Systemic Sclerosis: Elevated Levels of Leukotrienes in Saliva and Plasma Are Associated with Vascular Manifestations and Nailfold Capillaroscopic Abnormalities
Abstract
1. Introduction
2. Materials and Methods
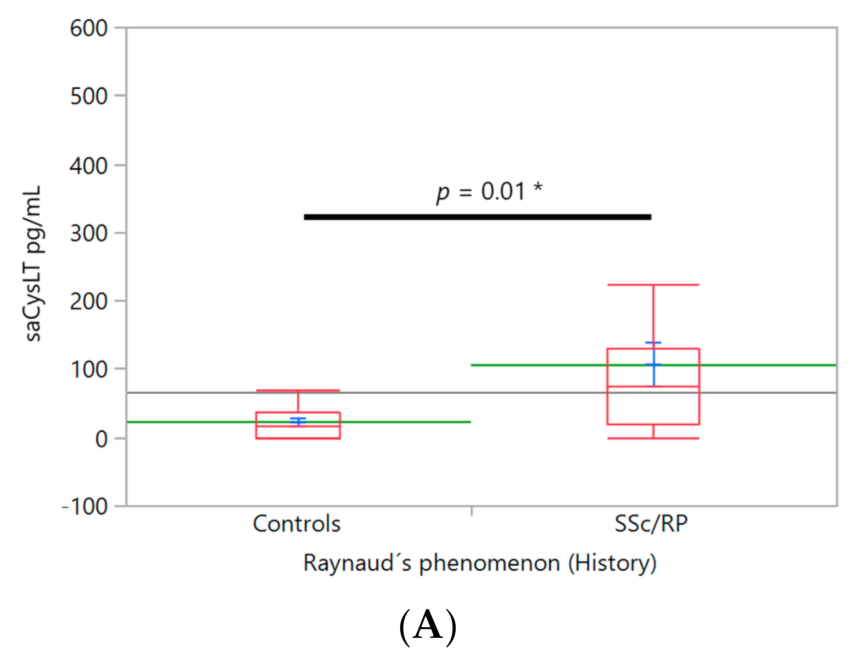
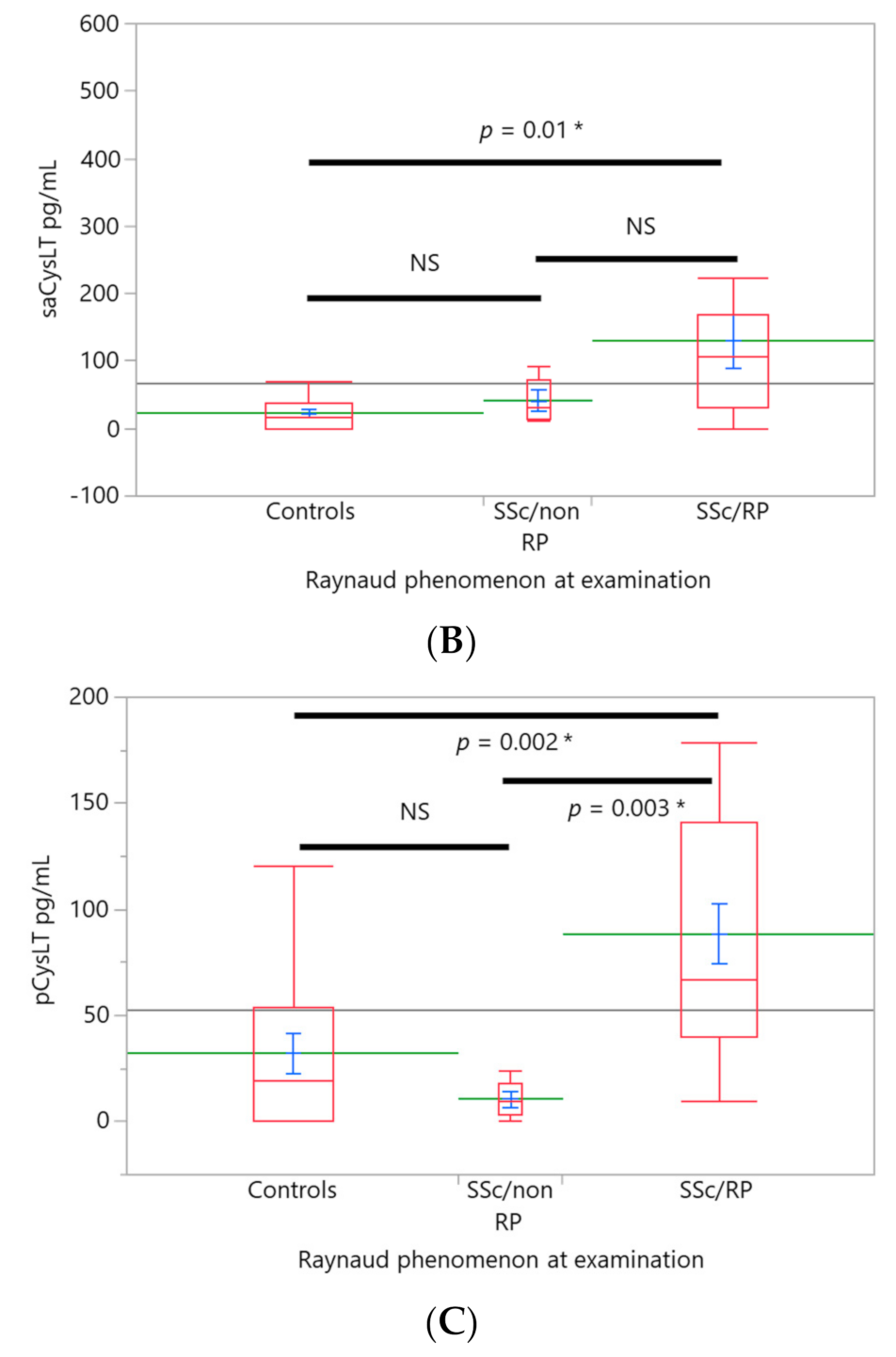
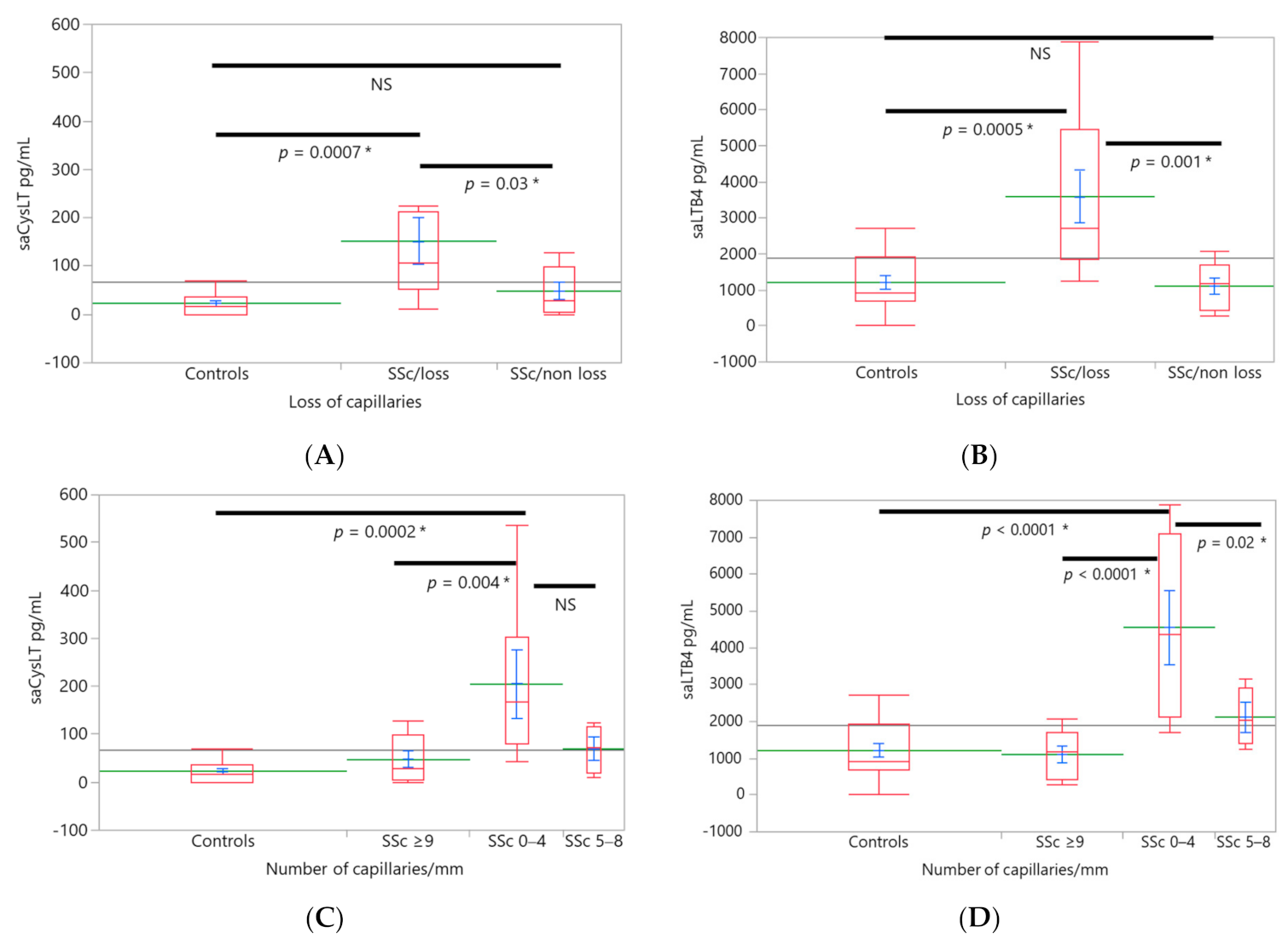
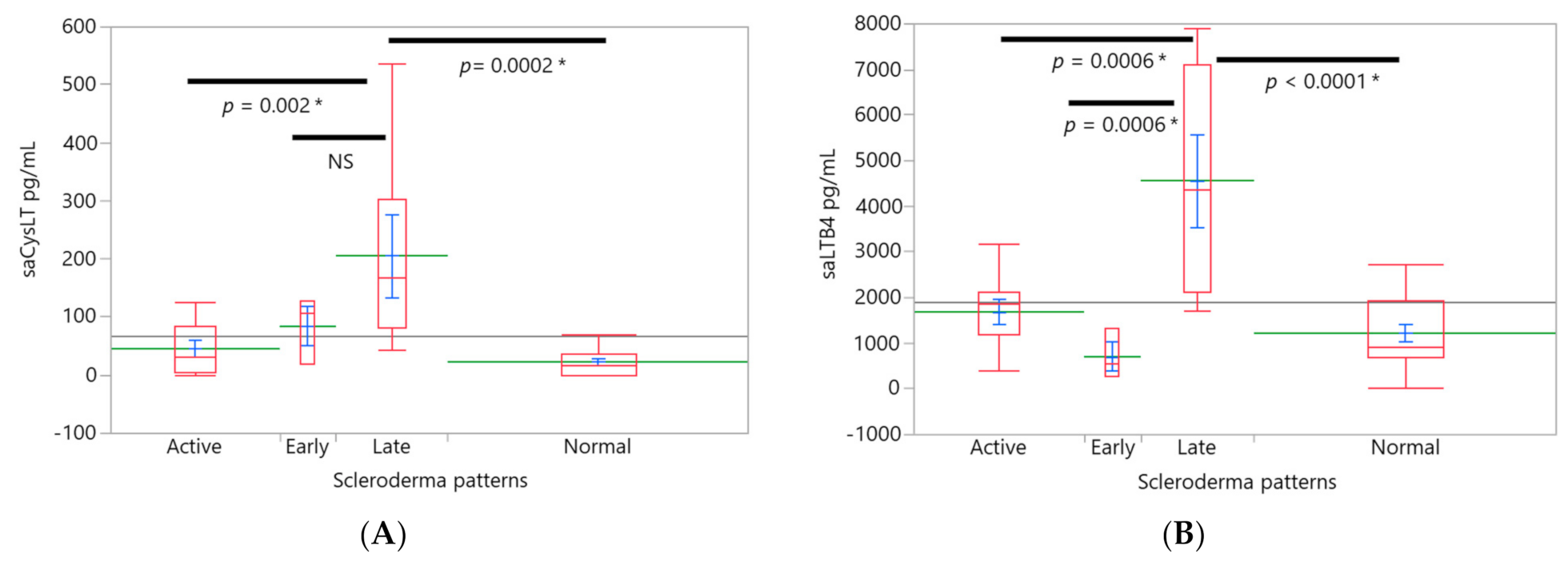
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef]
- Hughes, M.; Herrick, A.L. Systemic sclerosis. Br. J. Hosp. Med. 2019, 80, 530–536. [Google Scholar] [CrossRef]
- Orlandi, M.; Lepri, G.; Damiani, A.; Barsotti, S.; Di Battista, M.; Codullo, V.; Della Rossa, A.; Guiducci, S.; Allanore, Y. One year in review 2020: Systemic sclerosis. Clin. Exp. Rheumatol. 2020, 38, S3–S17. [Google Scholar]
- Steen, V.D.; Medgser, T.A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 2007, 66, 940–944. [Google Scholar] [CrossRef]
- Rubio-Rivas, M.; Royo, C.; Simeón, C.P.; Corbella, X.; Fonollosa, V. Mortality and survival in systemic sclerosis: Systematic review and meta-analysis. Semin. Arthritis. Rheum. 2014, 44, 208–219. [Google Scholar] [CrossRef]
- Varga, J.A.; Trojanowska, M. Fibrosis in systemic sclerosis. Rheum. Dis. Clin. N. Am. 2008, 34, 115–143. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wei, J.; Varga, J. Understanding fibrosis in systemic sclerosis: Shifting paradigms, emerging opportunities. Nat. Rev. Rheumatol. 2011, 8, 42–54. [Google Scholar] [CrossRef]
- Huang, S.K.; Peters-Golden, M. Eicosanoid Lipid Mediators in Fibrotic Lung Diseases: Ready for Prime Time? Chest 2008, 133, 1442–1450. [Google Scholar] [CrossRef]
- Boyce, J.A. Mast cells and eicosanoid mediators: A system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007, 217, 168–185. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Henderson, W.R. Leukotrienes. N. Engl. J. Med. 2007, 357, 1841–1854. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar]
- Li, X.; Xie, M.; Lu, C.; Mao, J.; Cao, Y.; Yang, Y.; Wei, Y.; Liu, X.; Cao, S.; Song, Y.; et al. Design and synthesis of Leukotriene A4 hydrolase inhibitors to alleviate idiopathic pulmonary fibrosis and acute lung injury. Eur. J. Med. Chem. 2020, 203, 112614. [Google Scholar] [CrossRef]
