T1DMicro: A Clinical Risk Calculator for Type 1 Diabetes Related Microvascular Complications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Rates of Diabetic Complications
3.2. Individual Risk Factors Associated with Diabetic Complications
3.3. Multivariate Predictive Model of Complications
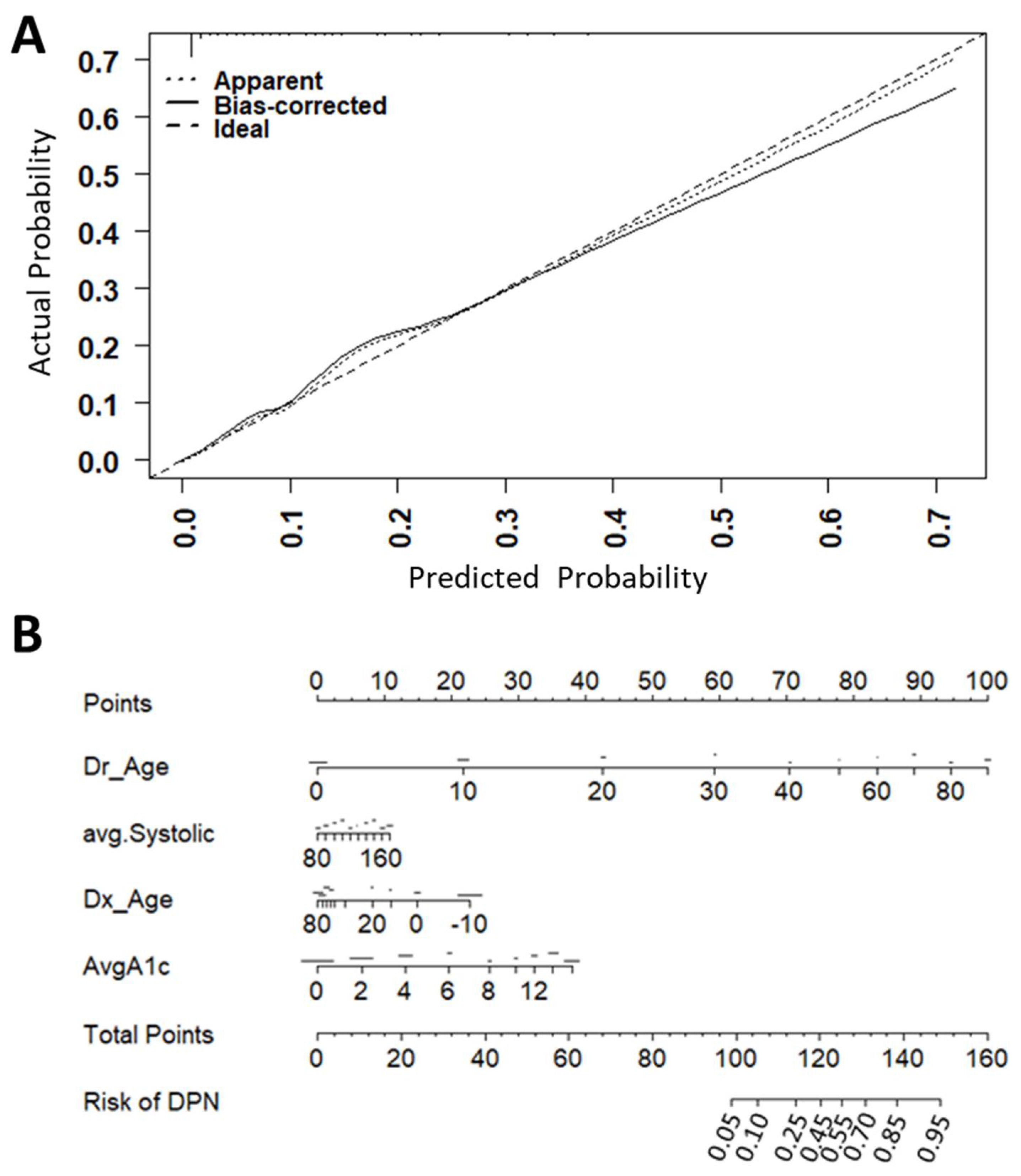
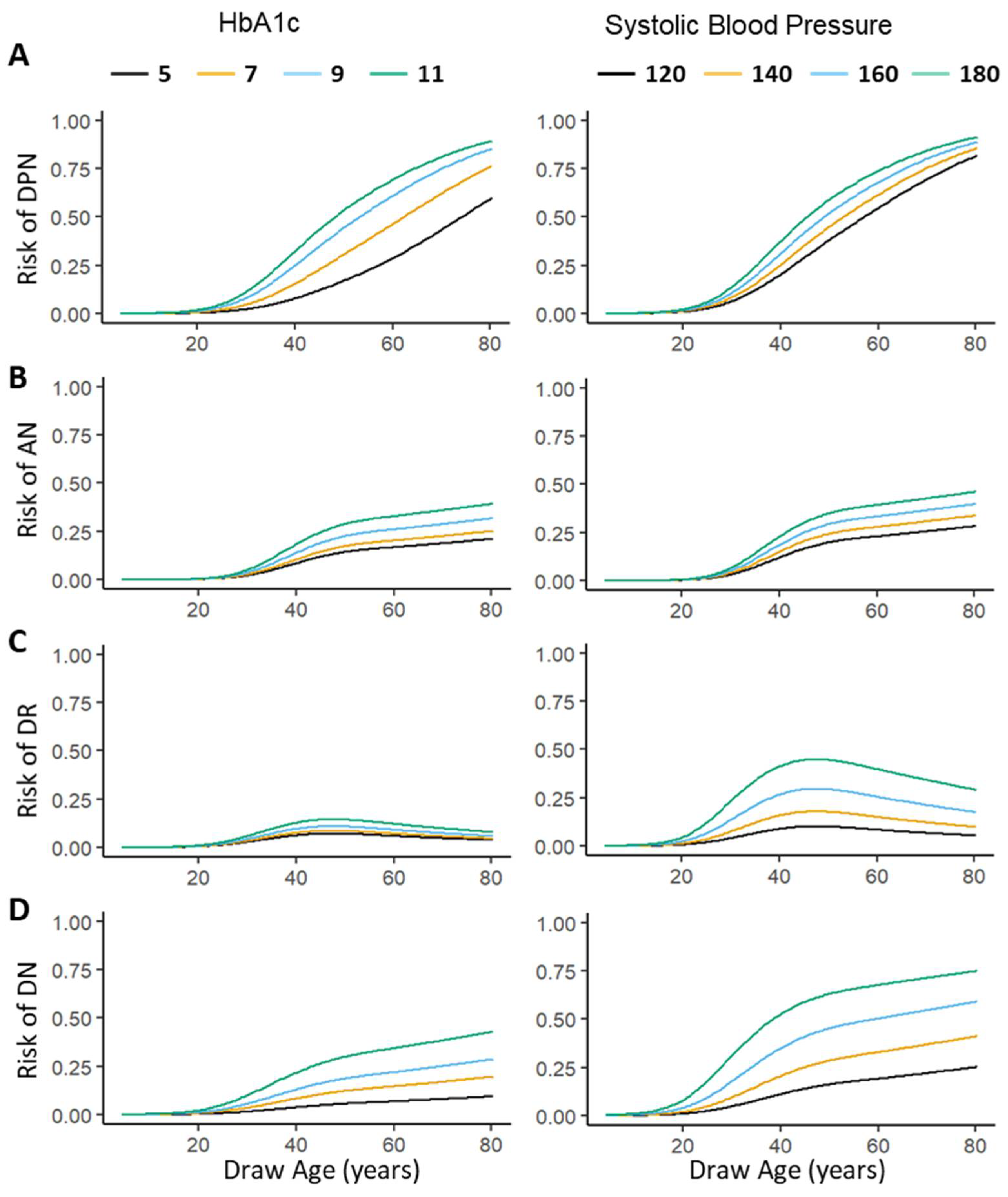
3.4. Web Interface to Predict Individual Risk of Diabetic Microvascular Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nathan, D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic autonomic neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.L.; De Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef] [Green Version]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Huxley, R.R.; Peters, S.A.; Mishra, G.D.; Woodward, M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Cho, Y.H.; Craig, M.E.; Donaghue, K.C. Puberty as an accelerator for diabetes complications. Pediatr. Diabetes 2014, 15, 18–26. [Google Scholar] [CrossRef]
- Bergenstal, R.M. Glycemic Variability and Diabetes Complications: Does It Matter? Simply Put, There Are Better Glycemic Markers! Diabetes Care 2015, 38, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Hilliard, M.E.; Mann, K.A.; Peugh, J.L.; Hood, K.K. How poorer quality of life in adolescence predicts subsequent type 1 diabetes management and control. Patient Educ. Couns. 