Lysophosphatidic Acid Receptor 6 (LPAR6) Is a Potential Biomarker Associated with Lung Adenocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. LPAR6 Gene Expression Level Analysis
2.3. Prognosis Analysis
2.4. Correlation Analysis of LPAR6 and Survival Rate
2.5. Methylation Analysis
2.6. Biological Network Analysis
2.7. LinkedOmics Analysis
2.8. Immune Infiltrates Level and Gene Correlation Analysis
2.9. Immunohistochemical Staining for LPAR6 in Lung Cancer Patient Cohort Tissue Microarrays
2.10. Statistical Analysis
3. Results
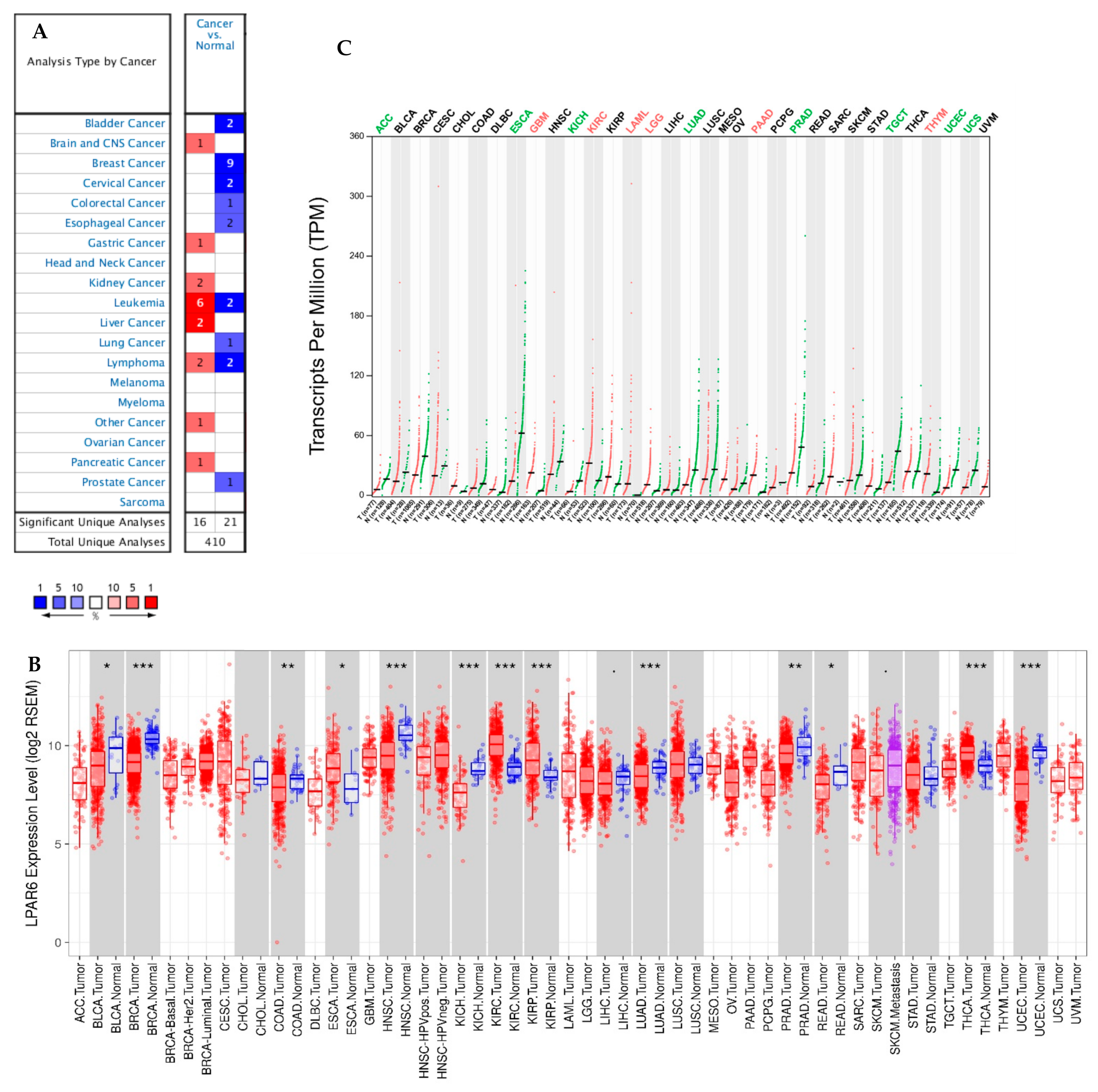
3.1. The Expression Levels of LPAR6 in Different Human Cancers
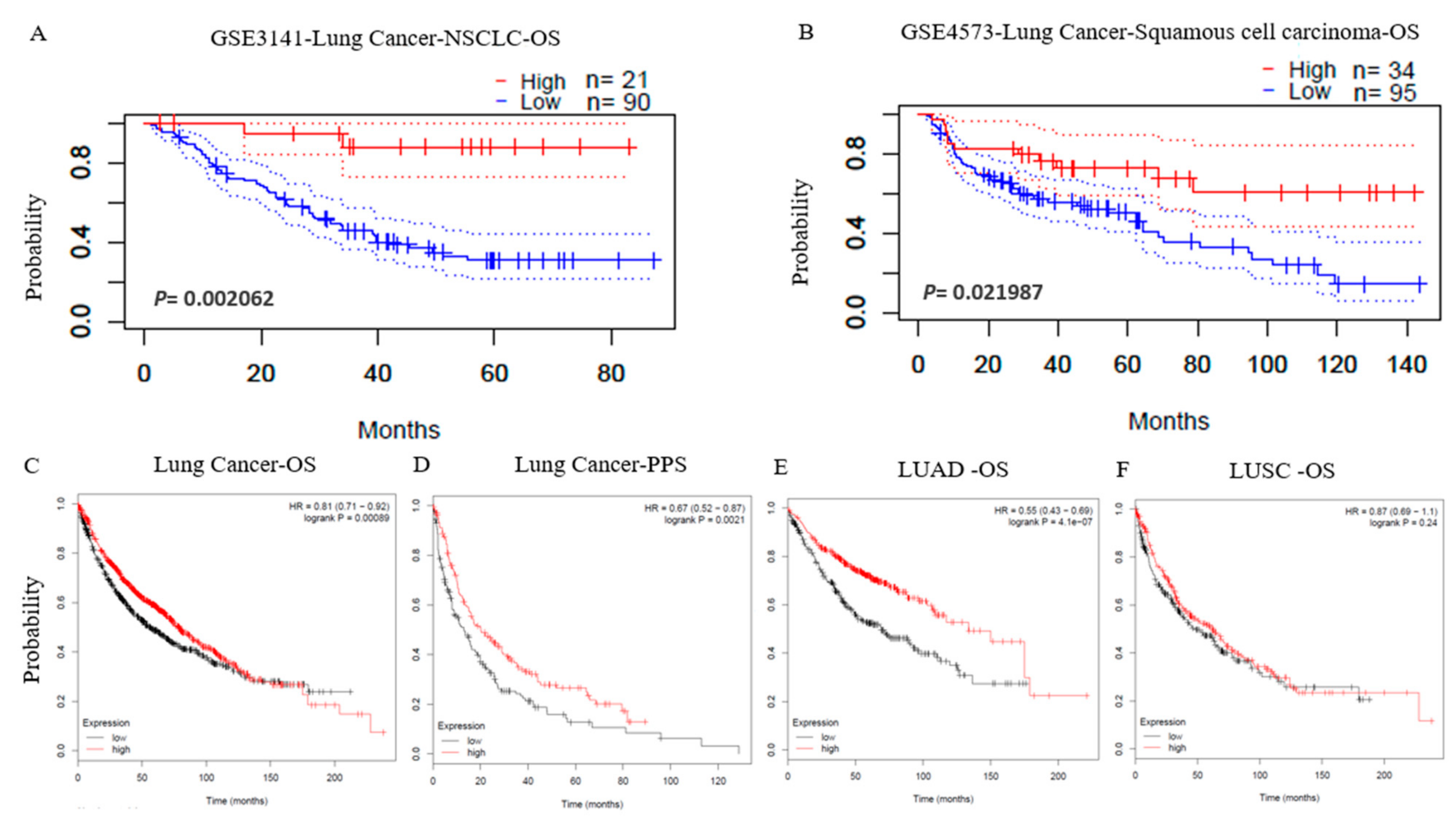
3.2. Prognostic Potential of LPAR6 in Various Types of Cancer
3.3. The mRNA Expression Level of LPAR6 Impacts the Lung Cancer Prognosis in Different Clinical Characteristics