- Castelino, F.V. Lipids and eicosanoids in fibrosis: Emerging targets for therapy. Curr. Opin. Rheumatol. 2012, 24, 649–655. [Google Scholar] [CrossRef]
- Chwieśko-Minarowska, S.; Kowal, K.; Bielecki, M.; Kowal-Bielecka, O. The role of leukotrienes in the pathogenesis of systemic sclerosis. Folia. Histochem. Cytobiol. 2012, 50, 180–185. [Google Scholar] [CrossRef][Green Version]
- Kim, N.D.; Luster, A.D. Regulation of immune cells by eicosanoid receptors. Sci. World. J. 2007, 7, 1307–1328. [Google Scholar] [CrossRef]
- Beller, T.C.; Friend, D.S.; Maekawa, A.; Lam, B.K.; Austen, K.F.; Kanaoka, Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc. Natl. Acad. Sci. USA 2004, 101, 3047–3052. [Google Scholar] [CrossRef]
- Lv, J.; Xiong, Y.; Li, W.; Yang, W.; Zhao, L.; He, R. BLT1 Mediates Bleomycin-Induced Lung Fibrosis Independently of Neutrophils and CD4+ T Cells. J. Immunol. 2017, 198, 1673–1684. [Google Scholar] [CrossRef]
- Topaloğlu, N.; Olgun, Y.; Şener, G.; Laçin, T.; Şehirli, O.; Bozkurtlar, E.; Çelikel, C.; Ceyhan, B. Protective effect of cysteinyl leukotriene receptor antagonist montelukast in bleomycin-induced pulmonary fibrosis. Turk. Gogus. Kalp. Dama. 2018, 26, 588–597. [Google Scholar] [CrossRef]
- Hirata, H.; Arima, M.; Fukushima, Y.; Sugiyama, K.; Tokuhisa, T.; Fukuda, T. Leukotriene C4 aggravates bleomycin-induced pulmonary fibrosis in mice. Respirology 2013, 18, 674–681. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Distler, O.; Kowal, K.; Siergiejko, Z.; Chwie?ko, J.; Sulik, A.; Gay, R.E.; Łukaszyk, A.B.; Gay, S.; Sierakowski, S. Elevated levels of leukotriene B4 and leukotriene E4 in bronchoalveolar lavage fluid from patients with scleroderma lung disease. Arthritis. Rheum. 2003, 48, 1639–1646. [Google Scholar] [CrossRef]
- Woo, C.H.; You, H.J.; Cho, S.H.; Eom, Y.W.; Chun, J.S.; Yoo, Y.J.; Kim, J.H. Leukotriene B4 stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J. Biol. Chem. 2002, 277, 8572–8578. [Google Scholar] [CrossRef]
- Goldstein, R.E. Involvement of leucocytes and leukotrienes in ischaemic dysfunction of the coronary microcirculation. Eur. Heart J. 1990, 11, 16–26. [Google Scholar] [CrossRef]