2013, 91, 120–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponirakis, G.; Petropoulos, I.N.; Alam, U.; Ferdousi, M.; Asghar, O.; Marshall, A.; Azmi, S.; Jeziorska, M.; Mahfoud, Z.R.; Boulton, A.J.M.; et al. Hypertension Contributes to Neuropathy in Patients With Type 1 Diabetes. Am. J. Hypertens 2019, 32, 796–803. [Google Scholar] [CrossRef]
- Sharot, T.; Korn, C.W.; Dolan, R.J. How unrealistic optimism is maintained in the face of reality. Nat. Neurosci. 2011, 14, 1475–1479. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, L.; Sansgiry, S. A systematic literature review of predicting diabetic retinopathy, nephropathy and neuropathy in patients with type 1 diabetes using machine learning. J. Med. Artif. Intell. 2020, 3, 6. [Google Scholar] [CrossRef]
- Kazemi, M.; Moghimbeigi, A.; Kiani, J.; Mahjub, H.; Faradmal, J. Diabetic peripheral neuropathy class prediction by multicategory support vector machine model: A cross-sectional study. Epidemiol. Health 2016, 38, e2016011. [Google Scholar] [CrossRef] [PubMed]
- Lagani, V.; Chiarugi, F.; Thomson, S.; Fursse, J.; Lakasing, E.; Jones, R.W.; Tsamardinos, I. Development and validation of risk assessment models for diabetes-related complications based on the DCCT/EDIC data. J. Diabetes Complicat. 2015, 29, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Braffett, B.H.; Gubitosi-Klug, R.A.; Albers, J.W.; Feldman, E.L.; Martin, C.L.; White, N.H.; Orchard, T.J.; Lopes-Virella, M.; Lachin, J.M.; Pop-Busui, R. