Assessment of Quality of Life and Pain Severity in Older Men with Osteoporosis: Cross-Sectional Study
Abstract
1. Introduction
Aim
- To determine whether, in men with osteoporosis, bone mass density and life quality components are related to each other and
- To assess the level of pain felt by men with osteoporosis and its impact on their quality of life.
2. Material and Methods
2.1. Study Design
2.2. Participants
2.3. Blinding
2.4. Outcomes
2.4.1. Bone Density
2.4.2. Quality of Life
2.4.3. Pain
2.5. Statistical Analysis
3. Results
4. Discussion
Study Limitation
5. Conclusions
Highlights
- −
- At present, the scientific literature does not provide sufficient, high-quality research and reports on the quality of life in older men with osteoporosis.
- −
- We studied the relationship between bone density and components of quality of life.
- −
- We assessed pain, quality of life, and bone density in 62 older men.
- −
- Bone mass density was related to the quality of life, mostly leisure and mobility.
- −
- Pain significantly affects general health perception in older men with osteoporosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haraldstad, K.; Network, T.L.; Wahl, A.; Andenæs, R.; Andersen, J.R.; Andersen, M.H.; Beisland, E.; Borge, C.R.; Engebretsen, E.; Eisemann, M.; et al. A systematic review of quality of life research in medicine and health sciences. Qual. Life Res. 2019, 28, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Nawrat-Szołtysik, A.; Polak, A.; Małecki, A.; Piejko, L.; Grzybowska-Ganszczyk, D.; Kręcichwost, M.; Opara, J. Effect of physical activity on the sequelae of osteoporosis in female residents of residential care facilities. Adv. Clin. Exp. Med. 2018, 27, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.B.; Dagar, M. Osteoporosis in Older Adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, E.J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Sirufo, M.M.; Polsinelli, M.; Placidi, G.; Di Silvestre, D.; Ginaldi, L. Gender Differences in Osteoporosis: A Single-Center Observational Study. World J. Men’s Health 2021, 39, 750. [Google Scholar] [CrossRef]