- Michelassi, F.; Shahinian, H.K.; Ferguson, M.K. Effects of leukotrienes B4, C4, and D4 on rat mesenteric microcirculation. J. Surg. Res. 1987, 42, 475–482. [Google Scholar] [CrossRef]
- Garcia, J.G.N.; Noonan, T.C.; Jubiz, W.; Malik, A.B. Leukotrienes and the pulmonary microcirculation. Am. Rev. Respir. Dis. 1987, 136, 161–169. [Google Scholar] [CrossRef]
- Steiner, D.R.; Gonzalez, N.C.; Wood, J.G. Leukotriene B4 promotes reactive oxidant generation and leukocyte adherence during acute hypoxia. J. Appl. Physiol. 2001, 91, 1160–1167. [Google Scholar] [CrossRef]
- Duah, E.; Adapala, R.; Al-Azzam, N.; Kondeti, V.; Gombedza, F.; Thodeti, C.K.; Paruchuri, S. Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT2 and CysLT1 receptors. Sci. Rep. 2013, 3, 3274. [Google Scholar] [CrossRef]
- Simms, R.-W.; Korn, J.H. The Role of Leukotrienes in alveolitis associated with scleroderma. Arthritis. Rheum. 2003, 48, 1478–1480. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Distler, O.; Neidhart, M.; Künzler, P.; Rethage, J.; Nawrath, M.; Carossino, A.; Pap, T.; Müller-Ladner, U.; Michel, B.A.; et al. Evidence of 5-lipoxygenase overexpression in the skin of patients with systemic sclerosis: A newly identified pathway to skin inflammation in systemic sclerosis. Arthritis. Rheum. 2001, 44, 1865–1875. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Kowal, K.; Distler, O.; Rojewska, J.; Bodzenta-Lukaszyk, A.; Michel, B.A.; Gay, R.E.; Gay, S.; Sierakowski, S. Cyclooxygenase- and lypoxygenase-derived eicosanoids in bronchoalveolar lavage fluid from patients with scleroderma lung disease: An imbalance between proinflammatory and antiinflammatory lipid mediators. Arthritis. Rheum. 2005, 52, 3783–3791. [Google Scholar] [CrossRef]
- Tufvesson, E.; Bozovic, G.; Hesselstrand, R.; Bjermer, L.; Scheia, A.; Wuttge, D.M. Increased cysteinyl-leukotrienes and 8-isoprostane in exhaled breath condensate from systemic sclerosis patients. Rheumatology 2010, 49, 2322–2326. [Google Scholar] [CrossRef]
- Xiao, R.; Yoshida, N.; Higashi, Y.; Lu, Q.J.; Fukushige, T.; Kanzaki, T.; Kanekura, T. Retinoic acids exhibit anti-fibrotic activity through the inhibition of 5-lipoxygenase expression in scleroderma fibroblasts. J. Dermatol. 2011, 38, 345–353. [Google Scholar] [CrossRef]
