Are Resistance Training-Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle–Brain–Renal Axis
Abstract
:1. Introduction
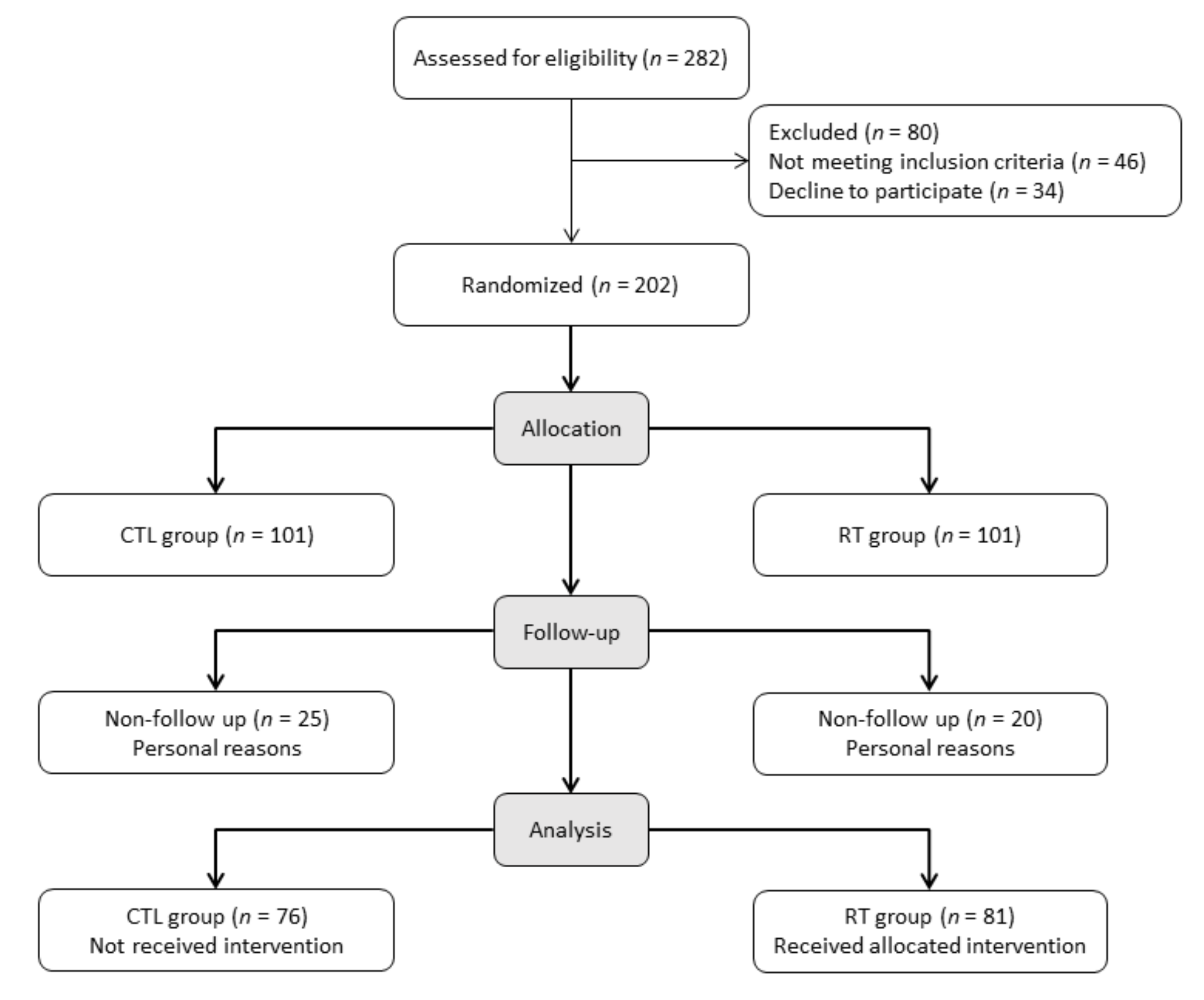
2. Methods
2.1. Study Population
2.2. Randomization
2.3. Physical Assessment
2.4. Handgrip Strength
2.5. Blood Samples and Biochemical Analysis
2.6. Quality of Life
2.7. Beck Depression Inventory
2.8. Resistance Training Protocol
2.9. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussien, H.; Apetrii, M.; Covic, A. Health-related quality of life in patients with chronic kidney disease. Expert Rev. Pharm. Outcomes Res. 2021, 21, 43–54. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013, 84, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhee, J.H.; Lee, E.; Cha, M.U.; Lee, M.; Kim, H.; Park, S.; Yun, H.R.; Jung, S.Y.; Kee, Y.K.; Yoon, C.Y.; et al. Prevalence of depression and suicidal ideation increases proportionally with renal function decline, beginning from early stages of chronic kidney disease. Medicine 2017, 96, e8476. [Google Scholar] [CrossRef]
- Duncan, L.E.; Hutchison, K.E.; Carey, G.; Craighead, W.E. Variation in brain-derived neurotrophic factor (BDNF) gene is associated with symptoms of depression. J. Affect. Disord. 2009, 115, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. : TEM 2014, 25, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoladz, J.A.; Smigielski, M.; Majerczak, J.; Nowak, L.R.; Zapart-Bukowska, J.; Smolenski, O.; Kulpa, J.; Duda, K.; Drzewinska, J.; Bartosz, G. Hemodialysis decreases serum brain-derived neurotrophic factor concentration in humans. Neurochem. Res. 2012, 37, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Eraldemir, F.C.; Ozsoy, D.; Bek, S.; Kir, H.; Dervisoglu, E. The relationship between brain-derived neurotrophic factor levels, oxidative and nitrosative stress and depressive symptoms: A study on peritoneal dialysis. Ren. Fail 2015, 37, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinus, N.; Hansen, D.; Feys, P.; Meesen, R.; Timmermans, A.; Spildooren, J. The Impact of Different Types of Exercise Training on Peripheral Blood Brain-Derived Neurotrophic Factor Concentrations in Older Adults: A Meta-Analysis. Sports Med. 2019, 49, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Maderova, D.; Krumpolec, P.; Slobodova, L.; Schon, M.; Tirpakova, V.; Kovanicova, Z.; Klepochova, R.; Vajda, M.; Sutovsky, S.; Cvecka, J.; et al. Acute and regular exercise distinctly modulate serum, plasma and skeletal muscle BDNF in the elderly. Neuropeptides 2019, 78, 101961. [Google Scholar] [CrossRef] [PubMed]