3.4. Low Promoter Methylation Levels of LPAR6 Impacts the Clinicopathological Parameters of Lung Cancer Patients
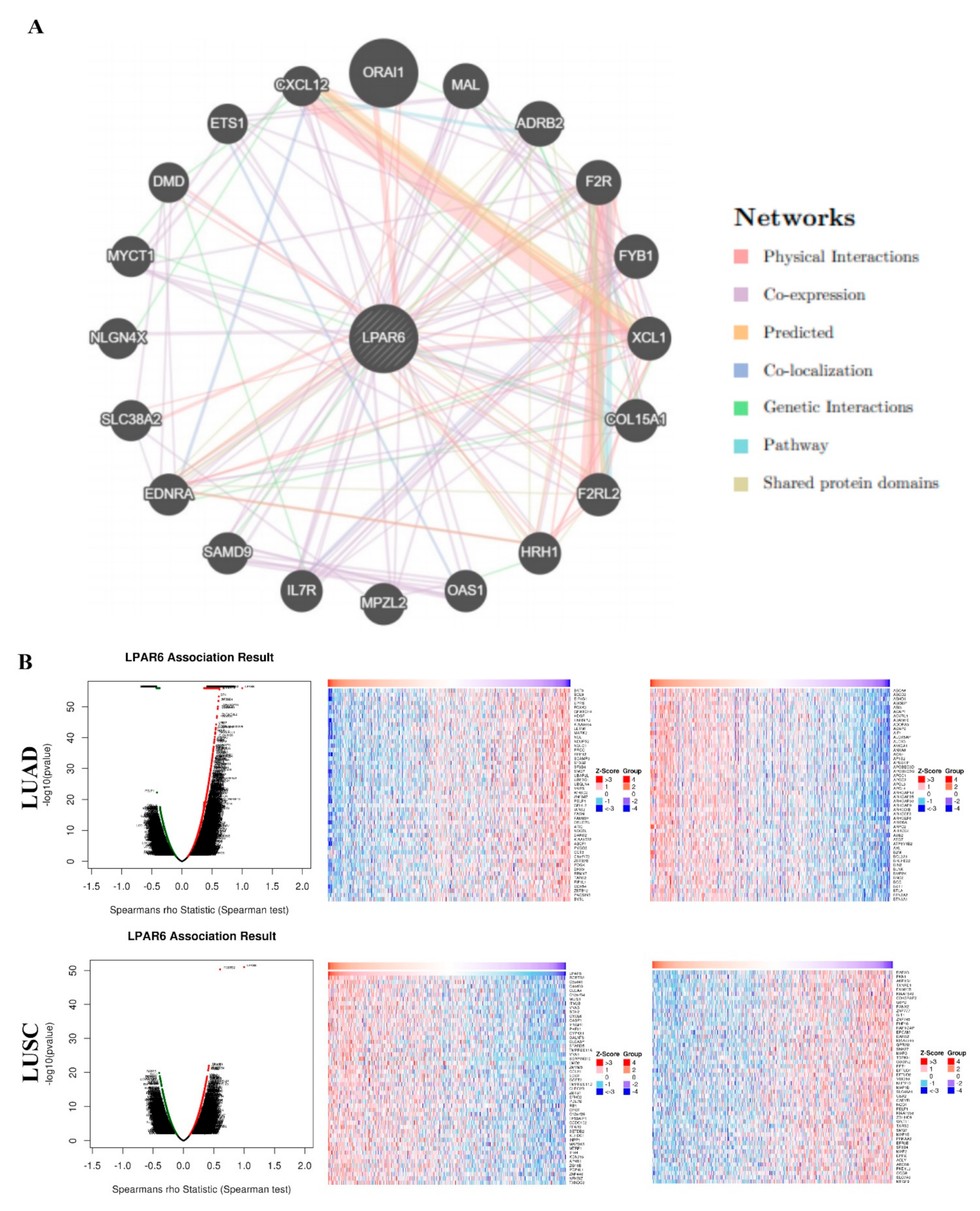
3.5. Interaction Network of LPAR6
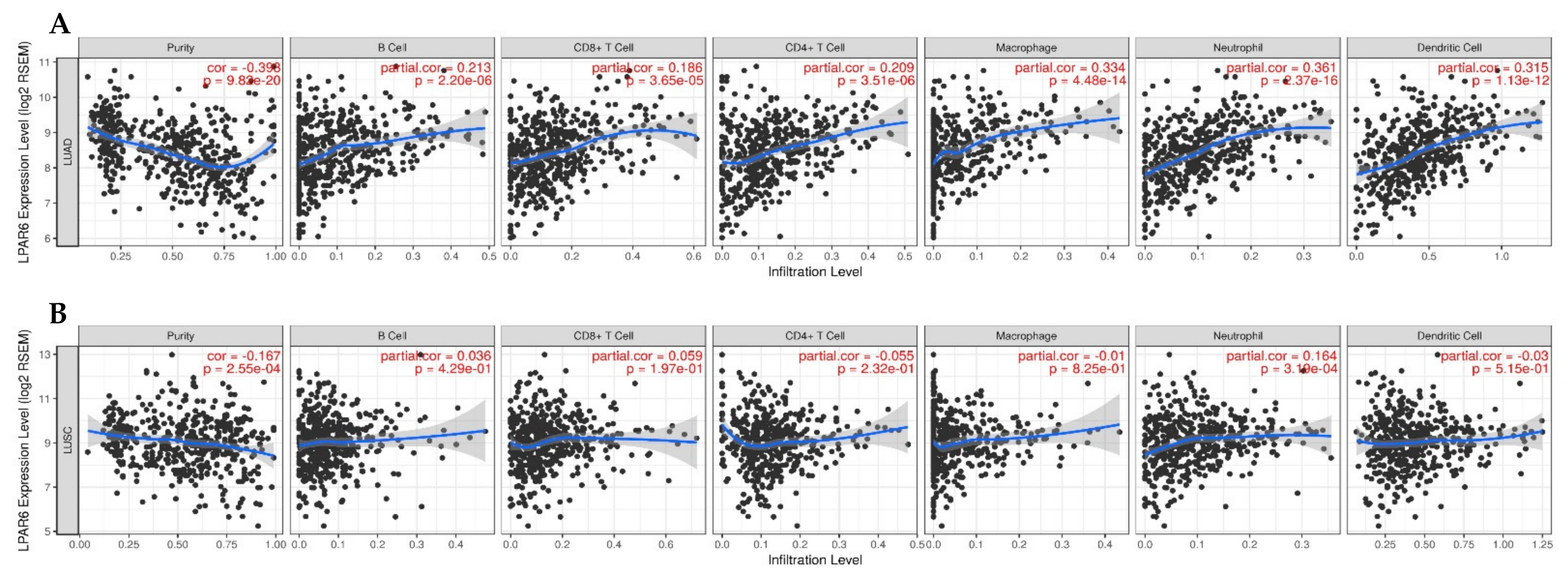
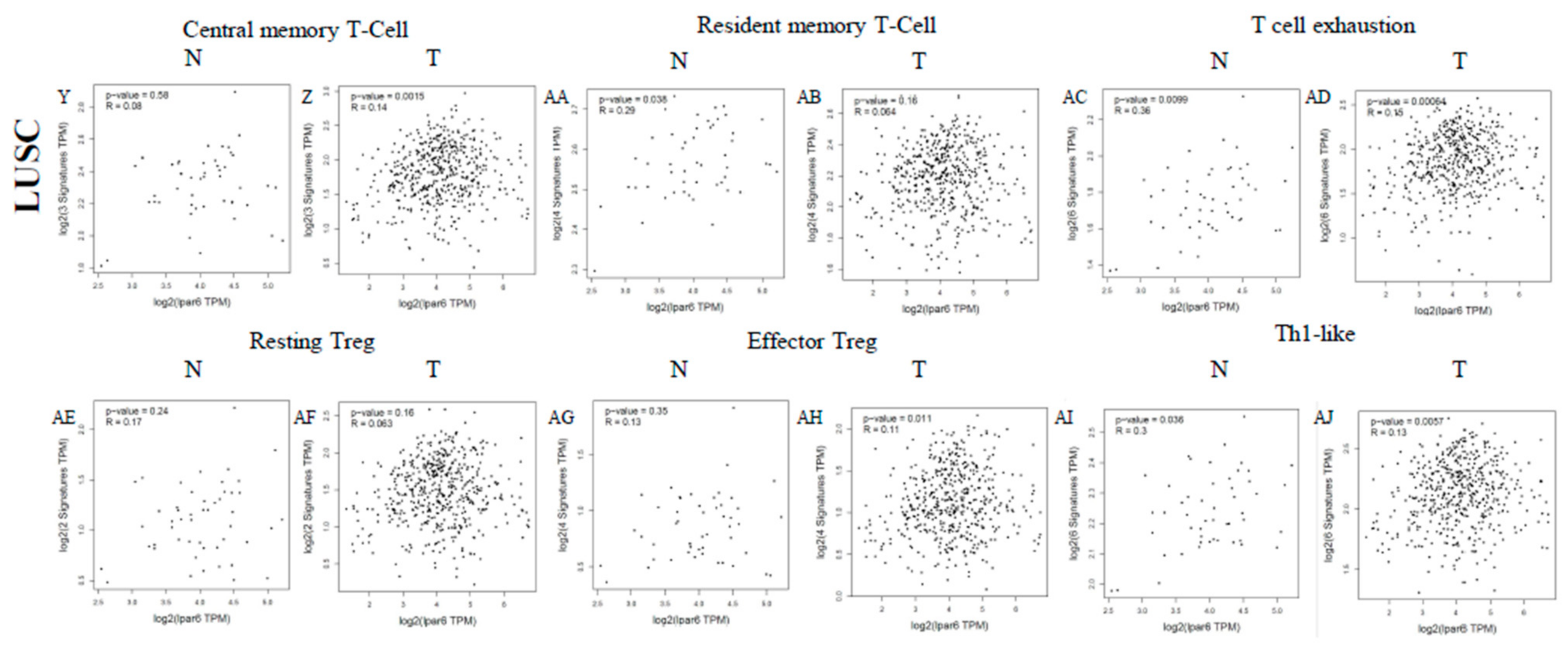
3.6. The Expression Level of LPAR6 Is Correlated with Immune Infiltration Level in Lung Cancers