- Liang, M.; Lv, J.; Jiang, Z.; He, H.; Chen, C.; Xiong, Y.; Zhu, X.; Xue, Y.; Yu, Y.; Yang, S.; et al. Promotion of myofibroblast differentiation and tissue fibrosis by the leukotriene B4-leukotriene B4 receptor axis in systemic sclerosis. Arthritis. Rheumatol. 2020, 72, 1013–1025. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Cutolo, M. Capillaroscopy. Best. Pract. Res. Clin. Rheumatol. 2008, 22, 1093–1108. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Smith, V. How to perform and interpret capillaroscopy. Best. Pract. Res. Clin. Rheumatol. 2013, 27, 237–248. [Google Scholar] [CrossRef]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gaetano, J. Holm-Bonferroni Sequential Correction: An EXCEL Calculator (ver.1.2); Microsoft Excel: Redmond, WA, USA, 2013. [Google Scholar]
- Bäck, M. Leukotriene receptors: Crucial components in vascular inflammation. Sci. World. J. 2007, 7, 1422–1439. [Google Scholar] [CrossRef] [PubMed]
- Rovati, G.E.; Capra, V. Cysteinyl-leukotriene receptors and cellular signals. Sci. World. J. 2007, 7, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; O’Dowd, A.; Belch, J.J. White blood cell activation in Raynaud’s phenomenon of systemic sclerosis and vibration induced white finger syndrome. Ann. Rheum. Dis. 1992, 51, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.N.; Bernardini, E.M.; Salles, E.F.; Heinzmann, F.; Marconde, F.; Mello, T.; Gomes, B.E. CysLT1 receptor inhibition in patients with Raynaud´s phenomenon- capillaroscopic evidence of the role of leukotriene. Rev. Bras. Reumatol. 2012, 52, 27–32. [Google Scholar]
- Lefebvre, B.; Pépin, J.L.; Baguet, J.P.; Tamisier, R.; Roustit, M.; Riedweg, K.; Bessard, G.; Lévy, P.; Stanke-Labesque, F. Leukotriene B4: Early mediator of atherosclerosis in obstructive sleep apnoea? Eur. Respir. J. 2008, 32, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Stanke-Labesque, F.; Pépin, J.L.; de Jouvencel, T.; Arnaud, C.; Baguet, J.P.; Petri, M.H.; Tamisier, R.; Jourdil, J.F.; Lévy, P.; Bäck, M. Leukotriene B4 pathway activation and atherosclerosis in obstructive sleep apnea. J. Lipid. Res. 2012, 53, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Kylhammar, D.; Rådegran, G. The principal pathways involved in the in vivo modulation of hypoxic pulmonary vasocontriction, pulmonary aarterial remodelling and pulmonary hypertension. Acta. Physiol. 