- Correa, H.L.; Neves, R.V.P.; Deus, L.A.; Souza, M.K.; Haro, A.S.; Costa, F.; Silva, V.L.; Santos, C.A.R.; Moraes, M.R.; Simoes, H.G.; et al. Blood Flow Restriction Training Blunts Chronic Kidney Disease Progression in Humans. Med. Sci. Sports Exerc. 2020, 53, 249–257. [Google Scholar] [CrossRef]
- Deus, L.A.; Neves, R.V.P.; Correa, H.L.; Reis, A.L.; Honorato, F.S.; Silva, V.L.; de Araujo, T.B.; Souza, M.K.; Sousa, C.V.; Simoes, H.G.; et al. Improving the prognosis of renal patients: The effects of blood flow-restricted resistance training on redox balance and cardiac autonomic function. Exp. Physiol. 2021, 106, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Correa, H.L.; Neves, R.V.P.; Deus, L.A.; Maia, B.C.H.; Maya, A.T.; Tzanno-Martins, C.; Souza, M.K.; Silva, J.A.B.; Haro, A.S.; Costa, F.; et al. Low-load resistance training with blood flow restriction prevent renal function decline: The role of the redox balance, angiotensin 1-7 and vasopressin(,). Physiol. Behav. 2021, 230, 113295. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, J.; Semenova, E.A.; Borisov, O.V.; Kostryukova, E.S.; Vepkhvadze, T.F.; Lysenko, E.A.; Andryushchenko, O.N.; Andryushchenko, L.B.; Lednev, E.M.; Larin, A.K.; et al. The BDNF-Increasing Allele is Associated with Increased Proportion of Fast-Twitch Muscle Fibers, Handgrip Strength, and Power Athlete Status. J. Strength Cond. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.A.; Aguiar, A.S., Jr.; Radak, Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants 2019, 8, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Pothula, S.; Liu, R.J.; Duman, C.H.; Terwilliger, R.; Vlasuk, G.P.; Saiah, E.; Hahm, S.; Duman, R.S. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J. Clin. Investig. 2019, 129, 2542–2554. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sujkowski, A.; Namkoong, S.; Gu, B.; Cobb, T.; Kim, B.; Kowalsky, A.H.; Cho, C.S.; Semple, I.; Ro, S.H.; et al. Sestrins are evolutionarily conserved mediators of exercise benefits. Nat. Commun. 2020, 11, 190. [Google Scholar] [CrossRef]
