Pediatric Hypothermia: An Ambiguous Issue
Abstract
1. Introduction
2. Basic Principles and Definitions
2.1. Pediatric vs. Adult Thermoregulation
2.2. Accidental vs. Induced Hypothermia
2.3. Induced Hypothermia vs. Natural Hibernation
2.4. Natural Hypometabolism vs. Energy Failure
3. Neonatal Hypothermia
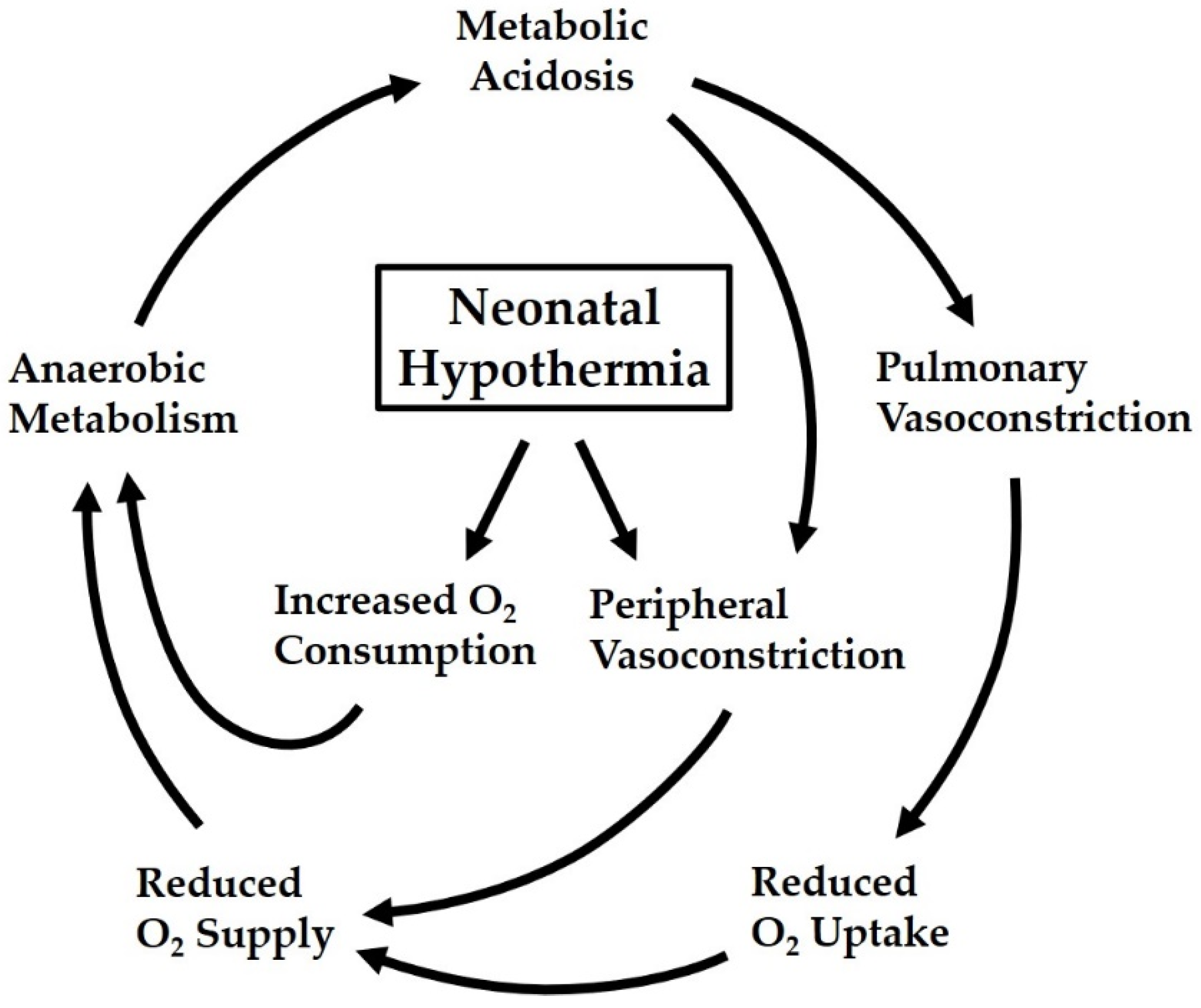
3.1. Hypothermia as a Risk Factor in Neonatal Transition
3.2. Hypothermia as an Outcome Predictor in Preterm Neonates
3.3. Hypothermia as a Self-Protective Mechanism in Perinatal Hypoxia