2017, 219, 728–756. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Haribabu, B.; Mathis, S.P.; Kim, J.; Gozal, D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am. J. Respir. Crit. Care. Med. 2011, 184, 124–131. [Google Scholar] [CrossRef]
- Colazzo, F.; Gelosa, P.; Tremoli, E.; Sironi, L.; Castiglioni, L. Role of the cysteinyl leukotrienes in the pathogenesis and progression of cardiovascular disease. Mediat. Inflamm. 2017, 2017, 2432958. [Google Scholar] [CrossRef]
- Hoxha, M.; Rovati, G.E.; Cavanillas, A.B. The leukotriene receptor antagonist montelukast and its posible role in the cardiovascular field. Eur. J. Clin. Pharmacol. 2017, 73, 799–809. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in aterosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [PubMed]
- Wang, B.; Lujin, W.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechnisms and potencial therapeutic targets. Signal. Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Carry, M.; Korley, V.; Willerson, J.T.; Weigelt, L.; Ford-Hutchinson, A.W.; Tagari, P. Increased urinary leukotriene excretion in patients with cardiac ischemia. In vivo Evidence for 5-Lipoxygenase activation. Circulation 1992, 85, 230–236. [Google Scholar] [CrossRef]
- Nair, J.; Shanker, J.; Arvind, P.; Jambunathan, S.; Kakkar, V.V. Association of leukotriene gene variants and plasma LTB4 levels with coronary artery disease in Asian Indians. ISRN Vasc. Med. 2013, 2013, 85743. [Google Scholar] [CrossRef]
- Nobili, E.; Salvado, M.D.; Folkersen, L.; Castiglioni, L.; Kastrup, J.; Wetterholm, A.; Tremoli, E.; Hansson, G.K.; Sironi, L.; Haeggström, J.Z.; et al. Cysteinyl leukotriene signaling aggravates myocardial hypoxia in experimental atherosclerotic heart disease. PLoS ONE 2012, 7, e41786. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, C.S.; Kalra, V.K. Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a-5p. J. Immunol. 2010, 184, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Dada, L.A.; Wu, M.; Kelly, A.; Trejo, H.; Zhou, Q.; Varga, J.; Sznajder, J.I. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2009, 297, L1120–L1130. [Google Scholar] [CrossRef]