- Correa, H.L.; Neves, R.V.P.; Deus, L.A.; Reis, A.L.; Simoes, H.G.; Navalta, J.W.; Prestes, J.; Moraes, M.R.; Rosa, T.S. Could sestrins 2 be the secret of resistance exercise benefiting dialytic patients? Nephrol. Dial Transpl. 2020, 35, 2198–2199. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Weber, K.; Volland, G.; Kashman, N. Reliability and validity of grip and pinch strength evaluations. J. Hand Surg. 1984, 9, 222–226. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Keller, S. SF-36 Physical and Mental Health Summary Scales: A User’s Manual; Health Assessment Lab: Boston, MA, USA, 1994. [Google Scholar]
- Ciconelli, R.M.; Ferraz, M.B.; Santos, W.; Meinao, I.M.; Quaresma, M.R. Brazilian-Portuguese version of the SF-36 questionnaire: A reliable and valid quality of life outcome measure. Arthritis Rheum. 1997, 40, 489. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Cunha, J.A. Manual da Versão em Português das Escalas Beck; Casa Do Psicólogo: São Paulo, Brazil, 2001; p. 256. [Google Scholar]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Gadelha, A.B.; Cesari, M.; Correa, H.L.; Neves, R.V.P.; Sousa, C.V.; Deus, L.A.; Souza, M.K.; Reis, A.L.; Moraes, M.R.; Prestes, J.; et al. Effects of pre-dialysis resistance training on sarcopenia, inflammatory profile, and anemia biomarkers in older community-dwelling patients with chronic kidney disease: A randomized controlled trial. Int. Urol. Nephrol. 2021, 53, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.C.; Lash, J.P.; Xie, D.; Pan, Q.; DeLuca, J.; Kanthety, R.; Kusek, J.W.; Lora, C.M.; Nessel, L.; Ricardo, A.C.; et al. Predictors and Outcomes of Health-Related Quality of Life in Adults with CKD. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Xia, J.; Zhang, X.; Gathirua-Mwangi, W.G.; Guo, J.; Li, Y.; McKenzie, S.; Song, Y. Associations of muscle mass and strength with all-cause mortality among US older adults. Med. Sci. Sports Exerc. 2018, 50, 458. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, E.; Brouillard, F.; Molet, J.; Claverie, D.; Cabungcal, J.H.; Cresto, N.; Doligez, N.; Rivat, C.; Do, K.Q.; Bernard, C.; et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatry 2017, 22, 1701–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctot, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Moller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Roh, H.T.; Cho, S.Y.; So, W.Y. A Cross-Sectional Study Evaluating the Effects of Resistance Exercise on Inflammation and