3.4. Hypothermia as a Treatment Option after Perinatal Asphyxia
4. Pediatric Hypothermia
4.1. Hypothermia Tolerance and Intolerance in Children
4.2. Adverse Effects of Unintentional Hypothermia in Children
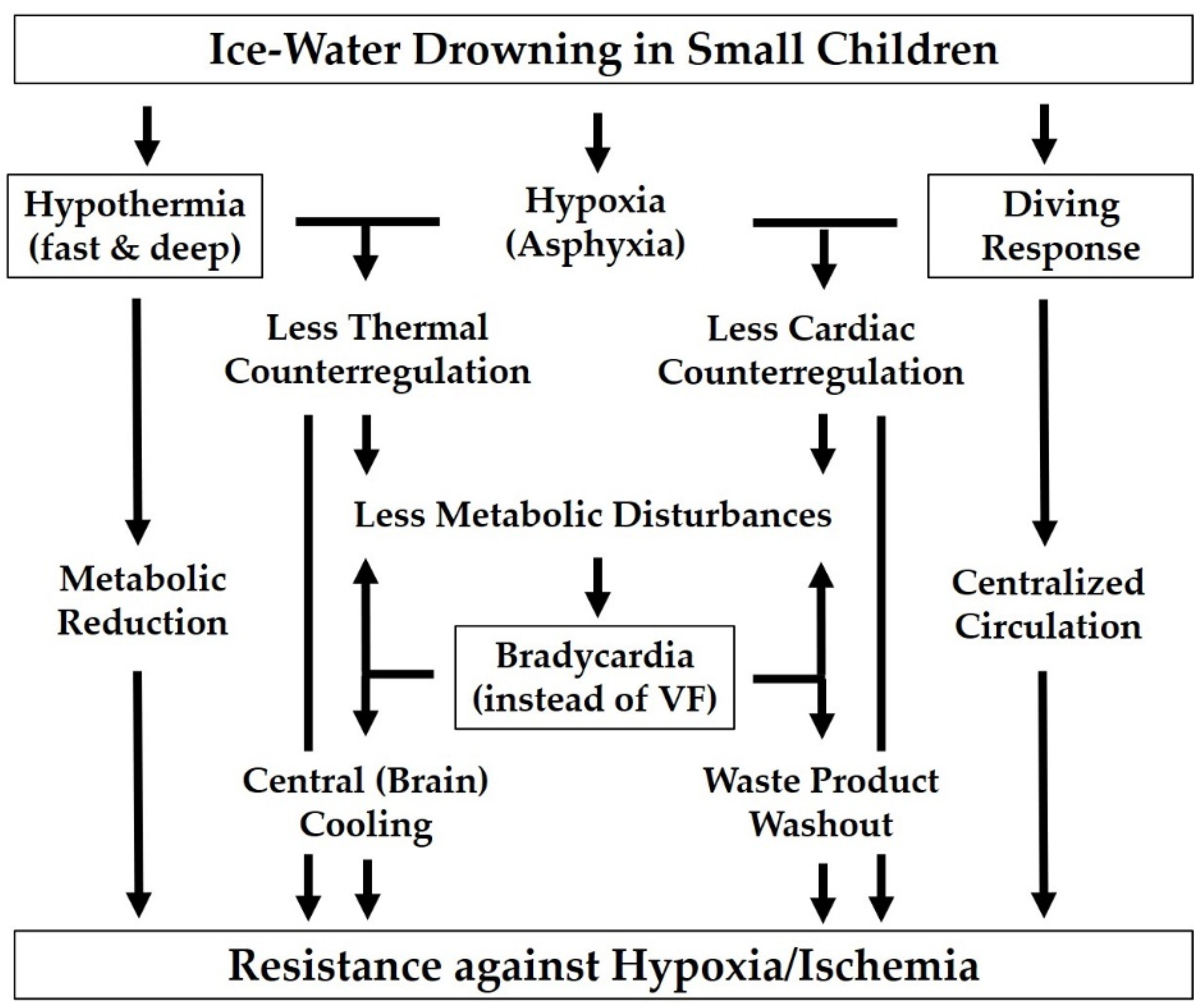
4.3. Surviving Profound Accidental Hypothermia in Young Children
- Due to the huge temperature gradient and the large surface-to-volume ratio, thermoregulation is overwhelmed so rapidly that the child is virtually “shock-frozen” and enters the state of hypothermia without major metabolic disturbances.