- Beyer, C.; Schett, G.; Gay, S.; Distler, O.; Distler, J.H. Hypoxia. Hypoxia in the pathogenesis of systemic sclerosis. Arthritis. Res. Ther. 2009, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.; Jüngel, A.; Pileckyte, M.; Zwerina, J.; Michel, B.A.; Gay, R.E.; Kowal-Bielecka, O.; Matucci-Cerinic, M.; Schett, G.; Marti, H.H.; et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis. Rheum. 2007, 56, 4203–4215. [Google Scholar] [CrossRef]
- Wright, L.; Tuder, R.M.; Wang, J.; Cool, C.D.; Lepley, R.A.; Voelkel, N.F. 5-lipoxygenase and 5-lipoxygenase activating protein (FLAP) immune reactivity in lungs from patients with primary pulmonary hypertension. Am. J. Respir. Crit. Care. Med. 1998, 157, 219–229. [Google Scholar] [CrossRef]
- Riccioni, G.; Bäck, M.; Capra, V. Leukotrienes and atherosclerosis. Curr. Drug Targets. 2010, 11, 882–887. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Grechko, A.V.; Myasedova, V.A.; Orekhov, A.N. Potential of anti-inflammatory agent for treatment of aterosclerosis. Exp. Mol. Pathol. 2018, 104, 114–124. [Google Scholar] [CrossRef]
- Riccioni, G.; Zanasi, A.; Vitulano, N.; Mancini, B.; D´Orazio, N. Leukotrienes in atherosclerosis: New target insights and future therapy perspectives. Mediators. Inflamm. 2009, 2009, 737282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Datta, Y.H.; Romano, M.; Jacobson, B.C.; Golan, D.E.; Serhan, C.N.; Ewenstein, B.M. Peptido-leukotrienes are potent agonists of von Willebrand factor secretion and P-Selectin surface expression in human umbilical vein endothelial cells. Circulation 1995, 92, 3304–3311. [Google Scholar] [CrossRef]