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2020, 69, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Development and validation of risk prediction equations to estimate future risk of blindness and lower limb amputation in patients with diabetes: Cohort study. BMJ 2015, 351, h5441. [Google Scholar] [CrossRef] [Green Version]
- Prochaska, J.O.; DiClemente, C.C. Stages and processes of self-change of smoking: Toward an integrative model of change. J. Consult. Clin. Psychol. 1983, 51, 390. [Google Scholar] [CrossRef]
- Nishimura, R.; LaPorte, R.E.; Dorman, J.S.; Tajima, N.; Becker, D.; Orchard, T.J. Mortality trends in type 1 diabetes: The Allegheny County (Pennsylvania) Registry 1965–1999. Diabetes Care 2001, 24, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Purohit, S.; Sharma, A.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Caldwell, R.; She, J.-X. Elevated Serum Levels of Soluble TNF Receptors and Adhesion Molecules Are Associated with Diabetic Retinopathy in Patients with Type-1 Diabetes. Mediat. Inflamm. 2015, 2015, 279393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, S.; Sharma, A.; Zhi, W.; Bai, S.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Reed, J.C.; Steed, R.D.; et al. Proteins of TNF-α and IL6 Pathways Are Elevated in Serum of Type-1 Diabetes Patients with Microalbuminuria. Front. Immunol. 2018, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Harrell, F.E., Jr. rms: Regression modeling strategies. R Package Vers. 2016, 5. Available online: https://cran.r-project.org/web/packages/rms/index.html (accessed on 2 October 2021).