Neurotrophic Factors in Elderly Women with Obesity. J. Clin. Med. 2020, 9, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuvagah Forti, L.; Van Roie, E.; Njemini, R.; Coudyzer, W.; Beyer, I.; Delecluse, C.; Bautmans, I. High Versus Low Load Resistance Training: The Effect of 24 Weeks Detraining on Serum Brain Derived-Neurotrophic Factor (BDNF) in Older Adults. J. Frailty Aging 2017, 6, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Marston, K.J.; Newton, M.J.; Brown, B.M.; Rainey-Smith, S.R.; Bird, S.; Martins, R.N.; Peiffer, J.J. Intense resistance exercise increases peripheral brain-derived neurotrophic factor. J. Sci. Med. Sport 2017, 20, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Quiles, J.M.; Klemp, A.; Dolan, C.; Maharaj, A.; Huang, C.J.; Khamoui, A.V.; Trexler, E.T.; Whitehurst, M.; Zourdos, M.C. Impact of resistance training program configuration on the circulating brain-derived neurotrophic factor response. Appl. Physiol. Nutr. Metab. 2020, 45, 667–674. [Google Scholar] [CrossRef]
- Kim, H.; Suzuki, T.; Kim, M.; Kojima, N.; Ota, N.; Shimotoyodome, A.; Hase, T.; Hosoi, E.; Yoshida, H. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: A randomized double blind, placebo-controlled, follow-up trial. PLoS ONE 2015, 10, e0116256. [Google Scholar] [CrossRef] [Green Version]
- Vieira, D.C.; Nascimento, D.C.; Tajra, V.; Teixeira, T.G.; Farias, D.L.; Tibana, R.A.; Silva, A.O.; Rosa, T.S.; De Moraes, M.R.; Voltarelli, F.A. High Supervised Resistance Training in Elderly Women: The Role of Supervision Ratio. Int. J. Exerc. Sci. 2020, 13, 597. [Google Scholar] [PubMed]
- Forti, L.N.; Van Roie, E.; Njemini, R.; Coudyzer, W.; Beyer, I.; Delecluse, C.; Bautmans, I. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp. Gerontol. 2015, 70, 144–149. [Google Scholar] [CrossRef] [PubMed]
| Exercise | Instrument | Observations |
|---|---|---|
| 1. Unilateral chest press | e-Lastic® | OMNI-RES 5 to 6 during 12 initial weeks. The exercise was performed with the contralateral arm to the fistula. A conservative measure intended to preserve arteriovenous fistula. The e-Lastic load record was the peak (maximum) load achieved in each movement to count the repetitions. |
| 2. Squat | Bodyweight | OMNI-RES 7 to 8 during 12 last weeks From 4 to 15 reps—weekly increasing the number of reps At the beginning of the protocol, we encourage patients to perform few squats (4reps) but weekly increasing the number of reps. |