- Sakata, K.; Dahlén, S.E.; Bäck, M. The contractile action of leukotriene B4 in the guinea-pig lung involves a vascular component. Br. J. Pharmacol. 2004, 141, 449–456. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc. Natl. Acad. Sci. USA 1986, 83, 2204–2208. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Bu, D.X.; Bränström, R.; Sheikine, Y.; Yan, Z.Q.; Hansson, G.K. Leukotriene B4 signaling through NF-?B-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc. Natl. Acad. Sci. USA 2005, 102, 17501–17506. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Walker, J.L.; Huang, A.; Keaney, J.F.; Clish, C.B.; Serhan, C.N.; Loscalzo, J. Expression of 5-lipoxygenase in pulmonary artery endothelial cells. Biochem. J. 2002, 361, 267–276. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Tuder, R.M.; Wade, K.; Höper, M.; Lepley, R.A.; Goulet, J.L.; Koller, B.H.; Fitzpatrick, F. Inhibition of 5-lipoxygenase-activating protein (FLAP) reduces pulmonary vascular reactivity and pulmonary hypertension in hypoxic rats. J. Clin. Investig. 1996, 97, 2491–2498. [Google Scholar] [CrossRef]
- Rola-Pleszczynski, M.; Stankova, J. Cytokine-leukotriene receptor interations. Sci. World J. 2007, 7, 1348–1358. [Google Scholar] [CrossRef]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef]
- Bäck, M.; Hlawaty, H.; Labat, C.; Michel, J.B.; Brink, C. The oral cavity and age: A site of chronic inflammation? PLoS ONE 2007, 2, e1351. [Google Scholar] [CrossRef]
- Ono, E.; Taniguchi, M.; Higashi, N.; Mita, H.; Yamaguchi, H.; Tatsuno, S.; Fukutomi, Y.; Tanimoto, H.; Sekiya, K.; Oshikata, C.; et al. Increase in salivary cysteinyl-leukotriene concentration in patients with aspirin-intolerant asthma. Allergol. Int. 2011, 60, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Allanore, Y.; Chung, L.; Pauling, J.D.; Denton, C.P.; Matucci-Cerinic, M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat. Rev. Rheumatol. 2020, 16, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Soldano, S.; Smith, V. Pathophysiology of systemic sclerosis: Current understanding and new insights. Expert. Rev. Clin. Immunol. 2019, 15, 753–764. [Google Scholar] [CrossRef] [PubMed]
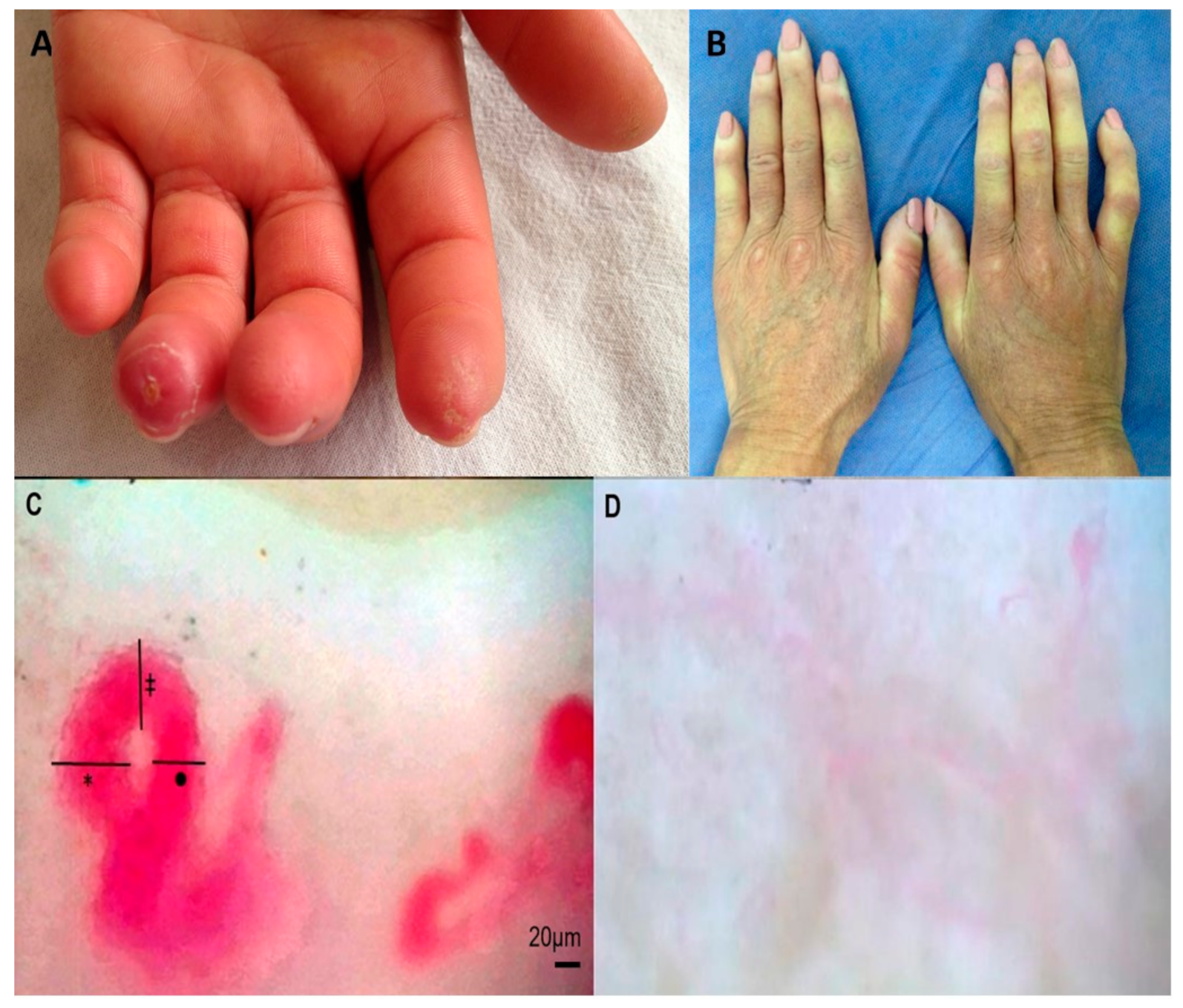
| Variable | SSc | Controls |
|---|---|---|
| Age, mean (range), years | 48.9 (18–61) | 36.8 (20–69) |
| BMI Kg/m2, mean | 24.4 | 23.6 |
| Sex, females/males, n | 20/0 | 16/0 |
| Smoking history, n | 1 | 1 |
| Disease duration since diagnosis mean (range), years | 6.64 (0–27) | - |