- Harrell, F.E., Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S55–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rewers, M.J.; Pillay, K.; de Beaufort, C.; Craig, M.E.; Hanas, R.; Acerini, C.L.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes 2014, 15 (Suppl. 20), 102–114. [Google Scholar] [CrossRef]
- Cheng, A.Y. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Introduction. Can. J. Diabetes 2013, 37 (Suppl. 1), S1–S3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jende, J.M.E.; Groener, J.B.; Rother, C.; Kender, Z.; Hahn, A.; Hilgenfeld, T.; Juerchott, A.; Preisner, F.; Heiland, S.; Kopf, S.; et al. Association of Serum Cholesterol Levels With Peripheral Nerve Damage in Patients With Type 2 Diabetes. JAMA Netw. Open 2019, 2, e194798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfaye, S.; Chaturvedi, N.; Eaton, S.E.; Ward, J.D.; Manes, C.; Ionescu-Tirgoviste, C.; Witte, D.R.; Fuller, J.H.; Group, E.P.C.S. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 2005, 352, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Maahs, D.M.; West, N.A.; Lawrence, J.M.; Mayer-Davis, E.J. Epidemiology of type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 481–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.L.; Yi, J.P.; Beyer, J.; Mayer-Davis, E.J.; Dolan, L.M.; Dabelea, D.M.; Lawrence, J.M.; Rodriguez, B.L.; Marcovina, S.M.; Waitzfelder, B.E.; et al. Type 1 and Type 2 diabetes in Asian and Pacific Islander U.S. youth: The SEARCH for Diabetes in Youth Study. Diabetes Care 2009, 32 (Suppl. 2), S133–S140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanewinckel, R.; van Oijen, M.; Ikram, M.A.; van Doorn, P.A. The epidemiology and risk factors of chronic polyneuropathy. Eur. J. Epidemiol. 2016, 31, 5–20. [Google Scholar] [CrossRef] [Green Version]
| Demographic/Clinical Variable | T1D (n = 1026) | T1D_wComp * (n = 621) | p |
|---|---|---|---|
| Male (n (%)) | 499 (48.6%) | 291 (46.9%) | |
| Females (n (%)) | 527 (51.4%) | 330 (53.1%) | n.s |
| Age (Years, median (range)) | 16.2 (12.0–25.7) | 48.2 (38.9–58.47) | <0.0001 |
| Duration of T1D (Years, median (range)) | 8.6 (2.1–12.5) | 25.3 (14.6–34.8) | <0.0001 |
| Complications, n (%) | |||
| DPN | 199 (32.0%) | <0.0001 | |
| AN | 63 (10.1%) | <0.0001 | |
| DR | 244 (39.3%) | <0.0001 | |
| DN | 88 (14.2%) | <0.0001 | |
| Photocoagulation | 167 (26.9%) | <0.0001 | |
| Blindness | 42 (6.8%) | <0.0001 | |
| Diabetic Foot Ulcer | 25 (4.0%) | <0.0001 | |
| Amputation | 17 (2.7%) | <0.0001 | |
| Past Medical History, n (%) | |||
| Hypertension | 287 (46.2%) | <0.0001 | |
| Dyslipidemia | 356 (57.3%) | <0.0001 | |
| Coronary Artery Disease | 102 (16.4%) | <0.0001 | |
| Prior Myocardial Infarction | 31 (5.0%) | <0.0001 | |
| Prior Angioplasty Stent | 61 (9.8%) | <0.0001 | |