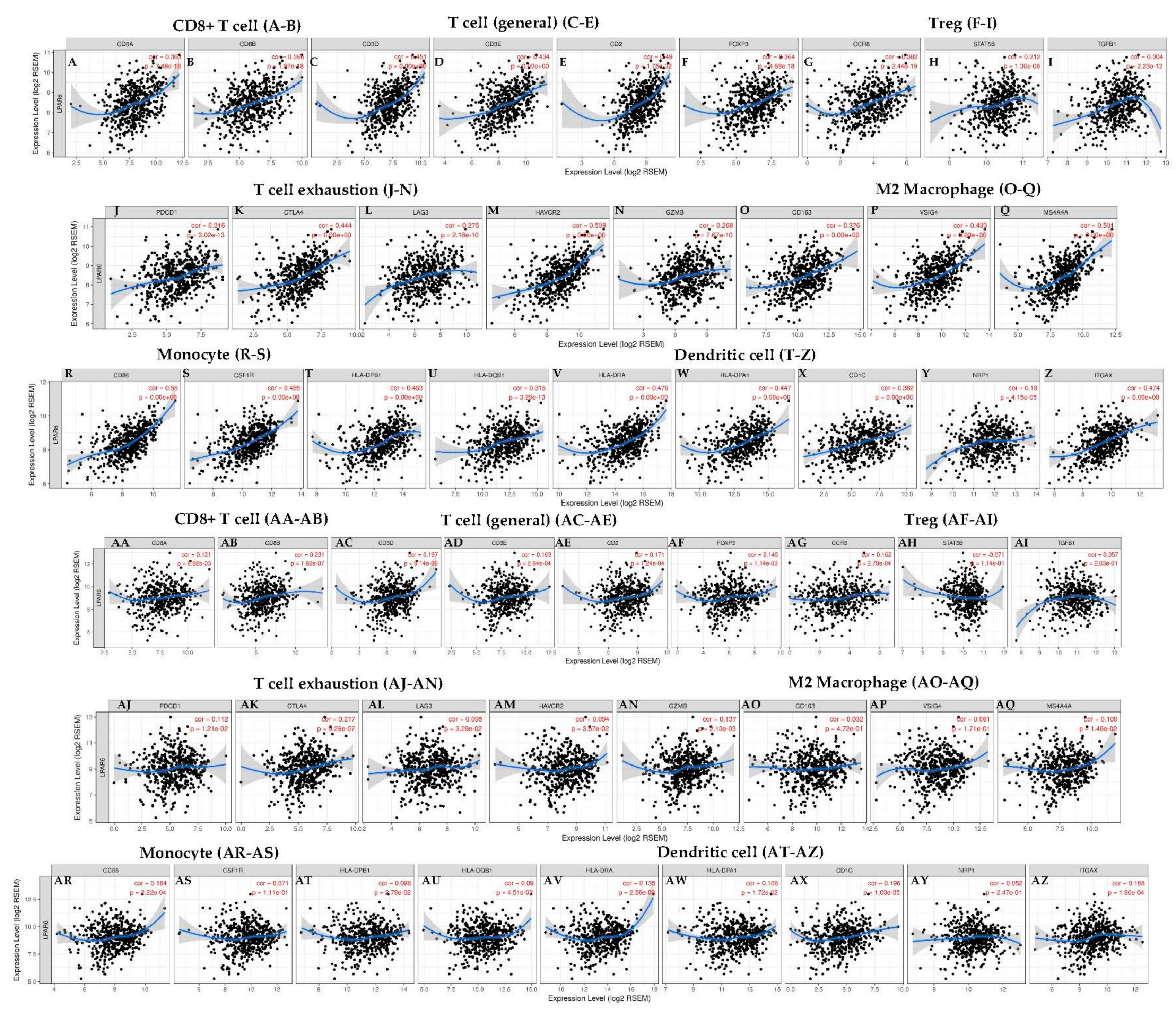
3.7. Correlation Analysis between LPAR6 Expression Level and the Immune Marker Sets
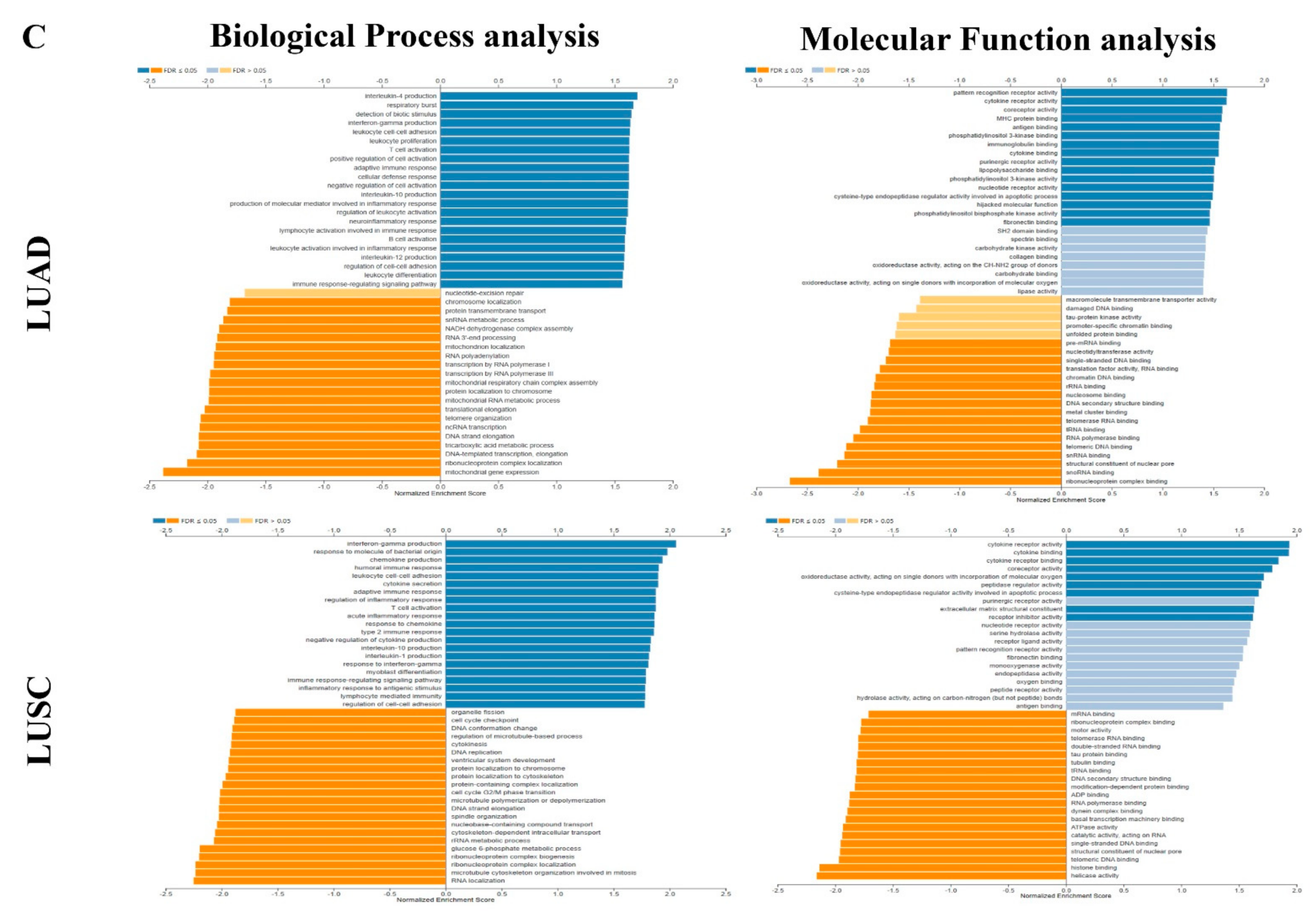
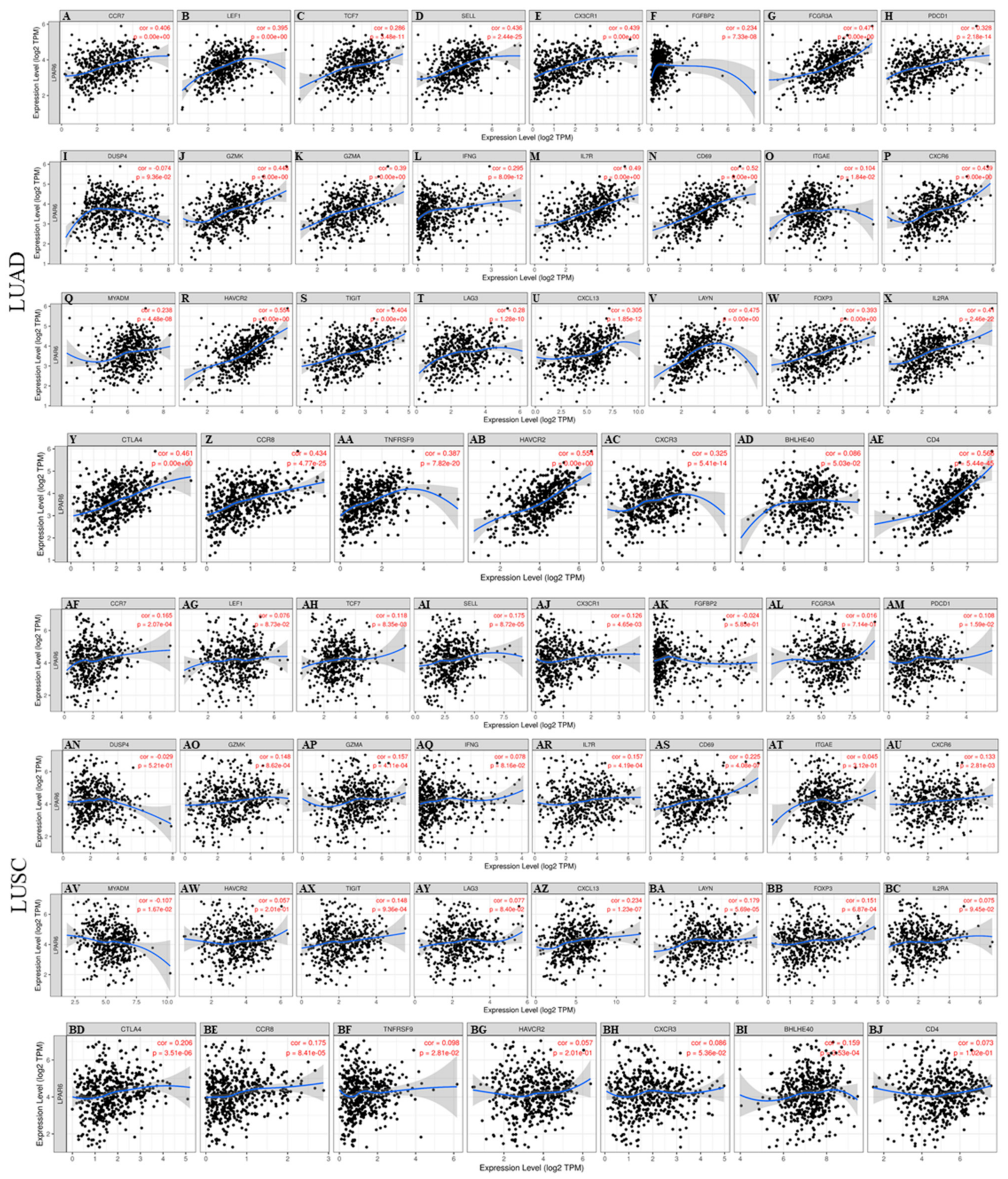
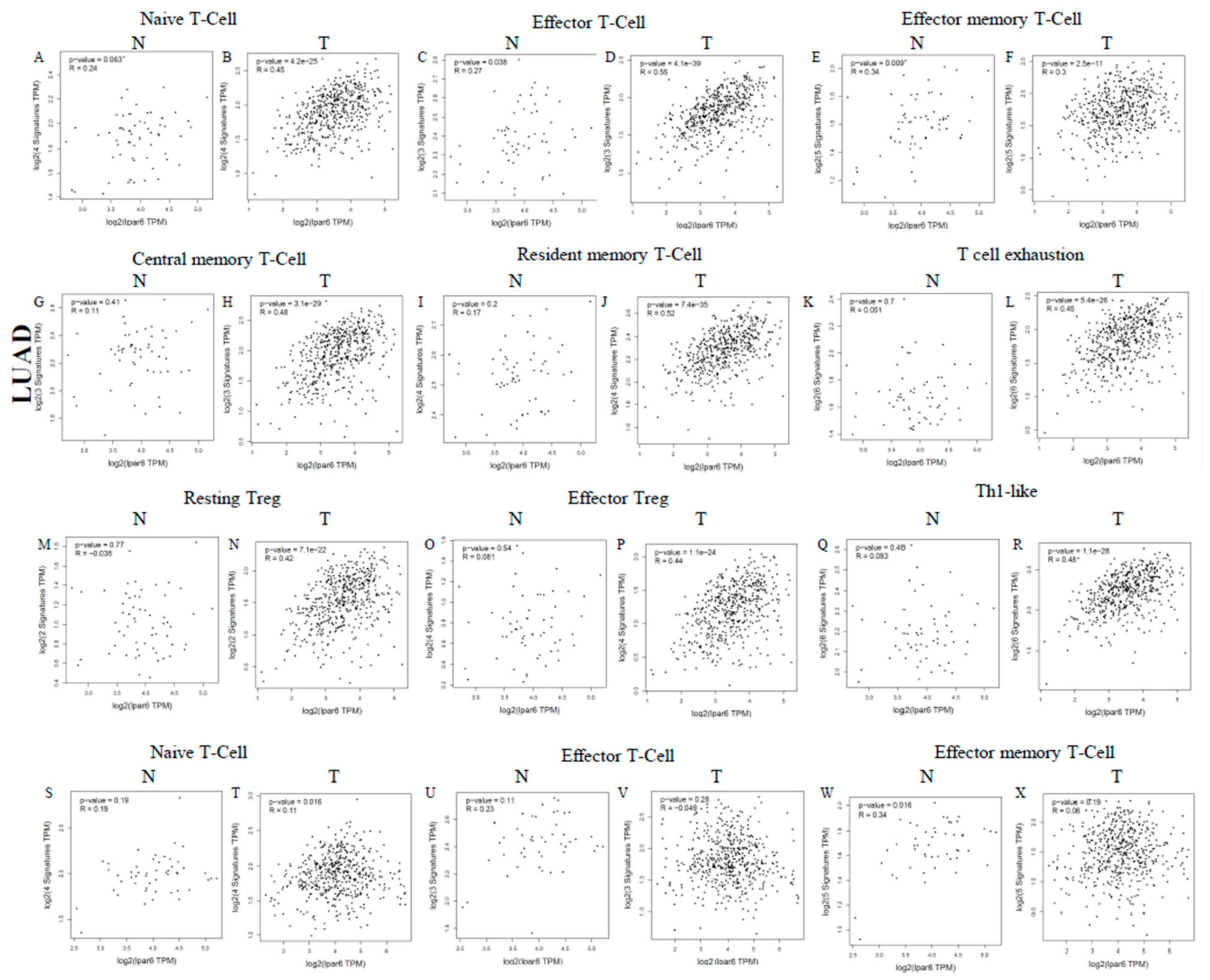
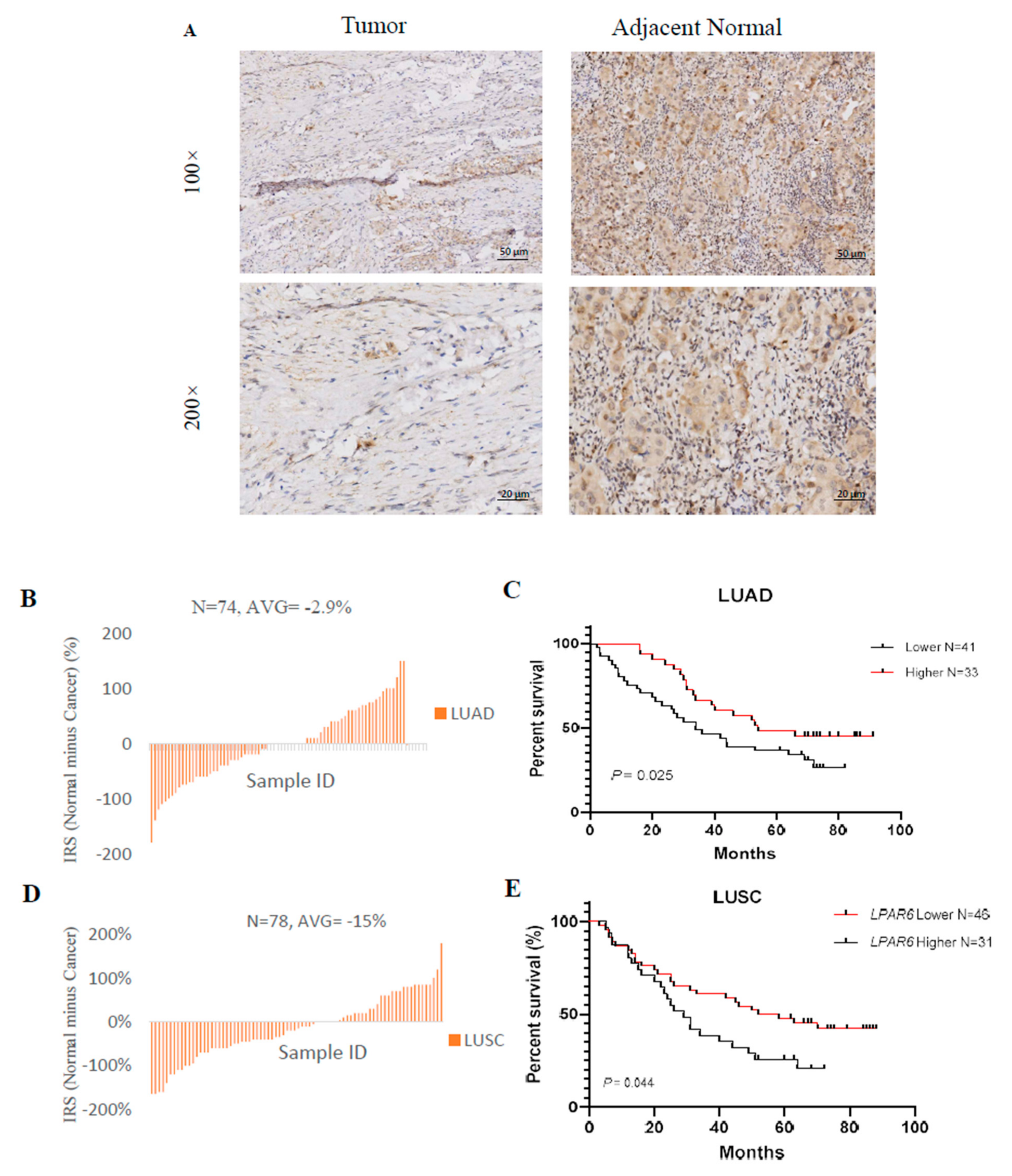
3.8. Different Correlation Patterns between Tumor and Normal Tissue in LUAD Patients
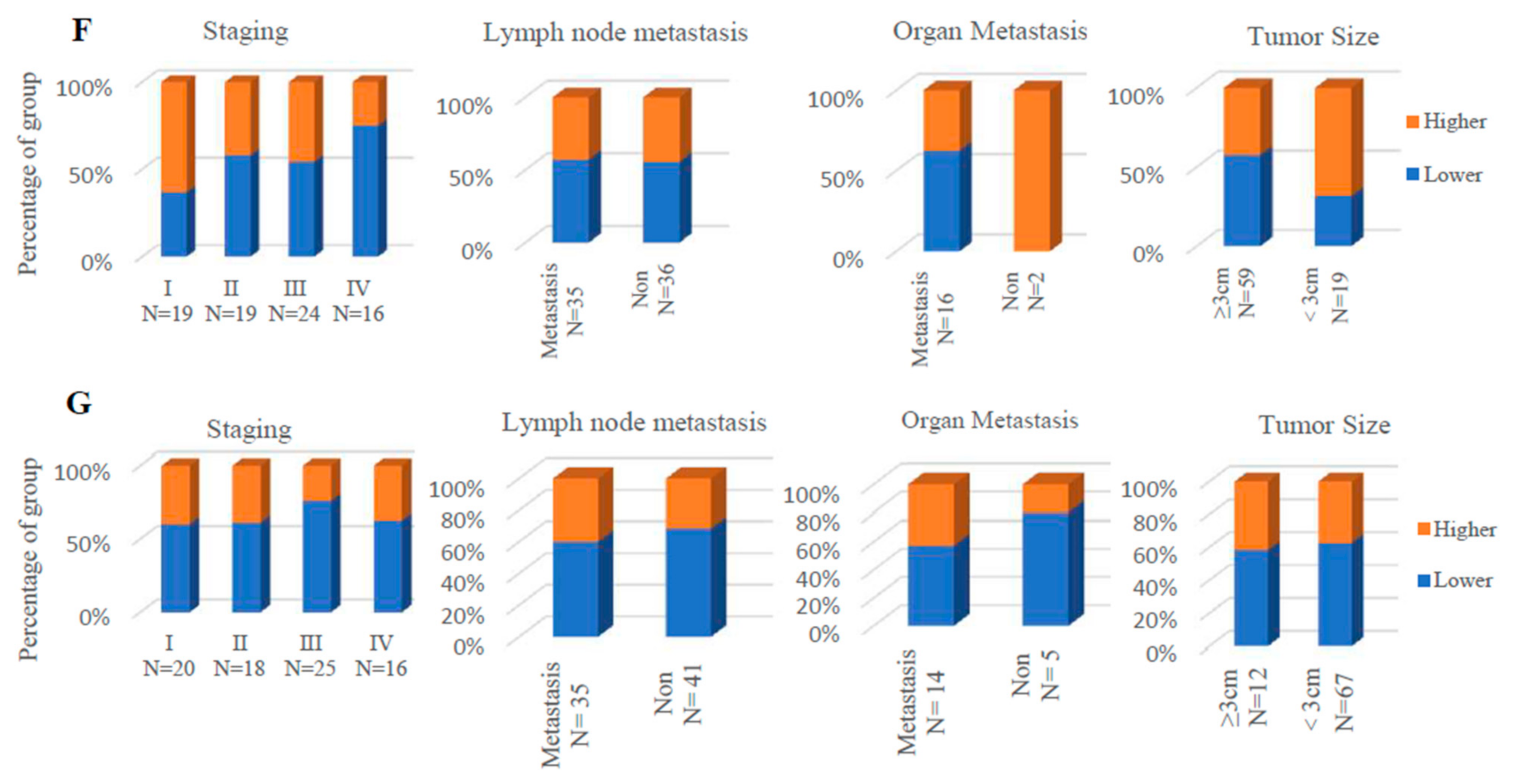
3.9. Higher Expression Level of LPAR6 Was Correlated with Clinicopathological Parameters in LUAD Cohort and Was Correlated with Increased OS of LUAD and LUSC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.F.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Lung Cancer. Available online: www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html (accessed on 31 December 2020).