- As a result of their aforementioned lower propensity to cardiac arrhythmias [65,66], small children are less likely to experience early ventricular fibrillation, even in cold water, than adult shipwreck victims who may suffer cardiac arrest (of whatever type) before a protective degree of hypothermia has been reached. A preserved heartbeat, albeit at slow pace, results in a residual tissue perfusion, delaying the progression of ischemia/acidosis and providing a supplementary “central” and thus more homogeneous cooling of the most jeopardized brain.
- The higher basal metabolic rate of small beings implies a larger distance to a minimal metabolic rate that may allow lower temperatures to be tolerated (cf. Figure 1).
4.4. Hypothermia as a Treatment Option after Hypoxic Events in Children
5. Conclusions
5.1. Propensity and Tolerance to Hypothermia in Children
5.2. Peculiar Role of Neonates with Regard to Hypothermia
5.3. Ambiguity of Being Cold in Childhood Emergencies
5.4. Current Limitations and Future Prospects of Therapeutic Hypothermia in Pediatrics
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brück, K. Heat production and temperature regulation. In Perinatal Physiology; Stave, U., Ed.; Plenum: New York, NY, USA, 1978; Chapter 21; pp. 455–498. [Google Scholar]
- Polin, R.A.; Fox, W.W.; Abman, S.H. Fetal and Neonatal Physiology, 4th ed.; Thermoregulation; Elesevier Saunders: Philadelphia, PA, USA, 2011; Section IX; pp. 615–670. [Google Scholar]
- Singer, D.; Schiffmann, H. Thermoregulatorische Besonderheiten des pädiatrischen Patienten. In Perioperative Hypothermie–Probleme, Prävention und Therapie; Weyland, W., Braun, U., Kettler, D., Eds.; Aktiv Druck & Verlag: Ebelsbach, Germany, 1997; pp. 110–122. [Google Scholar]
- Singer, D.; van der Meer, F.; Perez, A. What is the right temperature for a neonate. Pediatr. Adolesc. Med. 2020, 22, 95–111. [Google Scholar] [CrossRef]
- Singer, D. Phylogenese des Stoffwechsels der Säugetiere. Anaesthesiol. Intensivmed. Notfallmed. Schmerzther. 2002, 37, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Singer, D. Warum 37 °C? Evolutionäre Grundlagen der Thermoregulation. Anaesthesist 2007, 56, 899–906. [Google Scholar] [CrossRef]
- Geiser, F. Reduction of metabolism during hibernation and daily torpor in mammals and birds: Temperature effect or physiological inhibition? J. Comp. Physiol. B 1988, 158, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Heldmaier, G.; Ortmann, S.; Elvert, R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 2004, 141, 317–329. [Google Scholar] [CrossRef]
- Storey, K.B.; Heldmaier, G.; Rider, M.H. Mammalian hibernation: Physiology, cell signaling, and gene controls on metabolic rate depression. In Dormancy and Resistance to Harsh Environments (Topics in Current Genetics); Lubzens, E., Cerdà, J., Clark, M., Eds.; Springer: Heidelberg, Germany, 2010; Volume 21, Chapter 13; pp. 227–252. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Scaling: Why Is Animal Size So Important? Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Smil, V. Laying down the law: Every living thing obeys the rules of scaling discovered by Max Kleiber (Millenium Essay). Nature 2000, 403, 597. [Google Scholar] [CrossRef]
- Lindstedt, S.L. Body size, time and dimensions of oxygen diffusion. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2021, 252, 110847. [Google Scholar] [CrossRef]
- Singer, D.; Bretschneider, H.J. Metabolic reduction in hypothermia: Pathophysiological problems and natural examples—Part 1/2. Thorac. Cardiovasc. Surg. 1990, 38, 205–219. [Google Scholar] [CrossRef]
- Singer, D.; Bach, F.; Bretschneider, H.J.; Kuhn, H.J. Metabolic size allometry and the limits to beneficial metabolic reduction: Hypothesis of a uniform specific minimal metabolic rate. In Surviving Hypoxia: Mechanisms of Control and Adaptation; Hochachka, P.W., Lutz, P.L., Sick, T., Rosenthal, M., van den Thillart, G., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 447–458. [Google Scholar]