| 3. Unilateral row | e-Lastic® | The exercise was performed with the contralateral arm to the fistula. A conservative measure intended to preserve arteriovenous fistula. The e-Lastic load record was the peak (maximum) load achieved in each movement to count the repetitions. |
| 4. Bilateral knee extension | Weighted cuffs | Seated position Wrapped at the ankle. |
| 5. Unilateral shoulder press | Dumbbells | Exercise performed with the arm contralateral to fistula. A conservative measure intended to preserve arteriovenous fistula. |
| 6. Hip thrust | Weighted cuffs | Positioned at the hips. |
| 7. Unilateral knee flexion | Weighted cuffs | Stand position Wrapped at the ankle. |
| 8. Biceps curl | Dumbbells | The exercise was performed with the contralateral arm to the fistula. A conservative measure intended to preserve arteriovenous fistula. |
| 9. Unilateral hip adduction | e-Lastic® | The e-Lastic load record was the peak (maximum) load achieved in each movement to count the repetitions. |
| 10. Unilateral elbow extension | Dumbbells | The exercise was performed with the contralateral arm to the fistula. A conservative measure intended to preserve arteriovenous fistula. |
| 11. Unilateral hip abduction | e-Lastic® | The e-Lastic load record was the peak (maximum) load achieved in each movement to count the repetitions. |
| 12. Seated calf raise | Weighted cuffs | Wrapped across the quadriceps |
| Variables | CTL Group (n = 76) | RT Group (n = 81) |
|---|---|---|
| Time in hemodialysis (months) | ||
| Mean and SD. | 55.47 ± 8.171 | 54.09 ± 11.05 |
| Minimum | 35.00 | 35.00 |
| 25% Percentile | 49.25 | 44.00 |
| Median | 56.00 | 53.00 |
| 75% Percentile | 61.00 | 64.00 |
| Maximum | 70.00 | 71.00 |
| Age (years) | ||
| Mean and SD. | 66.33 ± 3.88 | 67.27 ± 3.24 |
| Minimum | 60.00 | 60.00 |
| 25% Percentile | 63.25 | 66.00 |
| Median | 66.00 | 68.00 |
| 75% Percentile | 70.00 | 69.00 |
| Maximum | 72.00 | 72.00 |
| BMI (kg/m2) | ||
| Mean and SD. | 26.82 ± 2.90 | 27.30 ± 3.77 |
| Minimum | 21.65 | 20.93 |
| 25% Percentile | 24.87 | 25.10 |
| Median | 27.23 | 27.40 |
| 75% Percentile | 28.52 | 28.65 |
| Maximum | 33.61 | 34.60 |
| Variables | Control Group (n = 76) | Resistance-Trained Group (n = 81) | p-Value | ||
|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | ||