| Prior CABG | 44 (7.1%) | <0.0001 | |
| Prior Transient Ischemic Attack | 14 (2.3%) | <0.0001 | |
| Prior Cerebrovascular Accident | 9 (1.4%) | <0.0001 | |
| Smoking Status† | |||
| Current | 32 (3.1%) | 73 (11.8%) | <0.0001 |
| Former | 58 (5.7%) | 157 (25.3%) | |
| Never | 898 (87.5%) | 383 (61.7) | |
| Physiologic Measurements and Laboratory Values, Median (IQR) | |||
| Systolic Blood Pressure (mmHg) | 114.7 (108.6–122) | 123.3 (115.7–131.7) | <0.0001 |
| Diastolic Blood Pressure (mmHg) | 70 (66.3–74.8) | 72.7 (70.0–78.0) | <0.0001 |
| Creatinine (mg/dL) | 0.77 (0.6–0.9) | 0.97 (0.83–1.1) | <0.0001 |
| Albumin Creatinine Ratio (mg/ug) | 9.04 (5.4–18.5) | 9.91 (5.2–43.4) | <0.05 |
| Lipid panel | |||
| Total Cholesterol (mg/dL) | 163.0 (146.0–185.3) | 175.5 (150.8–200.0) | <0.0001 |
| LDL (mg/dL) | 90.0 (77.0–106.0) | 96.5 (76.0–118.5) | <0.001 |
| HDL (mg/dL) | 53.0 (43.0–64.0) | 55.0 (44.8–68.0) | <0.01 |
| Triglycerides (mg/dL) | 76.0 (55.0–112.0) | 79.0 (58.0–119.0) | n.s. |
| HbA1C (NGSP, %) | 7.9 (7.1–8.9) | 7.8 (7.3–8.7) | n.s. |
| HbA1c (IFCC, mmol/mol) | 63.6 (54.1–73.0) | 62.1(55.2–71.2) | n.s. |
| DPN | AN | DR | DN | |||||
|---|---|---|---|---|---|---|---|---|
| Demographics | OR | p | OR | p | OR | p | OR | p |
| Age | 1.1 | <0.0001 | 1.1 | <0.0001 | 1.1 | <0.0001 | 1.0 | <0.0001 |
| Age at T1D diagnosis | 1.0 | <0.0001 | 1.0 | 0.0015 | 1.0 | 0.0039 | 1.0 | 0.0310 |
| Duration of T1D | 1.1 | <0.0001 | 1.1 | <0.0001 | 1.1 | <0.0001 | 1.1 | <0.0001 |
| Sex | 0.7 | 0.0150 | 1.1 | 0.6500 | 0.7 | 0.0038 | 0.9 | 0.4800 |
| Complications | ||||||||
| DPN | 25.4 | <0.0001 | 12.2 | <0.0001 | 7.9 | <0.0001 | ||
| AN | 25.4 | <0.0001 | 9.3 | <0.0001 | 6.0 | <0.0001 | ||
| DR | 12.2 | <0.0001 | 9.3 | <0.0001 | 13.7 | <0.0001 | ||
| DN | 7.9 | <0.0001 | 6.0 | <0.0001 | 13.7 | <0.0001 | ||
| Blindness | 6.6 | <0.0001 | 5.6 | <0.0001 | 286.0 | <0.0001 | 19.1 | <0.0001 |
| Photocoagulation | 10.5 | <0.0001 | 8.1 | <0.0001 | 1492.5 | <0.0001 | 14.5 | <0.0001 |
| Amputation | 36.2 | <0.0001 | 11.4 | <0.0001 | 19.6 | <0.0001 | 6.1 | 0.0021 |
| Diabetic Foot Ulcer | 198.3 | <0.0001 | 16.8 | <0.0001 | 32.8 | <0.0001 | 14.1 | <0.0001 |
| Past Medical History | ||||||||
| Smoking | 1.5 | 0.1400 | 1.0 | 0.9200 | 1.5 | 0.1200 | 1.4 | 0.4000 |
| Hypertension | 6.8 | <0.0001 | 4.9 | <0.0001 | 7.9 | <0.0001 | 8.1 | <0.0001 |
| Dyslipidemia | 5.0 | <0.0001 | 4.6 | <0.0001 | 3.7 | <0.0001 | 3.3 | <0.0001 |
| CAD | 13.3 | <0.0001 | 6.1 | <0.0001 | 6.0 | <0.0001 | 5.8 | <0.0001 |
| Prior Angioplasty/Stent | 18.5 | <0.0001 | 6.1 | <0.0001 | 8.9 | <0.0001 | 7.4 | <0.0001 |
| Prior CABG | 12.0 | <0.0001 | 5.4 | 0.0001 | 5.6 | <0.0001 | 4.2 | 0.0004 |
| Prior CVA | 9.4 | 0.0009 | 13.5 | 0.0003 | 11.8 | 0.0005 | 15.0 | <0.0001 |
| Prior MI | 12.7 | <0.0001 | 4.0 | 0.0120 | 7.5 | <0.0001 | 5.5 | 0.0001 |
