Int. J. Stroke 2020, 15, 535–539. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Garcidueñas, L.; Stommel, E.W.; Rajkumar, R.P.; Mukherjee, P.S.; Ayala, A. Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters. Int. J. Environ. Res. Public Health 2021, 18, 11568. https://doi.org/10.3390/ijerph182111568
Calderón-Garcidueñas L, Stommel EW, Rajkumar RP, Mukherjee PS, Ayala A. Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters. International Journal of Environmental Research and Public Health. 2021; 18(21):11568. https://doi.org/10.3390/ijerph182111568
Chicago/Turabian StyleCalderón-Garcidueñas, Lilian, Elijah W. Stommel, Ravi Philip Rajkumar, Partha S. Mukherjee, and Alberto Ayala. 2021. "Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters" International Journal of Environmental Research and Public Health 18, no. 21: 11568. https://doi.org/10.3390/ijerph182111568
APA StyleCalderón-Garcidueñas, L., Stommel, E. W., Rajkumar, R. P., Mukherjee, P. S., & Ayala, A. (2021). Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters. International Journal of Environmental Research and Public Health, 18(21), 11568. https://doi.org/10.3390/ijerph182111568