- Yang, L.; Wang, L.; Zhang, Y. Immunotherapy for lung cancer: Advances and prospects. Am. J. Clin. Exp. Immunol. 2016, 5, 1–20. [Google Scholar]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest. Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [Green Version]
- Gelsomino, F.; Lamberti, G.; Parisi, C.; Casolari, L.; Melotti, B.; Sperandi, F.; Ardizzoni, A. The evolving landscape of immunotherapy in small-cell lung cancer: A focus on predictive biomarkers. Cancer Treat Rev. 2019, 79, 101887. [Google Scholar] [CrossRef]
- Vafadar, S. Immunotherapy for non-small cell lung cancer. JAAPA 2019, 32, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Neeve, S.C.; Robinson, B.W.; Fear, V.S. The role and therapeutic implications of T cells in cancer of the lung. Clin. Transl. Immunol. 2019, 8, e1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steven, A.; Fisher, S.A.; Robinson, B.W. Immunotherapy for lung cancer. Respirology 2016, 21, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C. Immunology: Another shot at cancer. Nature 2015, 527, S105–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbee, M.S.; Ogunniyi, A.; Horvat, T.Z.; Dang, T.O. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann. Pharmacother. 2015, 49, 907–937. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P. Pembrolizumab for the Treatment of Non-Small-Cell Lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.; Roviello, G.; Cretella, D.; Cavazzoni, A.; Biondi, A.; Cappelletti, M.R. Tumor-infiltrating lymphocytes and breast cancer: Beyond the prognostic and predictive utility. Tumour. Biol. 2017, 39, 1010428317695023. [Google Scholar] [CrossRef] [Green Version]
- Stanton, S.E.; Disis, M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef] [Green Version]
- Benevides, L.; da Fonseca, D.M.; Donate, P.B.; Tiezzi, D.G.; De Carvalho, D.D.; de Andrade, J.M. IL17 Promotes Mammary Tumor Progression by Changing the Behavior of Tumor Cells and Eliciting Tumorigenic Neutrophils Recruitment. Cancer Res. 2015, 75, 3788–3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toor, S.M.; Syed Khaja, A.S.; El Salhat, H.; Faour, I.; Kanbar, J.; Quadri, A.A. Myeloid cells in circulation and tumor microenvironment of breast cancer patients. Cancer Immunol. Immunother. 2017, 66, 753–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, Y.; Mai, J.; Li, X.; Mitchell-Flack, M.; Zhang, T.; Zhang, L. Targeting Autocrine CCL5-CCR5 Axis Reprograms Immunosuppressive Myeloid Cells and Reinvigorates Antitumor Immunity. Cancer Res. 2017, 77, 2857–2868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waniczek, D.; Lorenc, Z.; Snietura, M.; Wesecki, M.; Kopec, A.; Muc-Wierzgon, M. Tumor associated macrophages and regulatory T cells infiltration and the clinical outcome in colorectal cancer. Arch. Immunol. Ther. Exp. 2017, 65, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Gyamfi, J.; Jang, H.; Koo, J.S. The role of tumor associated macrophage in breast cancer biology. Histol. Histopathol. 2018, 33, 133–145. [Google Scholar]
- Tariq, M.; Zhang, J.; Liang, G.; Ding, L.; He, Q.; Yang, B. Macrophage Polarization: Anti-Cancer Strategies to Target Tumor-Associated Macrophage in Breast Cancer. J. Cell Biochem. 2017, 118, 2484–2501. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Romagnoli, E.; Saladino, T.; Foghini, L.; Guarino, S.; Capponi, M. Triple negative breast cancer: Key role of Tumor-Associated Macrophages in regulating the activity of anti-PD-1/PD-L1 agents. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 78–84. [Google Scholar] [CrossRef]
- Lopane, C.; Agosti, P.; Gigante, I. Implications of the lysophosphatidic acid signaling axis in liver cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 277–282. [Google Scholar] [CrossRef]
- Bailey, K.A.; Klymenko, Y.; Feist, P.E.; Hummon, A.B.; Stack, M.S.; Schultz, Z.D. Chemical analysis of morphological changes in lysophosphatidic acid-treated ovarian cancer cells. Sci. Rep. 2017, 7, 15295. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, Y.; Wajid, M.; Ishii, Y.; Shapiro, L.; Petukhova, L.; Gordon, D. Disruption of P2RY5, an orphan G protein-coupled receptor, underlies autosomal recessive woolly hair. Nat. Genet. 2008, 40, 335–339. [Google Scholar] [CrossRef]
- Taniguchi, R.; Inoue, A.; Sayama, M.; Uwamizu, A.; Yamashita, K.; Hirata, K.; Yoshida, M. Structural insights into ligand recognition by the lysophosphatidic acid receptor LPA6. Nature 2017, 548, 356–360. [Google Scholar] [CrossRef]
- Ketscher, A.; Jilg, C.A.; Willmann, D. LSD1 controls metastasis of androgen-independent prostate cancer cells through PXN and LPAR6. Oncogenesis 2014, 3, e120. [Google Scholar] [CrossRef]
- Mazzocca, A.; Dituri, F.; De Santis, F.; Filannino, A.; Lopane, C.; Betz, R.C. Lysophosphatidic acid receptor LPAR6 supports the tumorigenicity of hepatocellular carcinoma. Cancer Res. 2015, 75, 532–543. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, E.; Eheim, A.L.; Ahrens, W.A. Lysophosphatidic acid receptor expression and function in human hepatocellular carcinoma. J. Surg. Res. 2013, 180, 104–113. [Google Scholar] [CrossRef]