- Singer, D. Metabolic adaptation to hypoxia: Cost and benefit of being small. Respir. Physiol. Neurobiol. 2004, 141, 215–228. [Google Scholar] [CrossRef]
- Watts, P.D.; Øritsland, N.A.; Jonkel, C.; Ronald, K. Mammalian hibernation and the oxygen consumption of a denning black bear (Ursus americanus). Comp. Biochem. Physiol. 1981, 69, 121–123. [Google Scholar] [CrossRef]
- Tøien, Ø.; Blake, J.; Edgar, D.M.; Grahn, D.A.; Heller, H.C.; Barnes, B.M. Hibernation in black bears: Independence of metabolic suppression from body temperature. Science 2011, 331, 906–909. [Google Scholar] [CrossRef]
- Mortola, J.P. How newborn mammals cope with hypoxia. Respir. Physiol. 1999, 116, 95–103. [Google Scholar] [CrossRef]
- Singer, D. Neonatal tolerance to hypoxia. A comparative-physiological approach. Comp. Biochem. Physiol. 1999, 123, 221–234. [Google Scholar] [CrossRef]
- Singer, D. The human fetus and metabolic adaptations to hypoxia. In Hypoxic Respiratory Failure in the Newborn: From Origins to Clinical Management; Dakshinamurti, S., Ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2021; Chapter 2; pp. 6–11. [Google Scholar]
- Gagnon, M.H.; Wintermark, P. Effect of persistent pulmonary hypertension on brain oxygenation in asphyxiated term newborns treated with hypothermia. J. Matern. Fetal Neonatal Med. 2016, 29, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, J.R.G.; Lopriore, E.; Te Pas, A.B.; Rijken, M.; van Zwet, E.W.; de Bruine, F.T.; Steggerda, S.J. Persistent pulmonary hypertension in neonates with perinatal asphyxia and therapeutic hypothermia: A frequent and perilous combination. J. Matern. Fetal Neonatal Med. 2021, 1–7. [Google Scholar] [CrossRef]
- Okken, A.; Koch, J. (Eds.) Thermoregulation of Sick and Low Birth Weight Neonates; Springer: Berlin, Germany, 1995. [Google Scholar]
- Agren, J. The thermal environment of the intensive care nursery. In Fanaroff & Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant, 10th ed.; Martin, R.J., Fanaroff, A.A., Walsh, M.C., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2015; Chapter 36; pp. 502–512. [Google Scholar]
- Hey, E.N.; Katz, G. Temporary loss of a metabolic response to cold stress in infants of low birthweight. Arch. Dis. Child. 1969, 44, 323–330. [Google Scholar] [CrossRef][Green Version]
- Sargant, N.; Sen, E.S.; Marden, B. Too cold for comfort: A neonate with severe hypothermia. Emerg. Med. J. 2012, 29, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Miller, F.S.; Westin, B. Hypothermia in the treatment of asphyxia neonatorum. Biol. Neonat. 1964, 6, 148–163. [Google Scholar] [CrossRef]
- Miller, J.A.; Miller, F.S. Mechanisms of hypothermic protection against anoxia. Adv. Exp. Med. Biol. 1972, 33, 571–586. [Google Scholar] [CrossRef]
- Silverman, W.A.; Fertig, J.W.; Berger, A.P. The influence of the thermal environment upon the survival of newly born premature infants. Pediatrics 1958, 22, 876–886. [Google Scholar] [PubMed]
- Day, R.L.; Caliguiri, L.; Kamenski, C.; Ehrlich, F. Body temperature and survival of premature infants. Pediatrics 1964, 34, 171–181. [Google Scholar] [PubMed]
- Lyu, Y.; Shah, P.S.; Ye, X.Y.; Warre, R.; Piedboeuf, B.; Deshpandey, A.; Dunn, M.; Lee, S.K. (Canadian Neonatal Network): Association between admission temperature and mortality and major morbidity in preterm infants born at fewer than 33 weeks’ gestation. JAMA Pediatr. 2015, 169, e150277. [Google Scholar] [CrossRef]
- Wilson, E.; Maier, R.F.; Norman, M.; Misselwitz, B.; Howell, E.A.; Zeitlin, J.; Bonamy, A.K. (Effective Perinatal Intensive Care in Europe [EPICE] Research Group): Admission hypothermia in very preterm infants and neonatal mortality and morbidity. J. Pediatr. 2016, 175, 61–67.e4. [Google Scholar] [CrossRef]