| TSS (range), mean | 14.4 (2–41) | 0 |
| dcSSc/lcSSc, n | 8, 12 | 0 |
| RP history, n | 19 | 0 |
| RP at examination, n | 15 | 0 |
| DU history, n | 6 | 0 |
| Fingertip scars, n | 13 | 0 |
| SLD, n | 3 | - |
| PAH, n | 8 | - |
| ANA, n | 20 | - |
| Scl-70, n | 4 | - |
| ACA, n | 11 | - |
| Treatment | - | |
| Micofenolic acid, n (dose range) | 8 (0.5–2 g) | |
| Methotrexate, n (dose range) | 6 (12.5–15 mg) | |
| Prednisone, n (dose range) | 2 (10–15 mg) | |
| Nifedipine, n | 7 | |
| Angiotensin receptor blockers, n | 3 | |
| Angiotensin-converting enzyme inhibitors, n | 3 | |
| Acetylsalicylic acid, n | 2 (100 mg) | |
| Capillaroscopy, scleroderma patterns, n | ||
| Early | 5 | 0 |
| Active | 10 | 0 |
| Late | 5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandujano, A.; Méndez-Ramírez, I.; Silveira-Torre, L.H. Systemic Sclerosis: Elevated Levels of Leukotrienes in Saliva and Plasma Are Associated with Vascular Manifestations and Nailfold Capillaroscopic Abnormalities. Int. J. Environ. Res. Public Health 2021, 18, 10841. https://doi.org/10.3390/ijerph182010841
Mandujano A, Méndez-Ramírez I, Silveira-Torre LH. Systemic Sclerosis: Elevated Levels of Leukotrienes in Saliva and Plasma Are Associated with Vascular Manifestations and Nailfold Capillaroscopic Abnormalities. International Journal of Environmental Research and Public Health. 2021; 18(20):10841. https://doi.org/10.3390/ijerph182010841
Chicago/Turabian StyleMandujano, Angélica, Ignacio Méndez-Ramírez, and Luis Humberto Silveira-Torre. 2021. "Systemic Sclerosis: Elevated Levels of Leukotrienes in Saliva and Plasma Are Associated with Vascular Manifestations and Nailfold Capillaroscopic Abnormalities" International Journal of Environmental Research and Public Health 18, no. 20: 10841. https://doi.org/10.3390/ijerph182010841
APA StyleMandujano, A., Méndez-Ramírez, I., & Silveira-Torre, L. H. (2021). Systemic Sclerosis: Elevated Levels of Leukotrienes in Saliva and Plasma Are Associated with Vascular Manifestations and Nailfold Capillaroscopic Abnormalities. International Journal of Environmental Research and Public Health, 18(20), 10841. https://doi.org/10.3390/ijerph182010841