- Takahashi, K.; Fukushima, K.; Onishi, Y.; Inui, K.; Node, Y.; Fukushima, N. Lysophosphatidic acid (LPA) signaling via LPA4 and LPA6 negatively regulates cell motile activities of colon cancer cells. Biochem. Biophys. Res. Common. 2017, 483, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Katkoori, V.R.; Shanmugam, C.; Jia, X.; Vitta, S.P.; Sthanam, M.; Callens, T. Prognostic significance and gene expression profiles of p53 mutations in microsatellite- stable stage III colorectal adenocarcinomas. PLoS ONE 2012, 7, e30020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, S.; Hirane, M.; Fukushima, K. Diverse effects of LPA4, LPA5 and LPA6 on the activation of tumor progression in pancreatic cancer cells. Biochem. Biophys. Res. Common. 2015, 461, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, D.A.; Rhodes, D.R.; Sinha-Kumar, C.; Chinnaiyan, A.M. Bioinformatics approaches in the study of cancer. Curr. Mol. Med. 2007, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, H.; Kitada, K.; Nakai, K.; Sarai, A. PrognoScan: A new database for meta-analysis of the prognostic value of genes. BMC Med Genom. 2009, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Lanczky, A.; Nagy, A.; Bottai, G.; Munkacsy, G.; Szabo, A.; Santarpia, L. miRpower: A web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat 2016, 160, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Rodriguez, I.P.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Vasaikar, S.; Shi, Z.; Zhang, B.; Greer, M. WebGestalt: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017, 45, W130–W137. [Google Scholar] [CrossRef]
- Sousa, S.; Maatta, J. The role of tumour-associated macrophages in bone metastasis. J. Bone Oncol. 2016, 5, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aran, D.; Sirota, M.; Butte, A.J. Systematic pan-cancer analysis of tumour purity. Nat. Commun. 2015, 6, 8971. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaher, P.; Warren, S.; Dennis, L.; D’Amico, L.; White, A.; Disis, M.L. Gene expression markers of Tumor Infiltrating Leukocytes. J. Immunother. Cancer 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Duan, X.; Li, X.; Li, J.; Ba, Q.; Wang, H. HIPK2 suppresses tumor growth and progression of hepatocellular carcinoma through promoting the degradation of HIF-1α. Oncogene 2020, 39, 2863–2876. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Scolyer, R.A.; Rumcheva, P.; Moncrieff, M.; Murali, R.; McCarthy, S.W. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J. Clin. Oncol. 2012, 30, 2678–2683. [Google Scholar] [CrossRef]
- Ohtani, H. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun. 2007, 7, 4. [Google Scholar]
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591. [Google Scholar] [CrossRef]
- van Corven, E.J.; Groenink, A.; Jalink, K.; Eichholtz, T.; Moolenaar, W.H. Lysophosphatidate-induced cell proliferation: Identification and dissection of signaling pathways mediated by G proteins. Cell 1989, 59, 45–54. [Google Scholar] [CrossRef]
- Moolenaar, W.H.; van Meeteren, L.A.; Giepmans, B.N. The ins and outs of lysophosphatidic acid signaling. Bioessays 2004, 26, 870–881. [Google Scholar] [CrossRef]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [Green Version]
- Pasternack, S.M.; von Kugelgen, I.; Al Aboud, K.; Lee, Y.A.; Ruschendorf, F.; Voss, K. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat. Genet. 2008, 40, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.; Dituri, F.; Lupo, L.; Quaranta, M.; Antonaci, S.; Giannelli, G. Tumorsecreted lysophostatidic acid accelerates hepatocellular carcinoma progression by promoting differentiation of peritumoral fibroblasts in myofibroblasts. Hepatology 2011, 54, 920–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Zhu, H.; Wu, W. Clinical significance of lymphoid enhancer- binding factor 1 expression in acute myeloid leukemia. Leuk. Lymphoma 2014, 55, 371–377. [Google Scholar] [CrossRef]
- Ganapathi, S.K.; Beggs, A.D.; Hodgson, S.V.; Kumar, D. Expression and DNA methylation of TNF, IFNG and FOXP3 in colorectal cancer and their prognostic significance. Br. J. Cancer 2014, 111, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Fichtner-Feigl, S.; Strober, W.; Kawakami, K.; Puri, R.K.; Kitani, A. IL-13 signaling through the IL-13 alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 2006, 1, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Fujisawa, T.; Husain, S.R.; Joshi, B.; Puri, R.K. Interleukin 13 mediates signal transduction through interleukin 13 receptor alpha2 in pancreatic ductal adenocarcinoma: Role of IL-13 Pseudomonas exotoxin in pancreatic cancer therapy. Clin. Cancer Res. 2010, 2, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Clinicopathological Characteristics | Overall Survival (n = 364) | |||||
|---|---|---|---|---|---|---|
| LUAD (n = 720) | LUSC (n = 524) | |||||
| N | Hazard Ratio | p-Value | N | Hazard Ratio | p-Value | |
| Sex | ||||||
| Female | 318 | 0.39 (0.26–0.58) | 1.4 × 10−10 | 129 | 1.69 (0.94–3.01) | 0.075 |
| Male | 344 | 0.66 (0.48–0.93) | 0.015 | 342 | 0.79 (0.59–1.04) | 0.087 |
| Smoking history | ||||||
| Never | 143 | 0.4 (0.17–0.96) | 0.034 | 9 | --- | --- |
| Smoker | 246 | 0.49 (0.3–0.79) | 0.0029 | 820 | 0.89 (0.72–1.09) | 0.26 |
| Stage | ||||||
| 1 | 370 | 0.27 (0.17–0.42) | 4.6 × 10−10 | 172 | 0.75 (0.49–1.14) | 0.17 |
| 2 | 136 | 0.51 (0.31–0.84) | 0.0073 | 100 | 1.42 (0.76–2.65) | 0.27 |
| 3 | 24 | 2.1 (0.71–6.21) | 0.17 | 43 | 0.48 (0.24–0.96) | 0.035 |
| 4 | 4 | --- | --- | 0 | --- | --- |
| Description | Gene Markers | LUAD | LUSC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| None | Purity | None | Purity | ||||||
| Cor | p | Cor | p | Cor | p | Cor | p | ||
| CD8+ T cell | CD8A | 0.363 | *** | 0.238 | *** | 0.121 | * | 0.065 | 0.157 |
| CD8B | 0.366 | *** | 0.281 | *** | 0.231 | *** | 0.196 | *** | |
| T cell (general) | CD3D | 0.451 | *** | 0.318 | *** | 0.197 | *** | 0.136 | * |
| CD3E | 0.434 | *** | 0.283 | *** | 0.163 | ** | 0.092 | 0.045 | |
| CD2 | 0.49 | *** | 0.358 | *** | 0.171 | ** | 0.101 | 0.0277 | |
| Naive T-Cell | CCR7 | 0.39 | *** | 0.222 | *** | 0.175 | *** | 0.109 | 0.0175 |
| LEF1 | 0.351 | *** | 0.241 | *** | −0.002 | 0.962 | 0.014 | 0.766 | |
| TCF7 | 0.237 | *** | 0.102 | 0.0232 | 0.095 | 0.0336 | 0.049 | 0.289 | |
| SELL | 0.421 | *** | 0.26 | *** | 0.187 | *** | 0.115 | 0.12 | |
| Effector T-Cell | CX3CR1 | 0.41 | *** | 0.353 | *** | 0.113 | 0.0113 | 0.055 | 0.23 |
| FGFBP2 | 0.247 | *** | 0.185 | *** | −0.04 | 0.378 | −0.02 | 0.656 | |
| FCGR3A | 0.448 | *** | 0.354 | *** | 0.042 | 0.35 | −0.044 | 0.34 | |