| Prior TIA | 19.5 | <0.0001 | 4.5 | 0.0540 | 4.5 | 0.0059 | 14.4 | <0.0001 |
| Physiologic Measurements and Laboratory Values | ||||||||
| SBP | 1.1 | <0.0001 | 1.0 | 0.0001 | 1.1 | <0.0001 | 1.1 | <0.0001 |
| DBP | 1.0 | 0.0021 | 1.1 | 0.0005 | 1.0 | 0.0002 | 1.1 | 0.0007 |
| Hemoglobin | 0.8 | <0.0001 | 0.8 | 0.0600 | 0.7 | <0.0001 | 0.7 | <0.0001 |
| Albumin | 1.0 | 0.3200 | 0.7 | 0.3800 | 0.3 | <0.0001 | 0.4 | 0.0011 |
| BUN | 1.1 | <0.0001 | 1.0 | 0.0087 | 1.1 | <0.0001 | 1.1 | <0.0001 |
| Creatinine | 1.0 | 0.6700 | 1.0 | 0.9100 | 1.0 | 0.4500 | 1.1 | 0.0200 |
| Micro Albumin | 1.0 | 0.0084 | 1.0 | 0.8400 | 1.0 | <0.0001 | 1.0 | <0.0001 |
| ACR | 1.0 | 0.0220 | 1.0 | 0.8000 | 1.0 | <0.0001 | 1.0 | <0.0001 |
| Lipid panel | ||||||||
| Total Cholesterol | 1.0 | 0.1 | 1.0 | 0.5 | 1.0 | 0.0290 | 1.0 | 0.1 |
| LDL | 1.0 | 0.7 | 1.0 | 0.6 | 1.0 | 0.3 | 1.0 | 0.8 |
| HDL | 1.0 | 0.02 | 1.0 | 0.0027 | 1.0 | 0.02 | 1.0 | 0.8 |
| Triglycerides | 1.0 | 0.4 | 1.0 | 0.3 | 1.0 | 0.8 | 1.0 | 0.1 |
| HbA1c | 1.0 | 0.8 | 1.0 | 0.8 | 0.9 | 0.2 | 1.1 | 0.4 |
| HbA1c, SD | 0.9 | 0.3 | 0.8 | 0.4 | 0.8 | 0.0 | 1.0 | 0.8 |
| HbA1c, last visit | 1.0 | 0.8 | 1.0 | 1.0 | 0.9 | 0.1 | 1.1 | 0.4 |
| HbA1c, maximum | 1.0 | 1.0 | 1.0 | 0.9 | 0.9 | 0.1 | 1.1 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, P.M.H.; Kim, E.; Tran, L.K.H.; Khaled, B.S.; Hopkins, D.; Gardiner, M.; Bryant, J.; Bernard, R.; Morgan, J.; Bode, B.; et al. T1DMicro: A Clinical Risk Calculator for Type 1 Diabetes Related Microvascular Complications. Int. J. Environ. Res. Public Health 2021, 18, 11094. https://doi.org/10.3390/ijerph182111094
Tran PMH, Kim E, Tran LKH, Khaled BS, Hopkins D, Gardiner M, Bryant J, Bernard R, Morgan J, Bode B, et al. T1DMicro: A Clinical Risk Calculator for Type 1 Diabetes Related Microvascular Complications. International Journal of Environmental Research and Public Health. 2021; 18(21):11094. https://doi.org/10.3390/ijerph182111094
Chicago/Turabian StyleTran, Paul Minh Huy, Eileen Kim, Lynn Kim Hoang Tran, Bin Satter Khaled, Diane Hopkins, Melissa Gardiner, Jennifer Bryant, Risa Bernard, John Morgan, Bruce Bode, and et al. 2021. "T1DMicro: A Clinical Risk Calculator for Type 1 Diabetes Related Microvascular Complications" International Journal of Environmental Research and Public Health 18, no. 21: 11094. https://doi.org/10.3390/ijerph182111094
APA StyleTran, P. M. H., Kim, E., Tran, L. K. H., Khaled, B. S., Hopkins, D., Gardiner, M., Bryant, J., Bernard, R., Morgan, J., Bode, B., Reed, J. C., She, J.-X., & Purohit, S. (2021). T1DMicro: A Clinical Risk Calculator for Type 1 Diabetes Related Microvascular Complications. International Journal of Environmental Research and Public Health, 18(21), 11094. https://doi.org/10.3390/ijerph182111094