- Zwart, M.; Azagra, R.; Encabo, G.; Aguye, A.; Roca, G.; Güell, S.; Puchol, N.; Gene, E.; López-Expósito, F.; Solà, S.; et al. Measuring health-related quality of life in men with osteoporosis or osteoporotic fracture. BMC Public Health 2011, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.A.; McCabe, E.; Bergin, D.; Kearns, S.R.; McCabe, J.P.; Armstrong, C.; Heaney, F.; Carey, J.J. Osteoporotic Vertebral Fractures are Common in Hip Fracture Patients and are Under-recognized. J. Clin. Densitom. 2021, 24, 183–189. [Google Scholar] [CrossRef]
- Matthews, J.; Torres, S.J.; Milte, C.M.; Hopkins, I.; Kukuljan, S.; Nowson, C.A.; Daly, R.M. Effects of a multicomponent exercise program combined with calcium–vitamin D3-enriched milk on health-related quality of life and depressive symptoms in older men: Secondary analysis of a randomized controlled trial. Eur. J. Nutr. 2019, 59, 1081–1091. [Google Scholar] [CrossRef]
- Palmer, S.; Barnett, S.; Cramp, M.; Berry, A.; Thomas, A.; Clark, E.M. Effects of postural taping on pain, function and quality of life following osteoporotic vertebral fractures-A feasibility trial. Musculoskelet. Care 2018, 16, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Renna., M.D.; Conversano, F.; Cascario, E.; Di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 2016, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Caitriona, C.; Mark, M.G.; Elaine, H.; Claire, G.; Michelle, F.; Persson, U.M.; Sherrington, C.; Blake, C. Management of hospitalised osteoporotic vertebral fractures. Arch. Osteoporos. 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Ammendolia, A.; Gimigliano, A.; Tiberi, R.; Cisari, C.; Invernizzi, M. Spinal Orthoses Prescription for Vertebral Fragility Fractures by Italian Physical and Rehabilitation Medicine Physicians: The SPIN-VER Survey. Healthcare 2021, 9, 8922. [Google Scholar] [CrossRef]
- Compton, M.; Ben Mortenson, W.; Sale, J.; Crossman, A.; Ashe, M.C. Men’s perceptions of living with osteoporosis: A systematic review of qualitative studies. Int. J. Orthop. Trauma Nurs. 2019, 33, 11–17. [Google Scholar] [CrossRef]
- Adler, R.A. Osteoporosis in men: A review. Bone Res. 2014, 2, 14001. [Google Scholar] [CrossRef]
- Kerr, C.; Bottomley, C.; Shingler, S.; Giangregorio, L.; De Freitas, H.M.; Patel, C.; Randall, S.; Gold, D.T. The importance of physical function to people with osteoporosis. Osteoporos. Int. 2017, 28, 1597–1607. [Google Scholar] [CrossRef]