- Laptook, A.R.; Bell, E.F.; Shankaran, S.; Boghossian, N.S.; Wyckoff, M.H.; Kandefer, S.; Walsh, M.; Saha, S.; Higgins, R. (NICHD Neonatal Research Network): Admission temperature and associated mortality and morbidity among moderately and extremely preterm infants. J. Pediatr. 2018, 192, 53–59. [Google Scholar] [CrossRef] [PubMed]
- McCall, E.M.; Alderdice, F.; Halliday, H.L.; Vohra, S.; Johnston, L. Interventions to prevent hypothermia at birth in preterm and/or low birth weight infants. Cochrane Database Syst. Rev. 2018, 2, CD004210. [Google Scholar] [CrossRef] [PubMed]
- Boundy, E.O.; Dastjerdi, R.; Spiegelman, D.; Fawzi, W.W.; Missmer, S.A.; Lieberman, E.; Kajeepeta, S.; Wall, S.; Chan, G.J. Kangaroo mother care and neonatal outcomes: A meta-analysis. Pediatrics 2016, 137. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Díaz-Rossello, J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. Rev. 2016, CD002771. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Uhrig, C.; Sperling, P.; Pasel, K.; Wieland, C.; Versmold, H.T. Body temperatures and oxygen consumption during skin-to-skin (kangaroo) care in stable preterm infants weighing less than 1500 grams. J. Pediatr. 1997, 130, 240–244. [Google Scholar] [CrossRef]
- Heimann, K.; Ebert, A.M.; Abbas, A.K.; Heussen, N.; Leonhardt, S.; Orlikowsky, T. Thermoregulation of premature infants during and after skin-to-skin care. Z. Geburtshilfe Neonatol. 2013, 217, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, V.; Heinemann, A.B.; Sjörs, G.; Nykvist, K.H.; Agren, J. Early skin-to-skin care in extremely preterm infants: Thermal balance and care environment. J. Pediatr. 2012, 161, 422–426. [Google Scholar] [CrossRef]
- WHO Immediate KMC Study Group; Arya, S.; Naburi, H.; Kawaza, K.; Newton, S.; Anyabolu, C.H.; Bergman, N.; Rao, S.P.N.; Mittal, P.; Assenga, E.; et al. Immediate “Kangaroo Mother Care” and survival of infants with low birth weight. N. Engl. J. Med. 2021, 384, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Malan, A.; Mioskowski, E. pH-temperature interactions on protein function and hibernation: GDP binding to brown adipose tissue mitochondria. J. Comp. Physiol. B 1988, 158, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, D. Innate hypothermia after hypoxic ischaemic delivery. Neonatology 2015, 107, 220–223. [Google Scholar] [CrossRef]
- Serdarevich, C.; Fewell, J.E. Influence of core temperature on autoresuscitation during repeated exposure to hypoxia in normal rat pups. J. Appl. Physiol. 1999, 87, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Fewell, J.E. Protective responses of the newborn to hypoxia. Respir. Physiol. Neurobiol. 2005, 149, 243–255. [Google Scholar] [CrossRef]
- Manole, M.D.; Hickey, R.W.; Momoi, N.; Tobita, K.; Tinney, J.P.; Suciu, G.P.; Johnnides, M.J.; Clark, R.S.; Keller, B.B. Preterminal gasping during hypoxic cardiac arrest increases cardiac function in immature rats. Pediatr. Res. 2006, 60, 174–179. [Google Scholar] [CrossRef]
- Mortola, J.P. Implications of hypoxic hypometabolism during mammalian ontogenesis. Respir. Physiol. Neurobiol. 2004, 141, 345–356. [Google Scholar] [CrossRef]
- Simonsen, K.; Graem, N.; Rothman, L.P.; Degn, H. Iatrogenic radiant heat burns in severely asphyxic newborns. Acta Paediatr. 1995, 84, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.; Schröder, M.; Harms, K. Vorteile der wassergefilterten gegenüber herkömmlicher Infrarot-Strahlung in der Neonatologie. Z. Geburtsh. Neonatol. 2000, 204, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Laptook, A.R.; Robertson, N.J.; Barks, J.D.; Thoresen, M.; Wassink, G.; Bennet, L. Therapeutic hypothermia translates from ancient history in to practice. Pediatr. Res. 2017, 81, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Thoresen, M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb. Clin. Neurol. 2019, 162, 217–237. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Dhillon, S.K.; Zhou, K.; Bennet, L.; Thoresen, M.; Gunn, A.J. Therapeutic hypothermia in neonatal hypoxic-ischemic encephalopathy. Curr. Neurol. Neurosci. Rep. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 2013, CD003311. [Google Scholar] [CrossRef]