| Effector memory T-Cell | PDCD1 | 0.316 | *** | 0.175 | *** | 0.112 | 0.0121 | 0.045 | 0.323 |
| DUSP4 | −0.074 | 0.0915 | −0.075 | 0.0966 | −0.017 | 0.707 | −0.057 | 0.212 | |
| GZMK | 0.444 | *** | 0.309 | *** | 0.172 | ** | 0.106 | 0.0204 | |
| GZMA | 0.408 | *** | 0.295 | *** | 0.197 | *** | 0.143 | * | |
| IFNG | 0.304 | *** | 0.201 | *** | 0.101 | 0.0235 | 0.061 | 0.183 | |
| Resident memory T-Cell | CD69 | 0.518 | *** | 0.423 | *** | 0.247 | *** | 0.192 | *** |
| ITGAE | 0.295 | *** | 0.228 | *** | 0.109 | 0.0149 | 0.09 | 0.0493 | |
| CXCR6 | 0.434 | *** | 0.305 | *** | 0.161 | ** | 0.095 | 0.0389 | |
| MYADM | 0.162 | ** | 0.064 | 0.156 | −0.132 | * | −0.197 | *** | |
| B cell | CD19 | 0.341 | *** | 0.192 | *** | 0.157 | ** | 0.083 | 0.0694 |
| CD79A | 0.312 | *** | 0.171 | ** | 0.155 | ** | 0.076 | 0.096 | |
| Monocyte | CD86 | 0.55 | *** | 0.455 | *** | 0.164 | ** | 0.079 | 0.085 |
| CD115 (CSF1R) | 0.495 | *** | 0.388 | *** | 0.071 | 0.111 | −0.031 | 0.498 | |
| TAM | CCL2 | 0.424 | *** | 0.331 | *** | 0.148 | ** | 0.086 | 0.0611 |
| CD68 | 0.387 | *** | 0.291 | *** | 0.012 | 0.785 | −0.087 | 0.0576 | |
| IL10 | 0.523 | *** | 0.433 | *** | 0.211 | *** | 0.151 | ** | |
| M1 Macrophage | INOS (NOS2) | 0.14 | * | 0.075 | 0.0955 | 0.104 | 0.0203 | 0.106 | 0.0203 |
| IRF5 | 0346 | *** | 0.254 | *** | −0.101 | 0.0236 | −0.129 | ** | |
| COX2 (PTGS2) | 0.009 | 0.833 | 0.017 | 0.705 | 0.27 | *** | 0.244 | *** | |
| M2 Macrophage | CD163 | 0.376 | *** | 0.281 | *** | 0.032 | 0.472 | −0.058 | 0.209 |
| VSIG4 | 0.438 | *** | 0.358 | *** | 0.061 | 0.171 | −0.021 | 0.649 | |
| MS4A4A | 0.501 | *** | 0.412 | *** | 0.109 | 0.0145 | 0.028 | 0.536 | |
| Neutrophils | CD66b (CEACAM8) | 0.114 | * | 0.09 | 0.0464 | 0.023 | 0.613 | 0.006 | 0.894 |
| CD11b (ITGAM) | 0.405 | *** | 0.29 | *** | 0.091 | 0.0412 | −0.006 | 0.898 | |
| CCR7 | 0.39 | *** | 0.222 | *** | 0.175 | *** | 0.109 | 0.0175 | |
| Natural killer cell | KIR2DL1 | 0.117 | * | 0.064 | 0.155 | 0.081 | 0.0704 | 0.052 | 0.258 |
| KIR2DL3 | 0.209 | *** | 0.13 | * | 0.013 | 0.776 | 0.013 | 0.776 | |
| KIR2DL4 | 0.179 | *** | 0.11 | 0.0145 | 0.08 | 0.0744 | 0.039 | 0.4 | |
| KIR3DL1 | 0.149 | ** | 0.075 | 0.098 | 0.005 | 0.902 | −0.048 | 0.294 | |
| KIR3DL2 | 0.168 | ** | 0.078 | 0.083 | 0.015 | 0.737 | −0.038 | 0.41 | |
| KIR3DL3 | 0.039 | 0.38 | 0.006 | 0.899 | −0.116 | * | −0.142 | * | |
| KIR2DS4 | 0.143 | * | 0.065 | 0.149 | 0.052 | 0.245 | 0.026 | 0.572 | |
| Dendritic cell | HLA-DPB1 | 0.463 | *** | 0.353 | *** | 0.098 | 0.0279 | 0.014 | 0.759 |
| HLA-DQB1 | 0.315 | *** | 0.195 | *** | 0.098 | 0.0279 | 0.014 | 0.759 | |
| HLA-DRA | 0.476 | *** | 0.376 | *** | 0.135 | * | 0.062 | 0.178 | |
| HLA-DPA1 | 0.447 | *** | 0.343 | *** | 0.106 | 0.0172 | 0.029 | 0.521 | |
| BDCA-1 (CD1C) | 0.382 | *** | 0.294 | *** | 0.196 | *** | 0.131 | * | |
| BDCA-4 (NRP1) | 0.18 | *** | 0.137 | * | 0.052 | 0.247 | −0.014 | 0.767 | |
| CD11c (ITGAX) | 0.474 | *** | 0.364 | *** | 0.168 | ** | 0.082 | 0.074 | |
| Th1 | TBX21 (T-bet) | 0.355 | *** | 0.216 | *** | 0.119 | * | 0.051 | 0.266 |
| STAT4 | 0.394 | *** | 0.267 | *** | 0.195 | *** | 0.122 | * | |
| STAT1 | 0.193 | *** | 0.075 | 0.094 | 0.032 | 0.477 | −0.018 | 0.689 | |
| IFNG (IFN-g) | 0.304 | *** | 0.201 | *** | 0.101 | 0.0235 | 0.061 | 0.183 | |
| TNF-a (TNF) | 0.414 | *** | 0.291 | *** | 0.277 | *** | 0.229 | *** | |
| Th2 | GATA3 | 0.365 | *** | 0.231 | *** | 0.187 | *** | 0.143 | * |
| STAT6 | 0.007 | 0.873 | 0.019 | 0.681 | 0.257 | *** | 0.259 | *** | |
| STAT5A | 0.459 | *** | 0.33 | *** | 0.15 | ** | 0.082 | 0.0722 | |
| IL13 | 0.209 | *** | 0.128 | 0.0213 | 0.076 | 0.0818 | 0.037 | 0.423 | |
| Tfh | BCL6 | 0.022 | 0.612 | 0.018 | 0.684 | 0.08 | 0.0729 | 0.105 | 0.0223 |
| IL21 | 0.118 | ** | 0.038 | 0.402 | 0.034 | 0.452 | −0.013 | 0.782 | |
| Th17 | STAT3 | −0.138 | ** | −0.147 | ** | 0.129 | * | 0.109 | 0.0168 |
| IL17A | 0.177 | *** | 0.11 | 0.014 | 0.051 | 0.254 | 0.025 | 0.58 | |
| Treg | FOXP3 | 0.364 | *** | 0.207 | *** | 0.145 | * | 0.063 | 0.172 |
| CCR8 | 0.382 | *** | 0.242 | *** | 0.162 | ** | 0.083 | 0.0706 | |
| STAT5B | 0.212 | *** | 0.18 | *** | −0.071 | 0.114 | −0.076 | 0.096 | |
| TGFB1 (TGFb) | 0.304 | *** | 0.201 | *** | 0.123 | ** | 0.123 | *** | |
| T cell exhaustion | PDCD1 (PD-1) | 0.316 | *** | 0.175 | *** | 0.112 | 0.0121 | 0.045 | 0.323 |
| CTLA4 | 0.444 | *** | 0.304 | *** | 0.217 | *** | 0.152 | ** | |
| LAG3 | 0.275 | *** | 0.152 | *** | 0.095 | 0.0329 | 0.034 | 0.457 | |
| HAVCR2 (TIM-3) | 0.539 | *** | 0.442 | *** | 0.094 | 0.0357 | 0.008 | 0.867 | |
| GZMB | 0.268 | *** | 0.15 | *** | 0.137 | ** | 0.077 | 0.0926 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Gao, R.; Meng, M.; Yu, M.; Liu, C.; Li, J.; Song, Y.; Wang, H. Lysophosphatidic Acid Receptor 6 (LPAR6) Is a Potential Biomarker Associated with Lung Adenocarcinoma. Int. J. Environ. Res. Public Health 2021, 18, 11038. https://doi.org/10.3390/ijerph182111038
He J, Gao R, Meng M, Yu M, Liu C, Li J, Song Y, Wang H. Lysophosphatidic Acid Receptor 6 (LPAR6) Is a Potential Biomarker Associated with Lung Adenocarcinoma. International Journal of Environmental Research and Public Health. 2021; 18(21):11038. https://doi.org/10.3390/ijerph182111038
Chicago/Turabian StyleHe, Jian, Rui Gao, Mei Meng, Miao Yu, Chengrong Liu, Jingquan Li, Yizhi Song, and Hui Wang. 2021. "Lysophosphatidic Acid Receptor 6 (LPAR6) Is a Potential Biomarker Associated with Lung Adenocarcinoma" International Journal of Environmental Research and Public Health 18, no. 21: 11038. https://doi.org/10.3390/ijerph182111038
APA StyleHe, J., Gao, R., Meng, M., Yu, M., Liu, C., Li, J., Song, Y., & Wang, H. (2021). Lysophosphatidic Acid Receptor 6 (LPAR6) Is a Potential Biomarker Associated with Lung Adenocarcinoma. International Journal of Environmental Research and Public Health, 18(21), 11038. https://doi.org/10.3390/ijerph182111038