- Iolascon, G.; De Sire, A.; Curci, C.; Paoletta, M.; Liguori, S.; Calafiore, D.; Gimigliano, F.; Moretti, A. Osteoporosis guidelines from a rehabilitation perspective: Systematic analysis and quality appraisal using AGREE II. Eur. J. Phys. Rehabil. Med. 2021, 57, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Rinonapoli, G.; Ruggiero, C.; Meccariello, L.; Bisaccia, M.; Ceccarini, P.; Caraffa, A. Osteoporosis in Men: A Review of an Underestimated Bone Condition. Int. J. Mol. Sci. 2021, 22, 2105. [Google Scholar] [CrossRef]
- Scaturro, D.; Rizzo, S.; Sanfilippo, V.; Giustino, V.; Messina, G.; Martines, F.; Falco, V.; Cuntrera, D.; Moretti, A.; Iolascon, G.; et al. Effectiveness of Rehabilitative Intervention on Pain, Postural Balance, and Quality of Life in Women with Multiple Vertebral Fragility Fractures: A Prospective Cohort Study. J. Funct. Morphol. Kinesiol. 2021, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.D.O.; Arthuso, M.; da Silva, R.; Pedro, A.O.; Neto, A.M.P.; Costa-Paiva, L. Quality of life in women with postmenopausal osteoporosis: Correlation between QUALEFFO 41 and SF-36. Maturitas 2009, 62, 85–90. [Google Scholar] [CrossRef]
- Bączyk, G. Measuring quality of life in patients with osteoporosis—A review of generic and disease-specific quality of life measurement scales. Reumatology 2009, 47, 300–306. [Google Scholar]
- Bączyk, G.; Opala, T.; Kleka, P. Quality of life in postmenopausal women with reduced bone mineral density: Psychometric evaluation of the Polish version of QUALEFFO-41. Arch. Med. Sci. 2011, 7, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; van Schoor, N.M. Quality of life in patients with osteoporosis. Osteoporos Int. 2005, 16, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Wai Kan Yeung, A.; Sui Miu Wong, N. The Historical Roots of Visual Analog Scale in Psychology as Revealed by Reference Publication Year Spectroscopy. Front. Hum. Neurosci. 2019, 13, 86. [Google Scholar] [CrossRef]
- Schulz, K.; Lehnert, H. Osteoporosis-specific treatment when and how? Internist Berl. 2020, 61, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, C.; Poivret, D.; Lespessailles, E.; Thevenot, C.; Aubraye, D.; Ziegler, L.E.; Beranger, M.; Filaire, E.; Gendarme, S.; Rat, A.C.; et al. Understanding Patients’ Perspectives and Educational Needs by Type of Osteoporosis in Men and Women and People with Glucocorticosteroid-Induced Osteoporosis: A Qualitative Study to Improve Disease