- Natarajan, G.; Laptook, A.; Shankaran, S. Therapeutic hypothermia: How can we optimize this therapy to further improve outcomes? Clin. Perinatol. 2018, 45, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Thayyil, S.; Pant, S.; Montaldo, P.; Shukla, D.; Oliveira, V.; Ivain, P.; Bassett, P.; Swamy, R.; Mendoza, J.; Moreno-Morales, M.; et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): A randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health 2021, 9, e1273–e1285. [Google Scholar] [CrossRef]
- Krishnan, V.; Kumar, V.; Shankaran, S.; Thayyil, S. Rise and fall of therapeutic hypothermia in low-resource settings: Lessons from the HELIX trial. Indian J. Pediatr. 2021. [Google Scholar] [CrossRef]
- Fleiss, B.; Gressens, P. Tertiary mechanisms of brain damage: A new hope for treatment of cerebral palsy? Lancet Neurol. 2012, 11, 556–566. [Google Scholar] [CrossRef]
- Titomanlio, L.; Kavelaars, A.; Dalous, J.; Mani, S.; El Ghouzzi, V.; Heijnen, C.; Baud, O.; Gressens, P. Stem cell therapy for neonatal brain injury: Perspectives and challenges. Ann. Neurol. 2011, 70, 698–712. [Google Scholar] [CrossRef]
- Nair, J.; Kumar, V.H.S. Current and emerging therapies in the management of hypoxic ischemic encephalopathy in neonates. Children 2018, 5, 99. [Google Scholar] [CrossRef]
- Bigelow, W.G.; Lindsay, W.K.; Greenwood, W.F. Hypothermia; its possible role in cardiac surgery: An investigation of factors governing survival in dogs at low body temperatures. Ann. Surg. 1950, 132, 849–866. [Google Scholar] [CrossRef]
- Lewis, F.J.; Taufic, M. Closure of atrial septal defects with the aid of hypothermia; experimental accomplishments and the report of one successful case. Surgery 1953, 33, 52–59. [Google Scholar] [PubMed]
- Barratt-Boyes, B.G.; Neutze, J.M.; Seelye, E.R.; Simpson, M. Complete correction of cardiovascular malformations in the first year of life. Prog. Cardiovasc. Dis. 1972, 15, 229–253. [Google Scholar] [CrossRef]
- Kirklin, J.W.; Barratt-Boyes, B.G. Hypothermia, circulatory arrest, and cardiopulmonary bypass. In Cardiac Surgery, 2nd ed.; Kirklin, J.W., Barrat-Boyes, B.G., Eds.; Churchill Livingstone/Wiley Medical: New York, NY, USA, 1993; pp. 61–127. [Google Scholar]
- Walsh, C.K.; Krongrad, E. Terminal cardiac electrical activity in pediatric patients. Am. J. Cardiol. 1983, 51, 557–561. [Google Scholar] [CrossRef]
- Smith, B.T.; Rea, T.D.; Eisenberg, M.S. Ventricular fibrillation in pediatric cardiac arrest. Acad. Emerg. Med. 2006, 13, 525–529. [Google Scholar] [CrossRef]
- Ewald, M.B.; Baum, C.R. Environmental emergencies. In Textbook of Pediatric Emergency Medicine, 5th ed.; Fleisher, G.R., Ludwig, S., Henretig, F.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 1009–1031. [Google Scholar]
- Paal, P.; Gordon, L.; Strapazzon, G.; Brodmann-Maeder, M.; Putzer, G.; Walpoth, B.; Wanscher, M.; Brown, D.; Holzer, M.; Broessner, G.; et al. Accidental hypothermia-an update (The content of this review is endorsed by the International Commission for Mountain Emergency Medicine, ICAR MEDCOM). Scand. J. Trauma Resusc. Emerg. Med. 2016, 24, 111. [Google Scholar] [CrossRef]
- Mehrotra, S.; Misir, A. Special traumatized populations: Accidental hypothermia in children. Curr. Pediatr. Rev. 2018, 14, 28–33. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.R.; Abramo, T.J.; Maxson, R.T.; Albert, G.; Rettiganti, M.R.; Saylors, M.E.; Orsborn, J.W.; Hollingsworth, A.I. Hypothermia as an outcome predictor tool in pediatric trauma: A propensity-matched analysis. Pediatr. Emerg. Care. 2021, 37, e284–e291. [Google Scholar] [CrossRef]