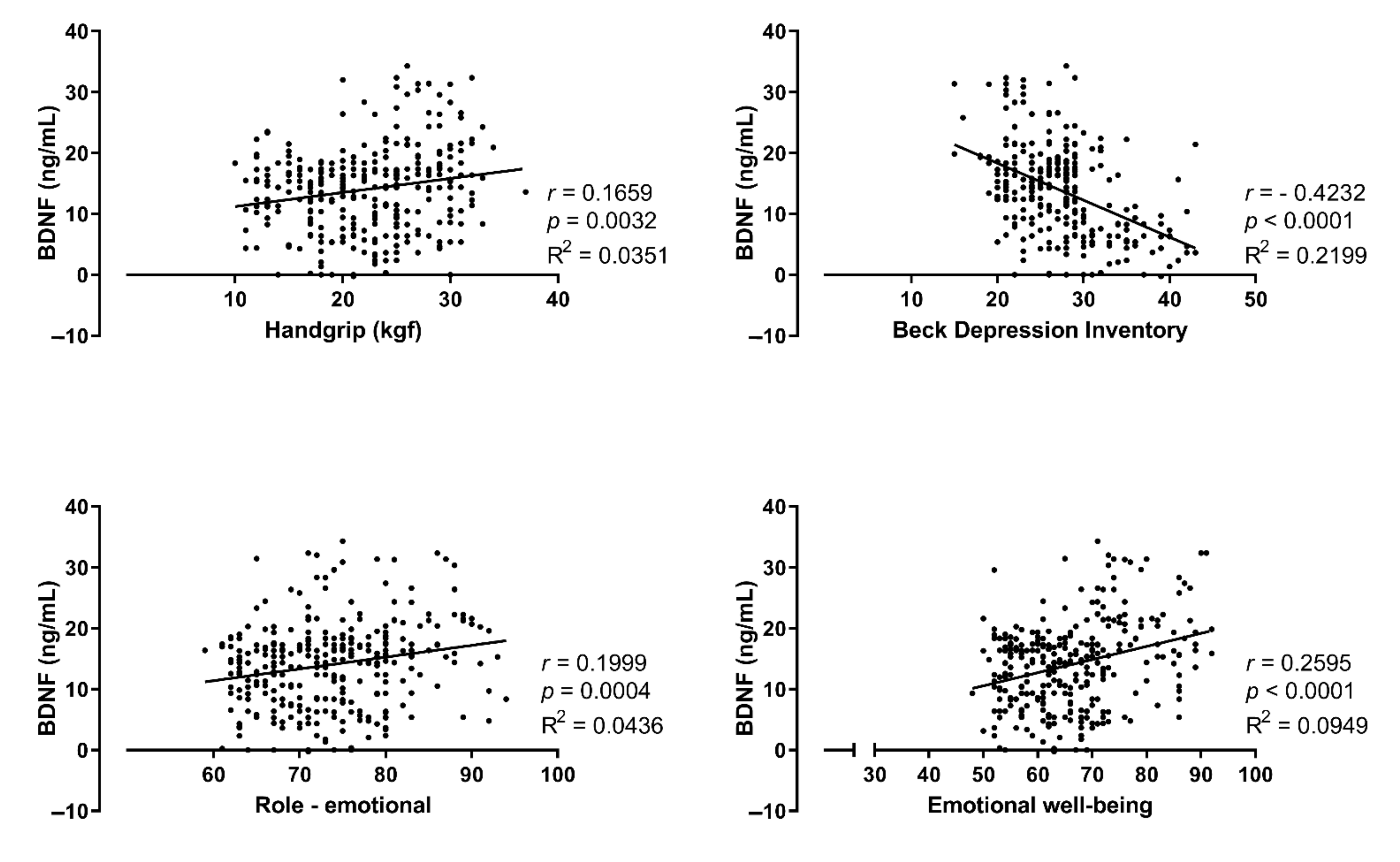
| BDNF (ng/mL) | |||||
| Mean and SD. | 14.40 ± 4.99 | 10.84 ± 5.94 a | 11.66 ± 5.20 | 19.60 ± 7.23 a,b | 0.0001 |
| Minimum | 4.380 | −0.2600 | 1.360 | 4.650 | |
| 25% Percentile | 12.10 | 6.378 | 6.390 | 15.31 | |
| Median | 15.34 | 10.10 | 12.35 | 20.23 | |
| 75% Percentile | 16.98 | 15.46 | 16.37 | 24.35 | |
| Maximum | 31.45 | 24.45 | 19.35 | 34.30 | |
| TROLOX (uM) | |||||
| Mean and SD. | 627.0 ± 191.6 | 589.5 ± 195.9 | 596.2 ± 218.3 | 680.8 ± 225.2 b | 0.001 |
| Minimum | 200.0 | 204.0 | 211.0 | 232.0 | |
| 25% Percentile | 497.0 | 451.0 | 415.5 | 490.5 | |
| Median | 643.0 | 622.5 | 647.0 | 734.0 | |
| 75% Percentile | 761.8 | 755.3 | 792.5 | 878.5 | |
| Maximum | 894.0 | 899.0 | 882.0 | 1027 | |
| GSH (µM) | |||||
| Mean and SD. | 4.66 ± 1.93 | 5.00 ± 2.96 | 4.23 ± 1.84 | 9.33 ± 2.09 a,b | 0.0001 |
| Minimum | 1.130 | 0.0800 | 1.090 | 4.580 | |
| 25% Percentile | 3.178 | 2.578 | 2.715 | 8.050 | |
| Median | 4.675 | 4.745 | 4.240 | 9.210 | |
| 75% Percentile | 5.843 | 7.110 | 5.445 | 10.68 | |
| Maximum | 9.500 | 13.40 | 8.690 | 14.53 | |
| TBARS (nmol/mL) | |||||
| Mean and SD | 13.26 ± 2.45 | 13.66 ± 2.62 | 14.17 ± 2.39 | 11.06 ± 2.95 a,b | 0.0001 |
| Minimum | 9.410 | 8.790 | 9.220 | 3.850 | |
| 25% Percentile | 11.19 | 11.70 | 12.05 | 8.735 | |
| Median | 12.65 | 13.43 | 14.63 | 11.38 | |
| 75% Percentile | 15.69 | 15.40 | 16.36 | 13.03 | |
| Maximum | 18.64 | 20.53 | 18.73 | 16.58 | |
| Handgrip (kgf) | |||||
| Mean and SD. | 20.09 ± 5.19 | 19.75 ± 5.54 | 21.17 ± 4.38 | 27.17 ± 4.34 a,b | 0.0001 |
| Minimum | 12.00 | 10.00 | 12.00 | 12.00 | |
| 25% Percentile | 16.00 | 16.25 | 18.00 | 25.00 | |
| Median | 19.00 | 19.50 | 22.00 | 27.00 | |
| 75% Percentile | 23.00 | 23.75 | 25.00 | 30.50 | |
| Maximum | 30.00 | 32.00 | 30.00 | 37.00 | |
| Medical Outcomes SF36 | Control Group (n = 76) | Resistance-Trained Group (n = 81) | p-Value | ||
|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | ||
| Physical functioning | |||||
| Mean and SD. | 70.17 ± 6.616 | 68.49 ± 7.057 | 69.19 ± 5.878 | 82.27 ± 6.166 a,b | 0.0001 |
| Minimum | 60.00 | 56.00 | 60.00 | 70.00 | |
| 25% Percentile | 64.25 | 64.00 | 64.50 | 77.50 | |
| Median | 70.00 | 68.50 | 69.00 | 82.00 | |
| 75% Percentile | 76.00 | 75.00 | 74.00 | 86.00 | |
| Maximum | 80.00 | 80.00 | 80.00 | 95.00 | |
| Physical role | |||||