Management. Calcif. Tissue Int. 2019, 105, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, C.K.; Oneda, B.; Bernardo, F.R.; Cardoso, C.G.; Forjaz, C.; Abrahao, S.B.; Mion, D.; Fonseca, .M.; Tinucci, T. A randomized, placebo-controlled trial of the effects of physical exercises and estrogen therapy on health-related quality of life in postmenopausal women. Menopause 2008, 15, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, J.; Kwolek, A.; Bazarnik, K.; Szeliga, E.; Wolan, A. Quality of life of nursing home residents and senior mature students. Prz. Med. Uniw. Rzesz. Inst. Lekow. 2007, 5, 225–232. (In Polish) [Google Scholar]
- Szczuka, E.; Gruszecka Marczyńska, K.; Jędruszewska, A.; Ostrowska, B. Quality of life of women with postmenopausal osteoporosis taking part in prophylactic health program. Polish J. Sport. Med. 2005, 21, 333–342. (In Polish) [Google Scholar]
- Hopman, W.; Berger, C.; Joseph, L.; Morin, S.; Towheed, T.; Anastassiades, T.; Adachi, J.; Hanley, D.; Prior, J.; the CaMos Research Group; et al. Longitudinal assessment of health-related quality of life in osteoporosis: Data from the population-based Canadian Multicentre Osteoporosis Study. Osteoporos. Int. 2019, 30, 1635–1644. [Google Scholar] [CrossRef]
- Nawrat-Szołtysik, A.; Ska, Z.M.; Opara, J.; Polak, A.; Matyja, B.; Małecki, A. Effect of Physical Activity on the Quality of Life in Osteoporotic Females Living in Residential Facilities: A Randomized Controlled Trial. J. Geriatr. Phys. Ther. 2019, 42, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Al Sari, U.A.; Tobias, J.H.; Clark, E.M. Health related quality of life in older people with osteoporotic vertebral fractures: A systematic review and meta-analysis. Osteoporos Int. 2016, 27, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Olsen, S.L.; Pasco, J.A.; Hosking, S.M.; Dobbins, A.G.; Williams, L.J. Poor quality of life in Australian men: Cross-sectional associations with obesity, mobility, lifestyle and psychiatric symptoms. Maturitas 2017, 103, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sanfelix-Genovés, J.; Hurtado, I.; Sanfélix-Gimeno, G.; Reig-Molla, B.; Peiró, S. Impact of osteoporosis and vertebral fractures on quality-of-life. a population-based study in Valencia, Spain (The FRAVO Study). Health Qual. Life Outcomes 2011, 9, 20. [Google Scholar] [CrossRef]