- Nemeth, M.; Miller, C.; Bräuer, A. Perioperative Hypothermia in Children. Int. J. Environ. Res. Public Health. 2021, 18, 7541. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.J.; Natale, A.M. Effect of hypothermia on the coagulation cascade. Crit. Care Med. 1992, 20, 1402–1405. [Google Scholar] [CrossRef]
- Trckova, A.; Stourac, P. Influence of perioperative hypothermia on blood clotting in children. Bratisl. Lek. Listy. 2018, 119, 294–297. [Google Scholar] [CrossRef]
- Singer, D. Ertrinkungsunfälle im Kindesalter. Notfallmed. Up2date 2007, 2, 301–320. [Google Scholar] [CrossRef]
- Romlin, B.S.; Winberg, H.; Janson, M.; Nilsson, B.; Björk, K.; Jeppsson, A.; Drake, G.; Claesson, A. Excellent outcome with extracorporeal membrane oxygenation after accidental profound hypothermia (13.8 °C) and drowning. Crit. Care Med. 2015, 43, e521–e525. [Google Scholar] [CrossRef] [PubMed]
- Elsner, R.; Gooden, B. Diving and Asphyxia: A Comparative Study of Animals and Man; Monographs of the Physiological Society No. 40; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Gooden, B.A. Why some people do not drown. Hypothermia versus the diving response. Med. J. Aust. 1992, 157, 629–632. [Google Scholar] [CrossRef]
- Boyd, J.; Brugger, H.; Shuster, M. Prognostic factors in avalanche resuscitation: A systematic review. Resuscitation 2010, 81, 645–652. [Google Scholar] [CrossRef]
- Mroczek, T.; Gladki, M.; Skalski, J. Successful resuscitation from accidental hypothermia of 11.8 °C: Where is the lower bound for human beings? Eur. J. Cardiothorac. Surg. 2020, 58, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
- Hilmo, J.; Naesheim, T.; Gilbert, M. “Nobody is dead until warm and dead”: Prolonged resuscitation is warranted in arrested hypothermic victims also in remote areas--a retrospective study from northern Norway. Resuscitation 2014, 85, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- van de Voorde, P.; Turner, N.M.; Djakow, J.; de Lucas, N.; Martinez-Mejias, A.; Biarent, D.; Bingham, R.; Brissaud, O.; Hoffmann, F.; Johannesdottir, G.B.; et al. European Resuscitation Council Guidelines 2021: Paediatric Life Support. Resuscitation 2021, 161, 327–387. [Google Scholar] [CrossRef] [PubMed]
- de Caen, A. Management of profound hypothermia in children without the use of extracorporeal life support therapy. Lancet 2002, 360, 1394–1395. [Google Scholar] [CrossRef]
- Skarda, D.; Barnhart, D.; Scaife, E.; Molitor, M.; Meyers, R.; Rollins, M. Extracorporeal cardiopulmonary resuscitation (EC-CPR) for hypothermic arrest in children: Is meaningful survival a reasonable expectation? J. Pediatr. Surg. 2012, 47, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Darocha, T.; Podsiadło, P.; Polak, M.; Hymczak, H.; Krzych, Ł.; Skalski, J.; Witt-Majchrzak, A.; Nowak, E.; Toczek, K.; Waligórski, S.; et al. Prognostic factors for nonasphyxia-related cardiac arrest patients undergoing extracorporeal rewarming–HELP registry study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Olfe, J.; Gottschalk, U.; Singer, D. Ertrinkungsunfälle bei Kindern und Jugendlichen. Notfallmed. Up2date 2018, 13, 187–207. [Google Scholar] [CrossRef]
- Biermann, D.; Gottschalk, U.; Köhne, M.; Holst, T.; Hüners, I.; von Stumm, M.; Mueller, G.; Stark, V.; van Rüth, V.; Kozlik-Feldmann, R.; et al. Outcomes of extracorporeal membrane oxygenation and cardiopulmonary bypass in children after drowning-related resuscitation. Perfusion 2021, 2676591211041229. [Google Scholar] [CrossRef]