| Mean and SD. | 75.43 ± 6.076 | 73.78 ± 6.378 | 74.63 ± 6.159 | 88.67 ± 6.253 a,b | 0.0001 |
| Minimum | 65.00 | 62.00 | 65.00 | 77.00 | |
| 25% Percentile | 70.00 | 68.25 | 70.00 | 84.00 | |
| Median | 75.00 | 74.00 | 73.00 | 88.00 | |
| 75% Percentile | 80.75 | 79.75 | 81.00 | 94.00 | |
| Maximum | 85.00 | 85.00 | 85.00 | 99.00 | |
| Pain | |||||
| Mean and SD. | 54.84 ± 7.005 | 53.50 ± 6.977 | 53.25 ± 7.680 | 71.32 ± 7.878 a,b | 0.0001 |
| Minimum | 42.00 | 38.00 | 42.00 | 58.00 | |
| 25% Percentile | 48.50 | 48.00 | 46.00 | 65.00 | |
| Median | 56.00 | 54.00 | 53.00 | 71.00 | |
| 75% Percentile | 62.00 | 60.00 | 60.00 | 78.00 | |
| Maximum | 65.00 | 65.00 | 65.00 | 86.00 | |
| General health | |||||
| Mean and SD. | 52.26 ± 6.363 | 50.89 ± 6.275 | 51.93 ± 6.249 | 63.90 ± 6.818 a,b | 0.0001 |
| Minimum | 42.00 | 38.00 | 42.00 | 51.00 | |
| 25% Percentile | 47.00 | 46.00 | 47.00 | 58.00 | |
| Median | 52.50 | 51.00 | 51.00 | 64.00 | |
| 75% Percentile | 58.00 | 57.00 | 57.00 | 69.50 | |
| Maximum | 62.00 | 63.00 | 62.00 | 77.00 | |
| Vitality | |||||
| Mean and SD. | 69.78 ± 6.168 | 68.34 ± 6.313 | 69.47 ± 6.044 | 76.99 ± 6.288 a,b | 0.0001 |
| Minimum | 60.00 | 57.00 | 60.00 | 65.00 | |
| 25% Percentile | 64.00 | 63.00 | 64.50 | 72.00 | |
| Median | 70.00 | 68.50 | 69.00 | 77.00 | |
| 75% Percentile | 75.75 | 74.00 | 74.00 | 82.00 | |
| Maximum | 80.00 | 80.00 | 80.00 | 90.00 | |
| Social function | |||||
| Mean and SD. | 63.20 ± 5.458 | 61.97 ± 5.535 | 62.94 ± 6.053 | 72.35 ± 6.118 a,b | 0.0001 |
| Minimum | 52.00 | 49.00 | 52.00 | 61.00 | |
| 25% Percentile | 58.00 | 58.00 | 58.00 | 67.00 | |
| Median | 63.50 | 62.00 | 64.00 | 73.00 | |
| 75% Percentile | 67.00 | 66.00 | 68.00 | 77.50 | |
| Maximum | 72.00 | 73.00 | 72.00 | 83.00 | |
| Emotional role | |||||
| Mean and SD. | 71.01 ± 5.375 | 69.64 ± 5.701 | 71.68 ± 5.922 | 81.31 ± 6.895 a,b | 0.0001 |
| Minimum | 62.00 | 59.00 | 62.00 | 68.00 | |
| 25% Percentile | 66.00 | 65.00 | 67.00 | 75.00 | |
| Median | 71.00 | 69.50 | 72.00 | 82.00 | |
| 75% Percentile | 75.00 | 74.75 | 77.00 | 87.00 | |
| Maximum | 80.00 | 80.00 | 80.00 | 94.00 | |
| Emotional well-being | |||||
| Mean and SD. | 62.38 ± 5.944 | 61.01 ± 6.245 | 61.06 ± 6.410 | 79.00 ± 6.624 a,b | 0.0001 |
| Minimum | 52.00 | 48.00 | 52.00 | 68.00 | |
| 25% Percentile | 57.00 | 56.25 | 55.00 | 73.00 | |
| Median | 63.00 | 61.00 | 60.00 | 78.00 | |