- Yoon, S.-P.; Lee, S.-H.; Ki, C.-H.; Lee, Y.-T.; Hong, S.-H.; Lee, H.-M.; Moon, S.-H. Quality of Life in Patients with Osteoporotic Vertebral Fractures. Asian Spine J. 2014, 8, 653–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cortet, B.; Chauvin, P.; Feron, J.-M.; Grange, L.; Coulomb, A.; Launois, R.; Alliot-Launois, F.; Sellami, R.; Touboul, C.; Vincent, B.; et al. Fragility fractures in France: Epidemiology, characteristics and quality of life (the EPIFRACT study). Arch. Osteoporos. 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, B. The shape of anterior-posterior spinal curvature in post-menopausal women with osteoporosis. Ort. Tr. Reh. 2006, 8, 537–542. (In Polish) [Google Scholar]
- Leidig-Bruckner, G.; Minne, H.W.; Schlaich, C.; Wagner, G.; Scheidt-Nave, C.; Bruckner, T.; Gebest, H.J.; Ziegler, R. Clinical grading of spinal osteoporosis: Quality of life components and spinal deformity in women with chronic low back pain and women with vertebral osteoporosis. J. Bone Miner. 1997, 12, 663–675. [Google Scholar] [CrossRef]
- Kanazawa, I.; Takeno, A.; Tanaka, K.-I.; Yamane, Y.; Sugimoto, T. Osteoporosis and vertebral fracture are associated with deterioration of activities of daily living and quality of life in patients with type 2 diabetes mellitus. J. Bone Miner. Metab. 2018, 37, 503–511. [Google Scholar] [CrossRef]
- Stanghelle, B.; Bentzen, H.; Giangregorio, L.; Pripp, A.H.; Bergland, A. Associations between health-related quality of life, physical function and pain in older women with osteoporosis and vertebral fracture. BMC Geriatr. 2019, 19, 298. [Google Scholar] [CrossRef]
| Group 1 (Osteoporosis) | Group 2 (without Osteoporosis) | |||||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Max | Min | Median | IQR | Max | Min | |
| Age | 79 | 15 | 85 | 65 | 71 | 6.75 | 80 | 65 |
| BMI | 27.10 | 6.99 | 29.03 | 23.34 | 25.86 | 1.65 | 36.79 | 19.29 |
| T-score | −3.10 | 1.48 | −4.3 | −2.5 | −1.90 | 0.30 | −2.4 | −1.4 |
| Group 1 (Osteoporosis) | Group 2 (No Osteoporosis) | MWW | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p | |
| QOL1 | 25 | 38 | 20 | 15 | 0.084 |
| QOL2 | 25 | 34 | 20 | 20 | 0.107 |
| QOL3 | 40 | 53 | 30 | 28 | 0.059 |
| QOL4 | 50 | 47 | 25 | 26 | 0.011 |
| QOL5 | 58 | 32 | 30 | 23 | <0.001 |
| QOL6 | 50 | 25 | 42 | 21 | 0.003 |
| QOL7 | 43 | 29 | 33 | 24 | 0.111 |