- Moler, F.W.; Hutchison, J.S.; Nadkarni, V.M.; Silverstein, F.S.; Meert, K.L.; Holubkov, R.; Page, K.; Slomine, B.S.; Christensen, J.R.; Dean, J.M. (Therapeutic Hypothermia After Pediatric Cardiac Arrest Out-of-Hospital Trial Investigators). Targeted temperature management after pediatric cardiac arrest due to drowning: Outcomes and complications. Pediatr. Crit. Care. Med. 2016, 17, 712–720. [Google Scholar] [CrossRef]
- Auerbach, P.S. Some people are dead when they’re cold and dead. JAMA 1990, 264, 1856–1857. [Google Scholar] [CrossRef] [PubMed]
- Spack, L.; Gedeit, R.; Splaingard, M.; Havens, P.L. Failure of aggressive therapy to alter outcome in pediatric near-drowning. Pediatr. Emerg. Care 1997, 13, 98–102. [Google Scholar] [CrossRef]
- Kieboom, J.K.; Verkade, H.J.; Burgerhof, J.G.; Bierens, J.J.; van Rheenen, P.F.; Kneyber, M.C.; Albers, M.J. Outcome after resuscitation beyond 30 minutes in drowned children with cardiac arrest and hypothermia: Dutch nationwide retrospective cohort study. BMJ 2015, 350, h418. [Google Scholar] [CrossRef]
- Moler, F.W.; Silverstein, F.S.; Holubkov, R.; Slomine, B.S.; Christensen, J.R.; Nadkarni, V.M.; Meert, K.L.; Clark, A.E.; Browning, B.; Pemberton, L.V.; et al. (THAPCA Trial Investigators). Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N. Engl. J. Med. 2015, 372, 1898–1908. [Google Scholar] [CrossRef]
- Moler, F.W.; Silverstein, F.S.; Holubkov, R.; Slomine, B.S.; Christensen, J.R.; Nadkarni, V.M.; Meert, K.L.; Browning, B.; Pemberton, V.L.; Page, K.; et al. (THAPCA Trial Investigators). Therapeutic hypothermia after in-hospital cardiac arrest in children. N. Engl. J. Med. 2017, 376, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Adelson, P.D.; Wisniewski, S.R.; Beca, J.; Brown, S.D.; Bell, M.; Muizelaar, J.P.; Okada, P.; Beers, S.R.; Balasubramani, G.K.; Hirtz, D. (Paediatric Traumatic Brain Injury Consortium). Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): A phase 3, randomised controlled trial. Lancet Neurol. 2013, 12, 546–553. [Google Scholar] [CrossRef]
- Crompton, E.M.; Lubomirova, I.; Cotlarciuc, I.; Han, T.S.; Sharma, S.D.; Sharma, P. Meta-Analysis of therapeutic hypothermia for traumatic brain injury in adult and pediatric patients. Crit. Care Med. 2017, 45, 575–583. [Google Scholar] [CrossRef]
- Bohman, L.E.; Levine, J.M. Fever and therapeutic normothermia in severe brain injury: An update. Curr. Opin. Crit. Care. 2014, 20, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, G.M.; Silverstein, F.S. Targeted temperature management in pediatric neurocritical care. Pediatr. Neurol. 2018, 88, 12–24. [Google Scholar] [CrossRef]
- Nordeen, C.A.; Martin, S.L. Engineering human stasis for long-duration spaceflight. Physiol. Bethesda 2019, 34, 101–111. [Google Scholar] [CrossRef]
- Hadj-Moussa, H.; Storey, K.B. Bringing nature back: Using hibernation to reboot organ preservation. FEBS J. 2019, 286, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Singer, D. Human hibernation for space flight: Utopistic vision or realistic possibility? J. Brit. Interplanet. Soc. 2006, 59, 139–143. [Google Scholar]
- Choukèr, A.; Bereiter-Hahn, J.; Singer, D.; Heldmaier, G. Hibernating astronauts-science or fiction? Pflügers Arch. 2019, 471, 819–828. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, D. Pediatric Hypothermia: An Ambiguous Issue. Int. J. Environ. Res. Public Health 2021, 18, 11484. https://doi.org/10.3390/ijerph182111484
Singer D. Pediatric Hypothermia: An Ambiguous Issue. International Journal of Environmental Research and Public Health. 2021; 18(21):11484. https://doi.org/10.3390/ijerph182111484
Chicago/Turabian StyleSinger, Dominique. 2021. "Pediatric Hypothermia: An Ambiguous Issue" International Journal of Environmental Research and Public Health 18, no. 21: 11484. https://doi.org/10.3390/ijerph182111484
APA StyleSinger, D. (2021). Pediatric Hypothermia: An Ambiguous Issue. International Journal of Environmental Research and Public Health, 18(21), 11484. https://doi.org/10.3390/ijerph182111484