| 75% Percentile | 67.00 | 66.00 | 67.50 | 86.00 | |
| Maximum | 72.00 | 73.00 | 72.00 | 92.00 | |
| Control Group (n = 76) | Resistance-Trained Group (n = 81) | |||
|---|---|---|---|---|
| Baseline | Post | Baseline | Post | |
| Beck Depression Inventory | ||||
| Mean and SD. | 26.83 ± 3.81 | 27.28 ± 4.35 | 28.10 ± 6.11 | 26.33 ± 6.28 |
| Minimum | 20.00 | 19.00 | 19.00 | 15.00 |
| 25% Percentile | 24.25 | 24.00 | 24.00 | 22.00 |
| Median | 26.00 | 28.00 | 26.00 | 25.00 |
| 75% Percentile | 29.00 | 29.00 | 31.50 | 30.00 |
| Maximum | 39.00 | 40.00 | 43.00 | 43.00 |
| ∆ Beck Depression score | 0.45 ± 1.67 | −1.76 ± 2.00 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deus, L.A.; Corrêa, H.d.L.; Neves, R.V.P.; Reis, A.L.; Honorato, F.S.; Silva, V.L.; Souza, M.K.; de Araújo, T.B.; de Gusmão Alves, L.S.; Sousa, C.V.; et al. Are Resistance Training-Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle–Brain–Renal Axis. Int. J. Environ. Res. Public Health 2021, 18, 11299. https://doi.org/10.3390/ijerph182111299
Deus LA, Corrêa HdL, Neves RVP, Reis AL, Honorato FS, Silva VL, Souza MK, de Araújo TB, de Gusmão Alves LS, Sousa CV, et al. Are Resistance Training-Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle–Brain–Renal Axis. International Journal of Environmental Research and Public Health. 2021; 18(21):11299. https://doi.org/10.3390/ijerph182111299
Chicago/Turabian StyleDeus, Lysleine Alves, Hugo de Luca Corrêa, Rodrigo Vanerson Passos Neves, Andrea Lucena Reis, Fernando Sousa Honorato, Victor Lopes Silva, Michel Kendy Souza, Thaís Branquinho de Araújo, Lucas Santos de Gusmão Alves, Caio Victor Sousa, and et al. 2021. "Are Resistance Training-Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle–Brain–Renal Axis" International Journal of Environmental Research and Public Health 18, no. 21: 11299. https://doi.org/10.3390/ijerph182111299
APA StyleDeus, L. A., Corrêa, H. d. L., Neves, R. V. P., Reis, A. L., Honorato, F. S., Silva, V. L., Souza, M. K., de Araújo, T. B., de Gusmão Alves, L. S., Sousa, C. V., Reis, T. L., de Aguiar, L. S., Simões, H. G., Prestes, J., Melo, G. F., & Rosa, T. S. (2021). Are Resistance Training-Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle–Brain–Renal Axis. International Journal of Environmental Research and Public Health, 18(21), 11299. https://doi.org/10.3390/ijerph182111299