| Qualeffo41 | 38 | 27 | 28 | 14 | 0.001 |
| Spearman’s Correlation | ||
|---|---|---|
| rho | p | |
| QOL1 | −0.36 | 0.004 |
| QOL2 | −0.44 | <0.001 |
| QOL3 | −0.46 | <0.001 |
| QOL4 | −0.57 | <0.001 |
| QOL5 | −0.66 | <0.001 |
| QOL6 | −0.59 | <0.001 |
| QOL7 | −0.39 | 0.002 |
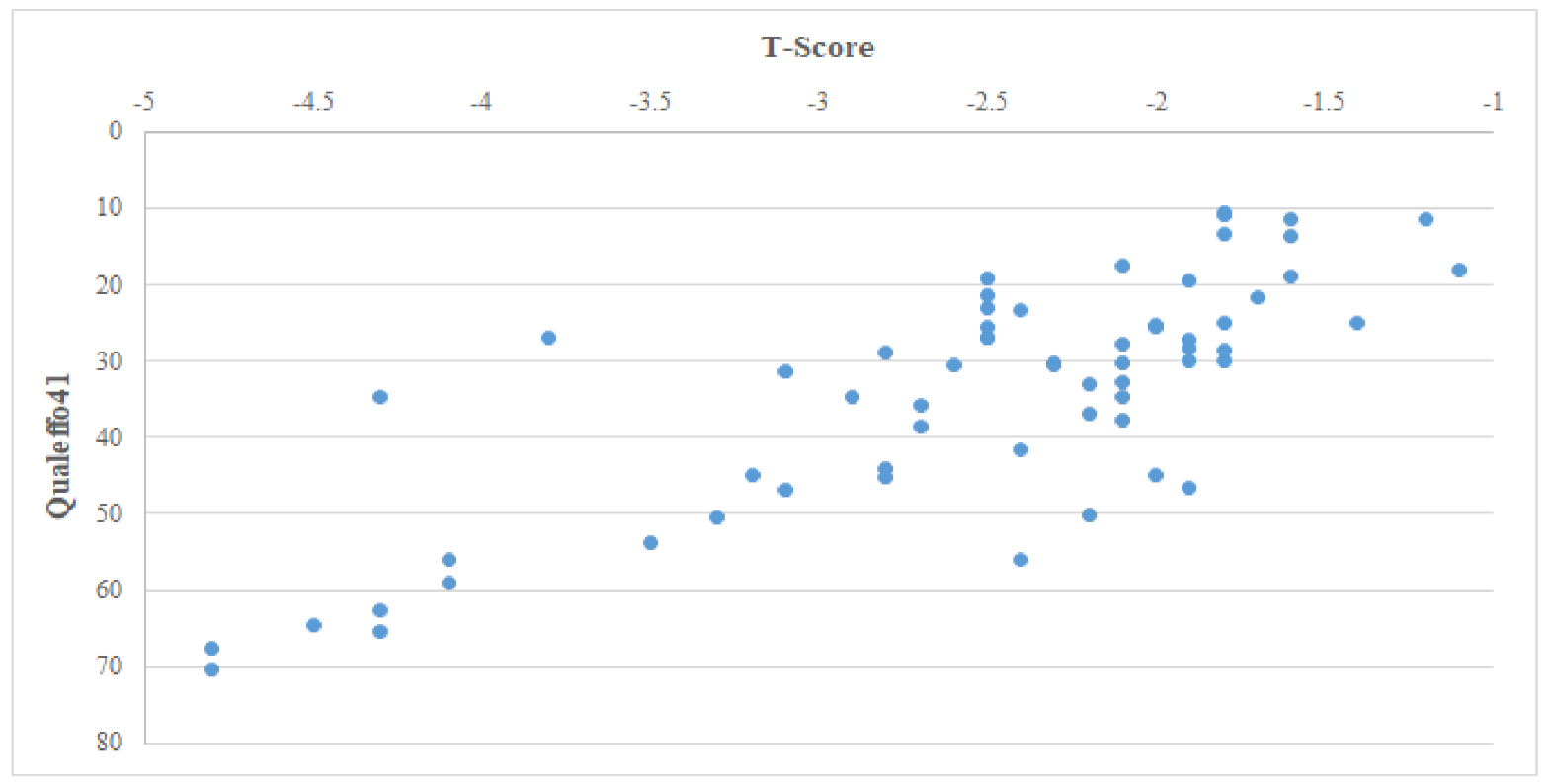
| Qualeffo41 | −0.72 | <0.001 |
| Spearman’s Correlation | ||
|---|---|---|
| rho | p | |
| Pain (QOL1) | 0.179 | 0.371 |
| Activities of daily living (QOL2) | 0.031 | 0.879 |
| Jobs around the house (QOL3) | 0.025 | 0.903 |
| Mobility (QOL4) | 0.324 | 0.099 |
| Leisure, social activities (QOL5) | 0.089 | 0.659 |
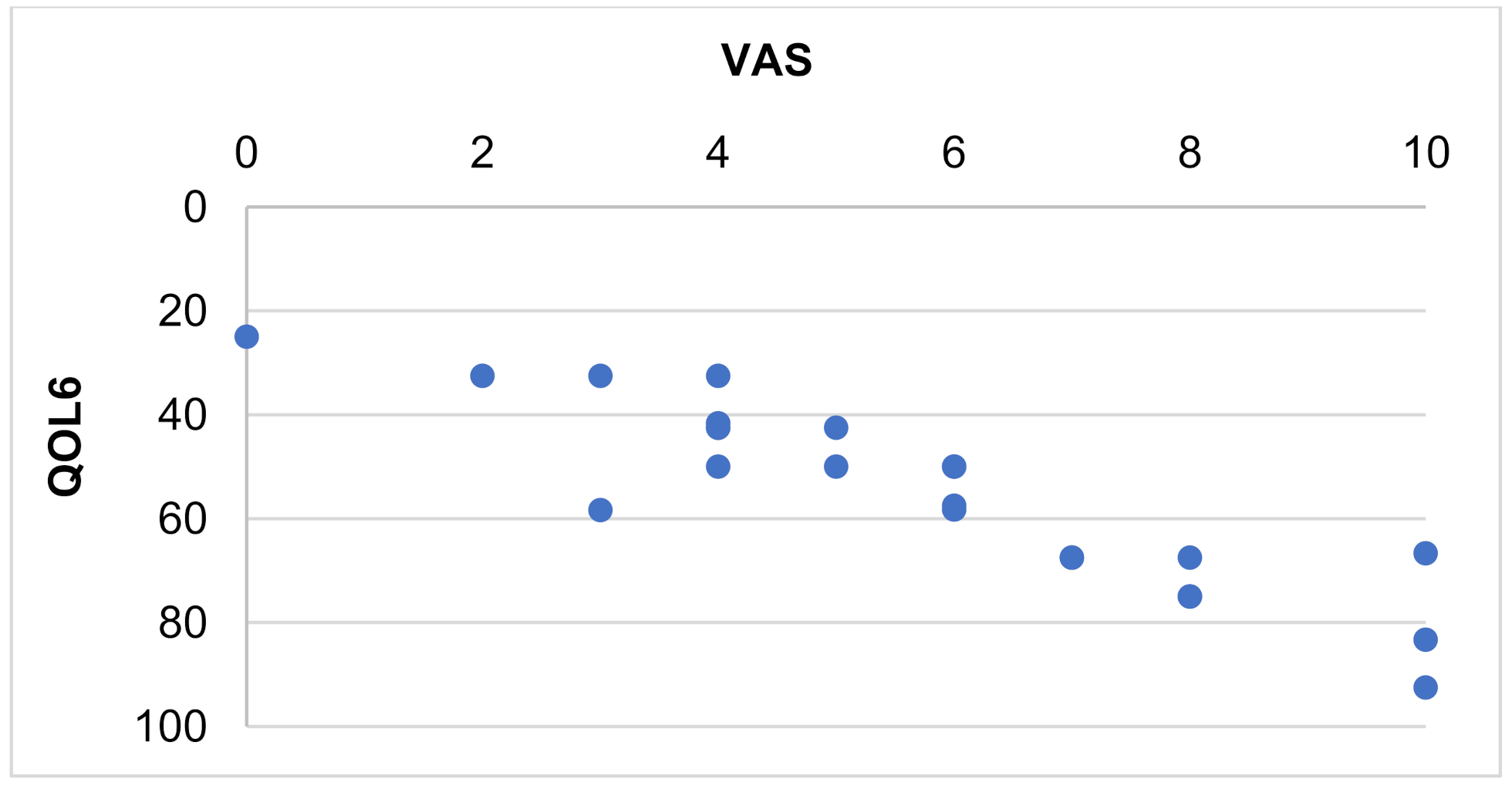
| General health perception (QOL6) | 0.888 | <0.001 |
| Mental function (QOL7) | 0.305 | 0.121 |
| Qualeffo41 | 0.284 | 0.152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrat-Szołtysik, A.; Miodońska, Z.; Piejko, L.; Szołtys, B.; Błaszczyszyn, M.; Matyja, B.; Zarzeczny, R.; Zając-Gawlak, I.; Kucio, E.; Polak, A. Assessment of Quality of Life and Pain Severity in Older Men with Osteoporosis: Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 11276. https://doi.org/10.3390/ijerph182111276
Nawrat-Szołtysik A, Miodońska Z, Piejko L, Szołtys B, Błaszczyszyn M, Matyja B, Zarzeczny R, Zając-Gawlak I, Kucio E, Polak A. Assessment of Quality of Life and Pain Severity in Older Men with Osteoporosis: Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2021; 18(21):11276. https://doi.org/10.3390/ijerph182111276
Chicago/Turabian StyleNawrat-Szołtysik, Agnieszka, Zuzanna Miodońska, Laura Piejko, Bogna Szołtys, Monika Błaszczyszyn, Beata Matyja, Ryszard Zarzeczny, Izabela Zając-Gawlak, Ewa Kucio, and Anna Polak. 2021. "Assessment of Quality of Life and Pain Severity in Older Men with Osteoporosis: Cross-Sectional Study" International Journal of Environmental Research and Public Health 18, no. 21: 11276. https://doi.org/10.3390/ijerph182111276
APA StyleNawrat-Szołtysik, A., Miodońska, Z., Piejko, L., Szołtys, B., Błaszczyszyn, M., Matyja, B., Zarzeczny, R., Zając-Gawlak, I., Kucio, E., & Polak, A. (2021). Assessment of Quality of Life and Pain Severity in Older Men with Osteoporosis: Cross-Sectional Study. International Journal of Environmental Research and Public Health, 18(21), 11276. https://doi.org/10.3390/ijerph182111276









